A Highly Selective and Sensitive Fluorescent Chemosensor for Detecting Al3+ Ion in Aqueous Solution and Plant Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Probe L
2.2. Plant Materials and Growth Conditions
2.3. Use of Probe L to Determine Al Concentration in Rice
2.4. In Situ Detection of Reactive Oxygen Species (ROS)
3. Results and Discussion
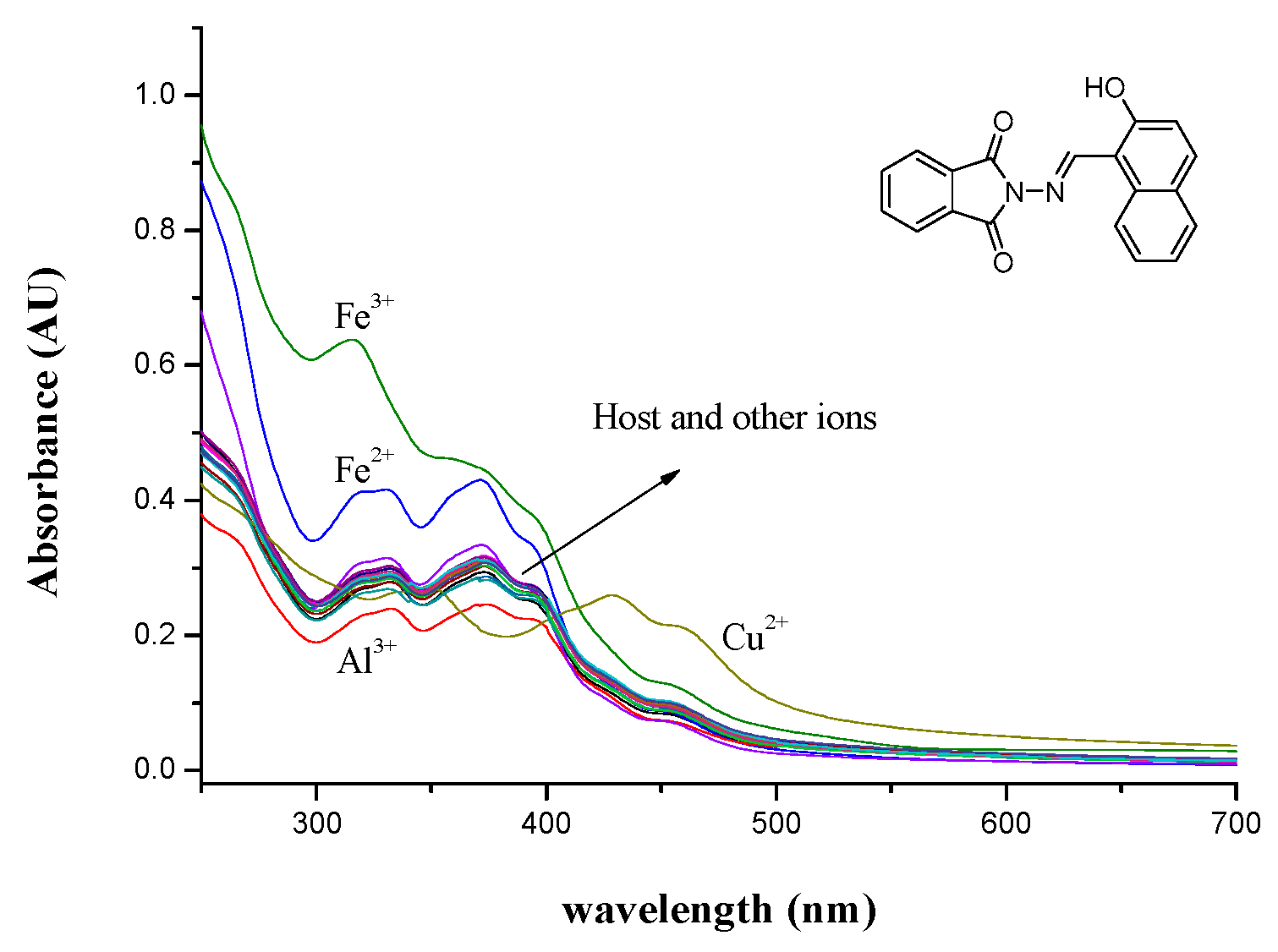
3.1. Absorption and Fluorescence Studies of Probe L Toward Various Metal Ions
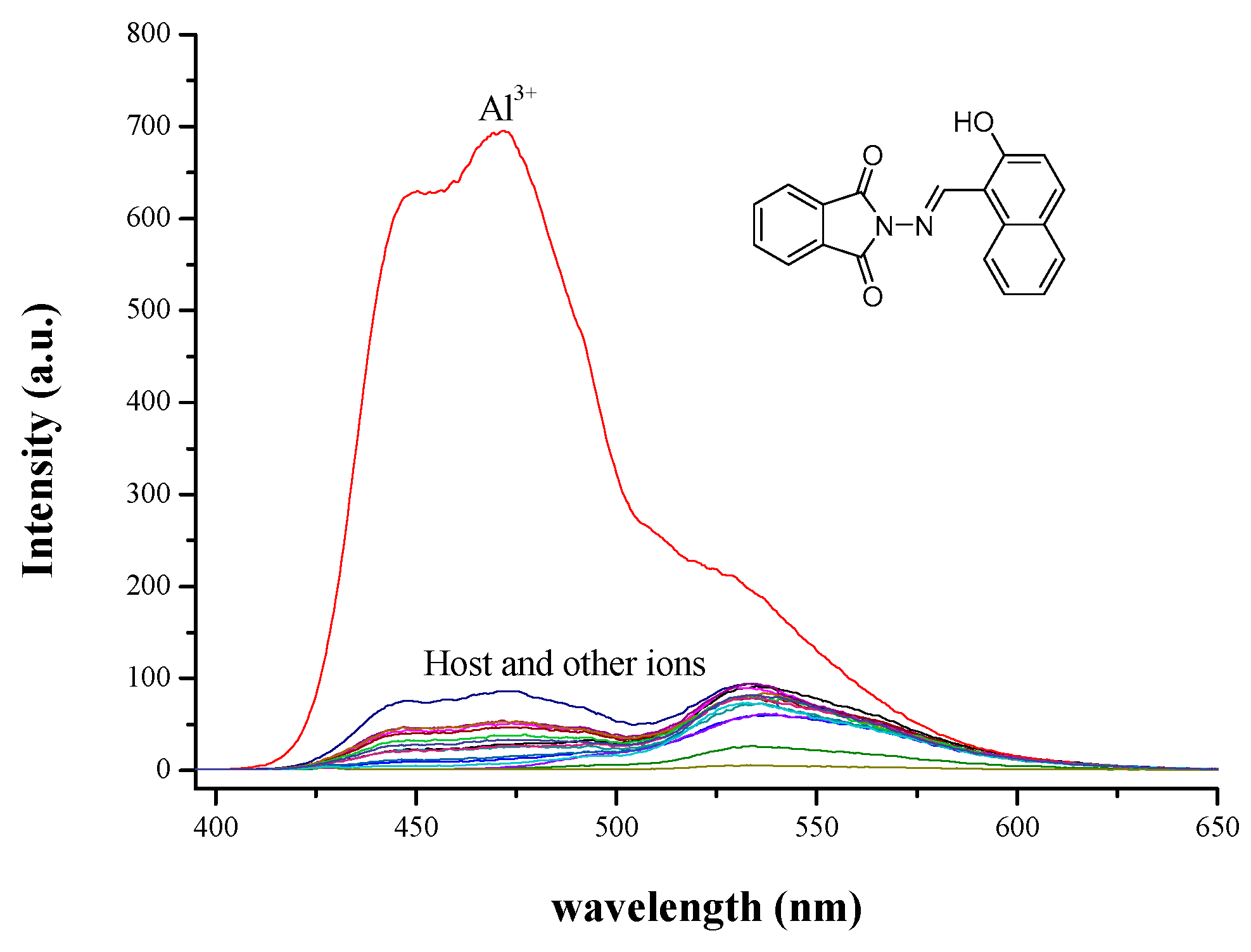
3.2. Fluorescence Titration and Binding Studies
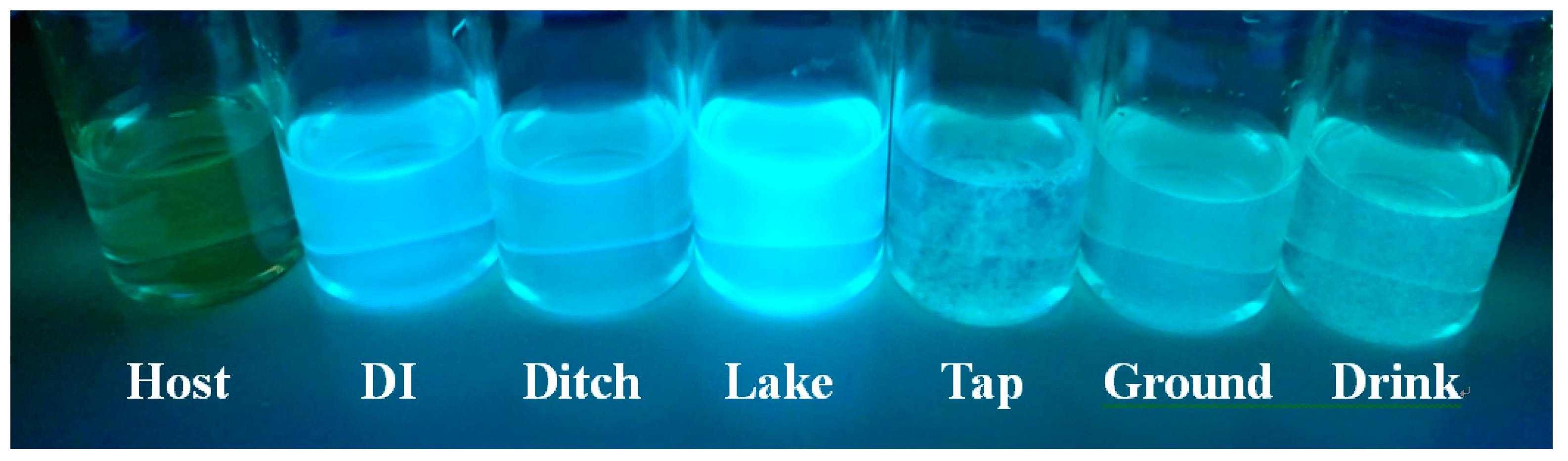
3.3. Application in Water
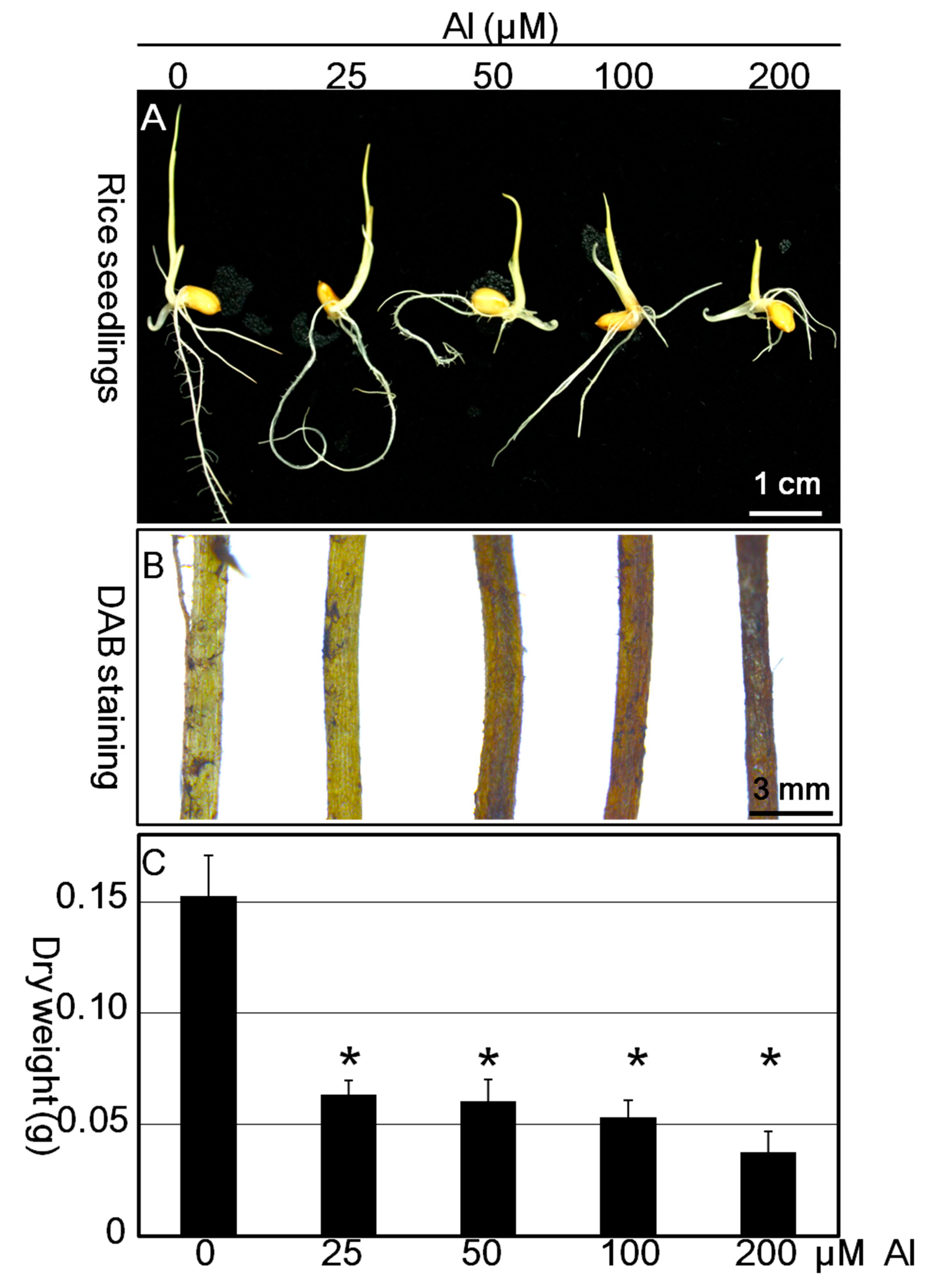
3.4. Effect of Al3+ Stress on Root Elongation and Oxidative Burst in Rice
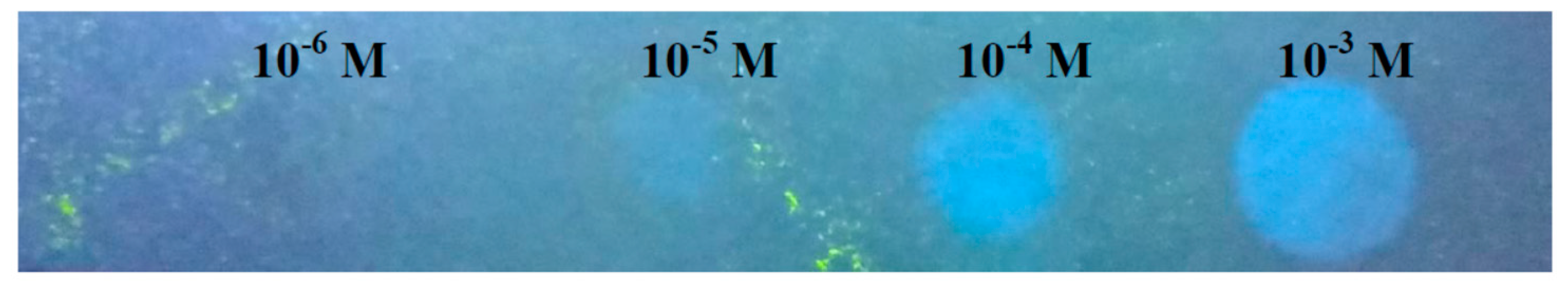
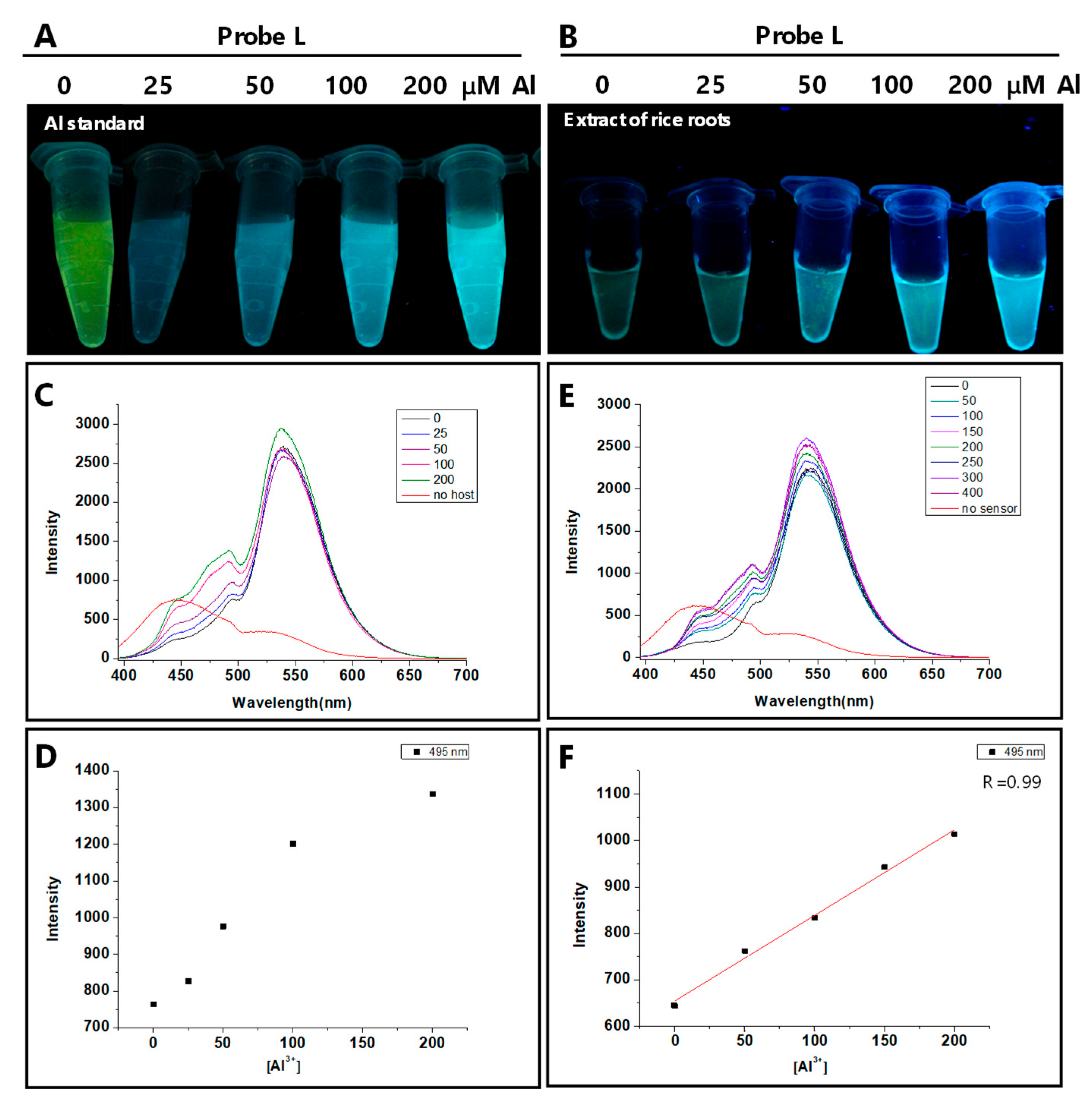
3.5. Practical Applications of Probe L for Quantifying Al Content in Rice Seedlings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Carvalho, J.R.P. Influence of aluminum in nutrient solutions on chemical composition in upland rice cultivars. Plant Soil 1982, 69, 31–44. [Google Scholar] [CrossRef]
