Cortical Network Response to Acupuncture and the Effect of the Hegu Point: An fNIRS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. NIRS Recording
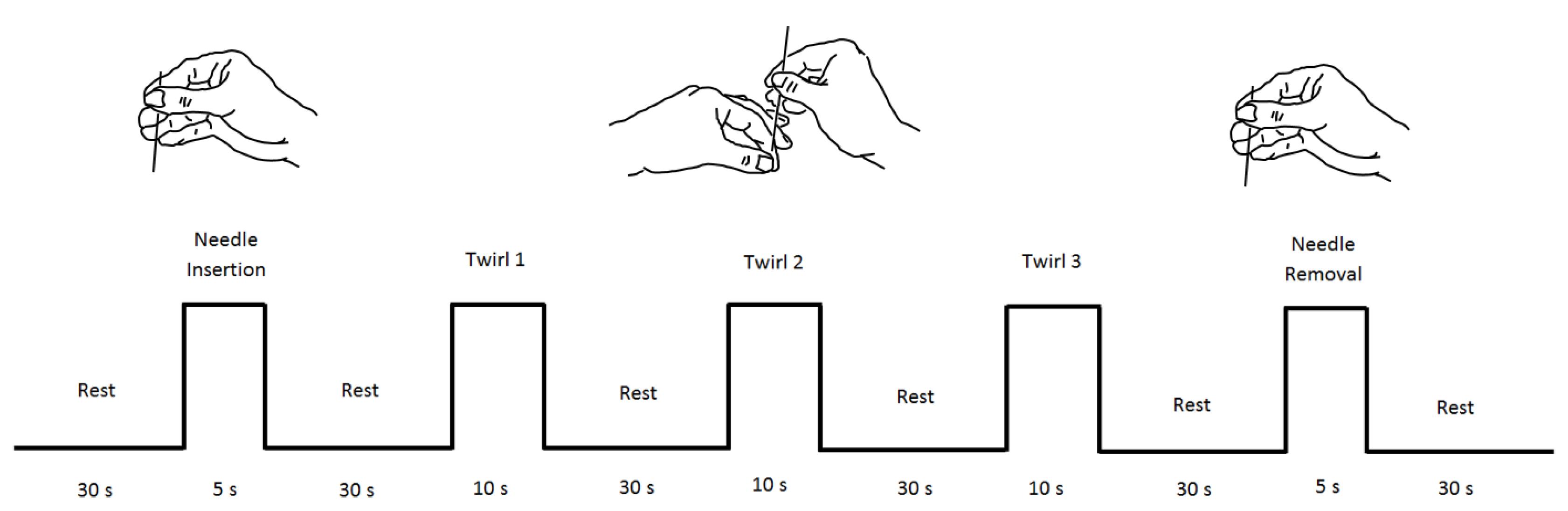
2.2. Stimuli
2.3. Participants
2.4. Data Analysis
2.4.1. Wavelet Transform
2.4.2. Wavelet Coherence
2.4.3. Bilateral Connectivity Analysis
2.5. Statistical Analysis
3. Results
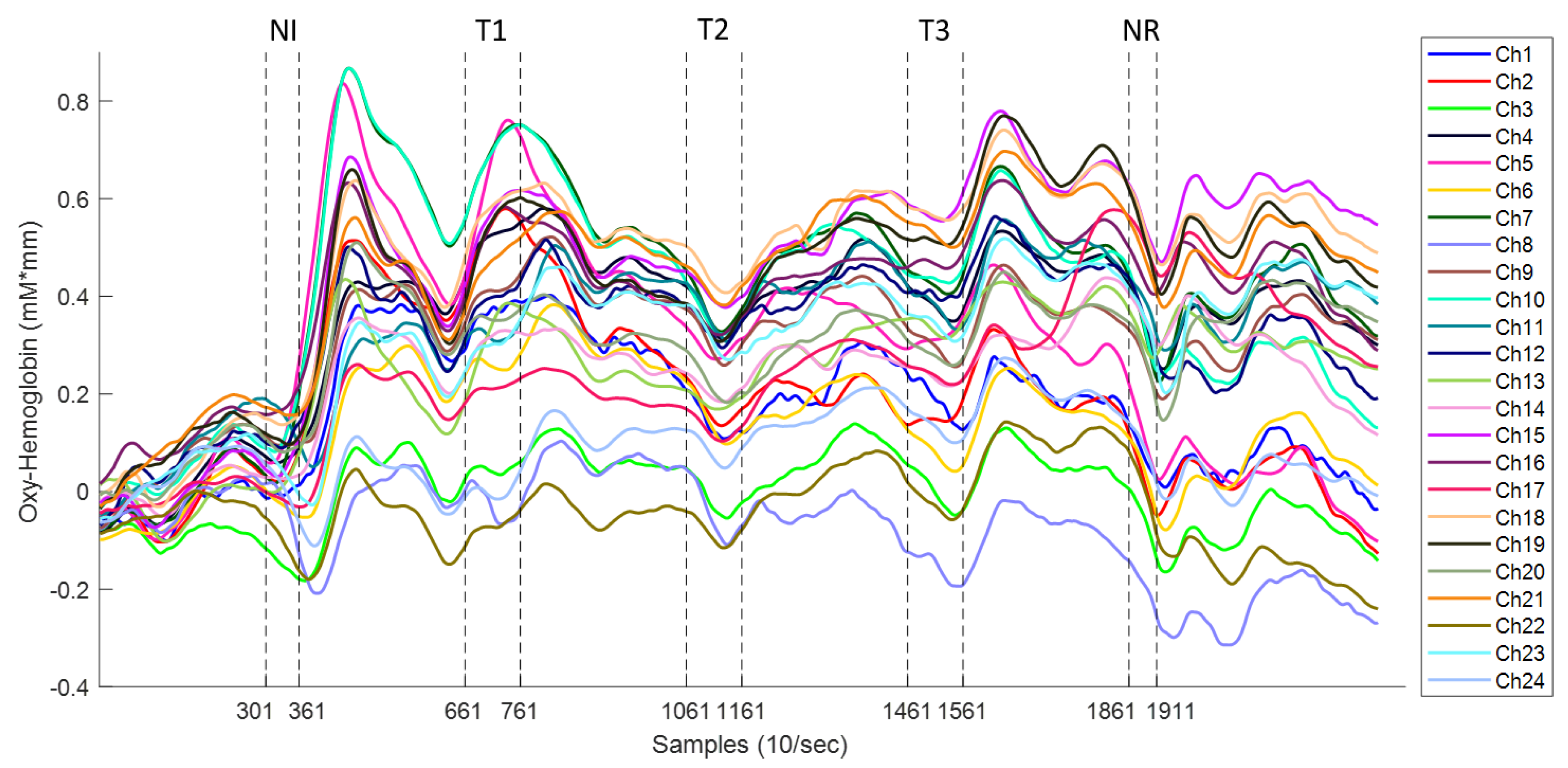
3.1. Hemodynamic Response to Acupuncture Stimulation
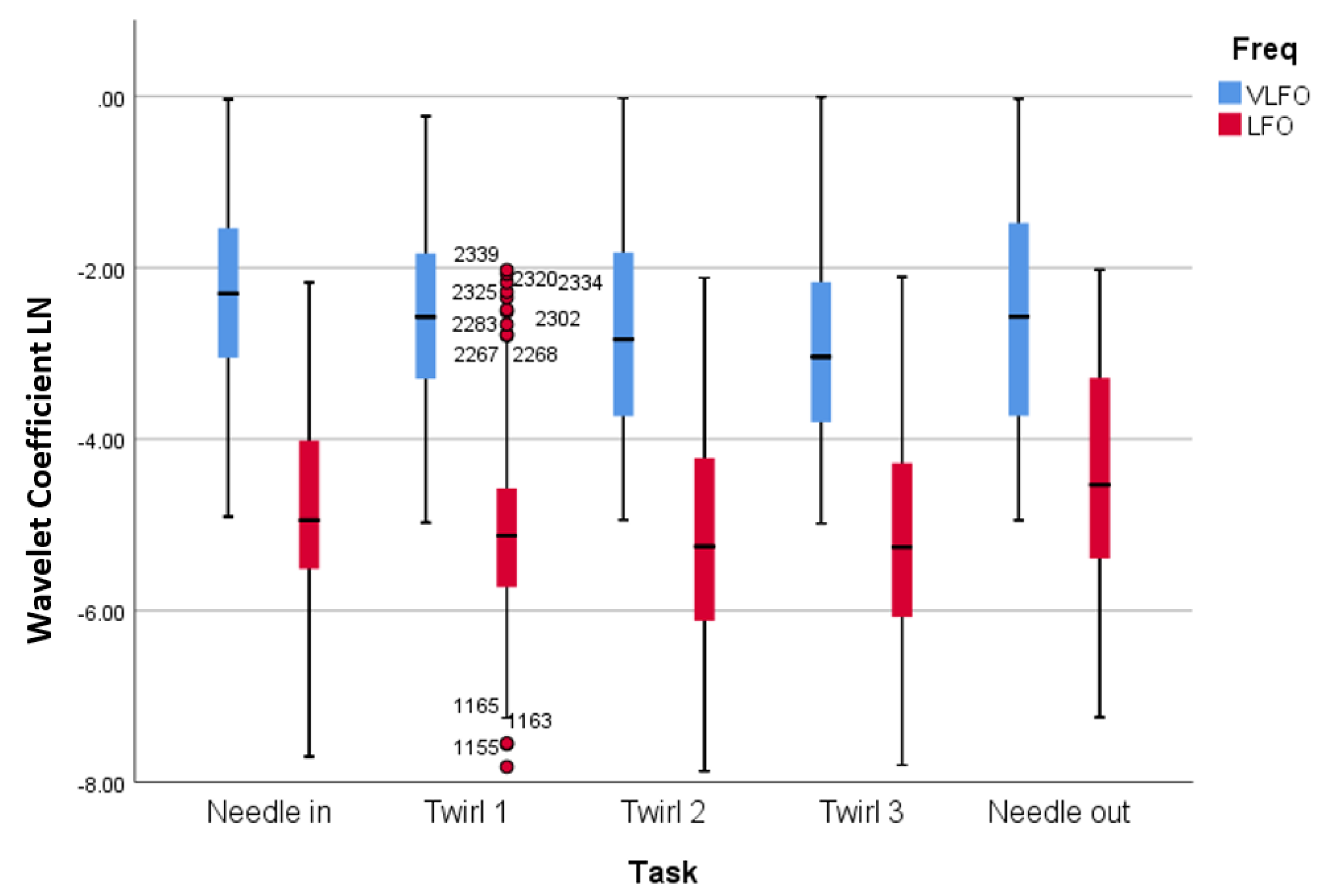
3.2. Wavelet Analysis
3.3. Cortical Connectivity
3.4. Effect of the Hegu Point
4. Discussions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindblom, U. Pain terms: A current list with definitions and notes on usage. Pain Suppl. 1986, 3, S215–S221. [Google Scholar]
- Dueñas, M.; Ojeda, B.; Salazar, A.; Mico, J.A.; Failde, I. A review of chronic pain impact on patients, their social environment and the health care system. J. Pain Res. 2016, 9, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Cronin, A.M.; Maschino, A.C.