- Vasconcelos, S.S.; Jacob-Neto, J.; Rossiello, R.O.P. Differential root responses to aluminum stress among Brazilian rice genotypes. J. Plant Nutr. 2002, 25, 655–669. [Google Scholar] [CrossRef]
- Alvarez, E.; Fernandez-Marcos, M.L.; Monterroso, C.; Fernandez-Sanjurjo, M.J. Application of aluminium toxicity indices to soils under various forest species. For. Ecol. Manag. 2005, 211, 227–239. [Google Scholar] [CrossRef]
- Savory, J.; Ghribi, O.; Forbes, M.S.; Herman, M.M. Aluminium and neuronal cell injury: Inter-relationships between neurofilamentous arrays and apoptosis. J. Inorg. Biochem. 2001, 87, 15–19. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 2006, 2, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Croom, J.; Taylor, I.L. Neuropeptide Y, peptide YY and aluminum in Alzheimer’s disease: Is there an etiological relationship? J. Inorg. Biochem. 2001, 87, 51–56. [Google Scholar] [CrossRef]
- Perlmutter, J.S.; Tempel, L.W.; Black, K.J.; Parkinson, D.; Todd, R.D. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology 1997, 49, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Hau, F.K.; He, X.M.; Lam, W.H.; Yam, V.W. Highly selective ion probe for Al3+ based on Au(I) … Au(I) interactions in a bis-alkynyl calix[4]arene Au(I) isocyanide scaffold. Chem. Commun. 2011, 47, 8778–8780. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Naphthaldehyde–Urea/Thiourea Conjugates as Turn-On Fluorescent Probes for Al3+ Based on Restricted C=N Isomerization. Eur. J. Inorg. Chem. 2011, 36, 5479–5489. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Conformationally Constrained (Coumarin−Triazolyl−Bipyridyl) Click Fluoroionophore as a Selective Al3+ Sensor. Inorg. Chem. 2010, 49, 7229–7231. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, S.S.; Liu, Y.Y.; He, S.; Zhao, L.C.; Zeng, X.S. Highly Selective and Sensitive Fluorescent Turn-on Chemosensor for Al3+ Based on a Novel Photoinduced Electron Transfer Approach. Org. Lett. 2011, 13, 5274–5277. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.K.; Nam, U.C.; Kwon, H.L.; Hwang, I.H.; Kim, C. A selective colorimetric and fluorescent chemosensor based-on naphthol for detection of Al3+ and Cu2+. Dyes Pigm. 2013, 99, 6–13. [Google Scholar] [CrossRef]
- Sahana, A.; Banerjee, A.; Lohar, S.; Sarkar, B.; Mukhopadhyay, S.K. Rhodamine-Based Fluorescent Probe for Al3+ through Time-Dependent PET–CHEF–FRET Processes and Its Cell Staining Application. Inorg. Chem. 2013, 52, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, H.S.; Kim, J.; Lee, S.J.; Quang, D.T.; Kim, J.S. Novel Optical/Electrochemical Selective 1,2,3-Triazole Ring-Appended Chemosensor for the Al3+ Ion. Org. Lett. 2010, 12, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.L.; Justino, L.L.G.; Salvador, A.I.N.; de Sousa, A.R.E.; Abreu, P.E.; Fonseca, S.M.; Burrows, H.D. NMR, DFT and luminescence studies of the complexation of Al(III) with 8-hydroxyquinoline-5-sulfonate. Dalton Trans. 2012, 41, 12478–12489. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Han, S.J.; Chun, J.; Lee, C.; Yoon, J. New thiazolothiazole derivatives as fluorescent chemosensors for Cr3+ and Al3+. Dyes Pigm. 2012, 94, 423–426. [Google Scholar] [CrossRef]
- Han, T.Y.; Feng, X.; Tong, B.; Shi, J.B.; Chen, L.; Zhi, J.G.; Dong, Y.P. A novel “turn-on” fluorescent chemosensor for the selective detection of Al3+ based on aggregation-induced emission. Chem. Commun. 2012, 48, 416–418. [Google Scholar] [CrossRef]
- Wang, F.; Nandhakumar, R.; Moon, J.H.; Kim, K.M.; Lee, J.Y.; Yoon, J. Ratiometric Fluorescent Chemosensor for Silver Ion at Physiological pH. Inorg. Chem. 2011, 50, 2240–2245. [Google Scholar] [CrossRef]
- Mahato, P.; Saha, S.; Suresh, E.; Liddo, R.D.; Parnigotto, P.P.; Conconi, M.T.; Kesharwani, M.K.; Ganguly, B.; Das, A. Ratiometric Detection of Cr3+ and Hg2+ by a Naphthalimide-Rhodamine Based Fluorescent Probe. Inorg. Chem. 2012, 51, 1769–1777. [Google Scholar] [CrossRef]
- Xuan, W.; Chen, C.; Cao, Y.; He, W.; Jiang, W.; Liu, K.; Wang, W. Rational design of a ratiometric fluorescent probe with a large emission shift for the facile detection of Hg2+. Chem. Commun. 2012, 48, 7292–7294. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.-W.; Peng, Y. A Selective and Ratiometric Bifunctional Fluorescent Probe for Al3+ Ion and Proton. Org. Lett. 2012, 14, 3420–3423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, L.; Geng, F.; Zhang, F.; Xu, M. Design of a dual-signaling sensing system for fluorescent ratiometric detection of Al3+ ion based on the inner-filter effect. Analyst 2011, 136, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Paul, S.; Manna, A. A differentially selective chemosensor for a ratiometric response to Zn2+ and Al3+ in aqueous media with applications for molecular switches. RSC Adv. 2013, 3, 25079–25085. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem. Commun. 2012, 48, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Aich, K.; Das, A.K.; Manna, A.; Das, S. A naphthalimide–quinolone based probe for selective, fluorescence ratiometric sensing of trivalent ions. RSC Adv. 2013, 3, 2412–2416. [Google Scholar] [CrossRef]
- Sahana, A.; Banerjee, A.; Lohar, S.; Banik, A.; Mukhopadhyay, S.K.; Safin, D.A.; Babashkina, M.G.; Bolte, M.; Garcia, Y.; Das, D. FRET based tri-color emissive rhodamine–pyrene conjugate as an Al3+ selective colorimetric and fluorescence sensor for living cell imaging. Dalton Trans. 2013, 42, 13311–13314. [Google Scholar] [CrossRef]
- Kumar, J.; Sarma, M.J.; Phukan, P.; Das, D.K. A new simple Schiff base fluorescence “on” sensor for Al3+ and its living cell imaging. Dalton Trans. 2015, 44, 4576–4581. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; You, G.R.; Choi, Y.W.; Jo, H.Y.; Kim, A.R.; Noh, I.; Kim, S.-J.; Kim, Y.; Kim, C. A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: An application to bioimaging. Dalton Trans. 2014, 43, 6650–6659. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Ali, S.S.; Maiti, K.; Manna, S.K.; Maji, R.; Mondal, S.; Uddin, M.R.; Mandal, S.; Sahoo, P. Aminomethylpyrene-based imino-phenols as primary fluorescence switch-on sensors for Al3+ in solution and in Vero cells and their complexes as secondary recognition ensembles toward pyrophosphate. RSC Adv. 2015, 5, 81203–81211. [Google Scholar] [CrossRef]
- Ranatunge, I.; Adikary, S.; Dasanayake, P.; Fernando, C.D.; Soysa, P. Development of a rapid and simple method to remove polyphenols from plant extracts. Int. J. Anal. Chem. 2017, 2017, 7230145. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.; Bahkali, A.; Moslem, M.; Amin, O.E.; Niessen, L. An optimized protocol for DNA extraction from wheat seeds and Loop-Mediated Isothermal Amplification (LAMP) to detect Fusarium graminearum contamination of wheat grain. Int. J. Mol. Sci. 2011, 12, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Nguyen, Q.T.T.; Fu, S.F.; Lin, C.Y.; Chen, Y.C.; Huang, H.J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef]
- Chen, G.K.; Li, X.B.; He, H.Z.; Li, H.S.; Zhang, Z.M. Varietal differences in the growth of rice seedlings exposed to perchlorate and their antioxidative defense mechanisms. Environ. Toxicol. Chem. 2015, 34, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
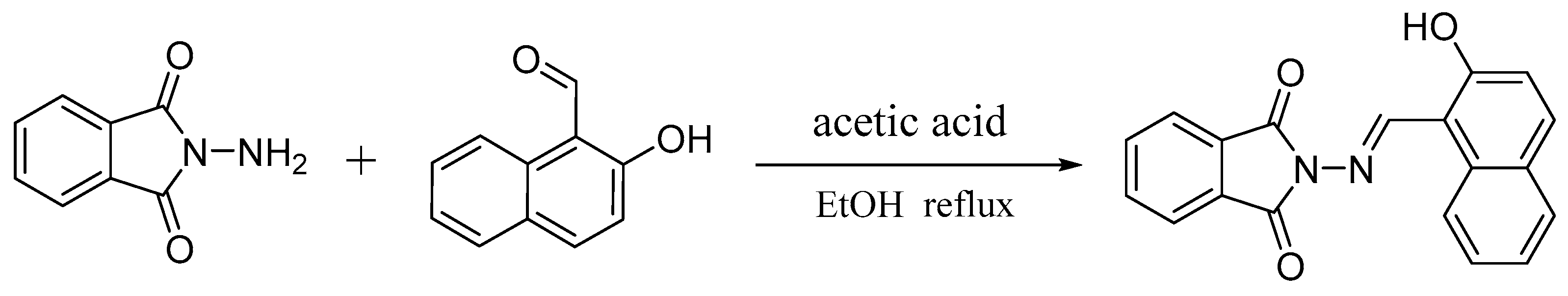


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Lu, P.-H.; Fu, S.-F.; Wu, A.-T. A Highly Selective and Sensitive Fluorescent Chemosensor for Detecting Al3+ Ion in Aqueous Solution and Plant Systems. Sensors 2019, 19, 623. https://doi.org/10.3390/s19030623
Li C-L, Lu P-H, Fu S-F, Wu A-T. A Highly Selective and Sensitive Fluorescent Chemosensor for Detecting Al3+ Ion in Aqueous Solution and Plant Systems. Sensors. 2019; 19(3):623. https://doi.org/10.3390/s19030623
Chicago/Turabian StyleLi, Chia-Lin, Ping-Hsuan Lu, Shih-Feng Fu, and An-Tai Wu. 2019. "A Highly Selective and Sensitive Fluorescent Chemosensor for Detecting Al3+ Ion in Aqueous Solution and Plant Systems" Sensors 19, no. 3: 623. https://doi.org/10.3390/s19030623