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Witt, C.M.; Linde, K.; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: Individual patient data meta-analysis. Arch. Intern. Med. 2012, 172, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Dung, H. Acupuncture: An Anatomical Approach; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Hopwood, V. Acupuncture in Physiotherapy: Key Concepts and Evidence-Based Practice; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Peets, J.; Pomeranz, B. CXBK mice deficient in opiate receptors show poor electroacupuncture analgesia. Nature 1978, 273, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.F.; Huang, X.; Ou, K.L. Bilateral connectivity in the somatosensory region using near-infrared spectroscopy (NIRS) by wavelet coherence. In Proceedings of the SPIE BioPhotonics Australasia, Adelaide, Australia, 17–19 October 2016; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; p. 100131Z. [Google Scholar]
- Zhang, W.T.; Jin, Z.; Luo, F.; Zhang, L.; Zeng, Y.W.; Han, J.S. Evidence from brain imaging with fMRI supporting functional specificity of acupoints in humans. Neurosci. Lett. 2004, 354, 50–53. [Google Scholar] [CrossRef]
- Sheng, L.; Chang, T. Electroacupuncture anaesthesia in oral surgery: A preliminary report. Chin. Med. J. 1960, 80, 97–99. [Google Scholar] [PubMed]
- Sun, P. The Treatment of Pain with Chinese Herbs and Acupuncture E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Kong, S.P.; Tan, Q.W.; Liu, Y.; Jing, X.H.; Zhu, B.; Huo, Y.J.; Nie, B.B.; Yang, D.H. Specific correlation between the Hegu point (LI4) and the orofacial part: Evidence from an fMRI study. Evid. Based Complement. Altern. Med. 2015, 2015, 585493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, L.; Zhi, X.; Huang, J.B.; Liu, D.X.; Wang, H.; Kong, X.Q.; Xu, H.B. Study on the regulatory effect of electro-acupuncture on hegu point (LI4) in cerebral response with functional magnetic resonance imaging. Chin. J. Integr. Med. 2007, 13, 10–16. [Google Scholar] [CrossRef]
- Hawrysz, D.J.; Sevick-Muraca, E.M. Developments Toward Diagnostic Breast Cancer Imaging Using Near-Infrared Optical Measurements and Fluorescent Contrast Agents 1. Neoplasia 2000, 2, 388–417. [Google Scholar] [CrossRef]
- Minagawa-Kawai, Y.; Van Der Lely, H.; Ramus, F.; Sato, Y.; Mazuka, R.; Dupoux, E. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cereb. Cortex 2010, 21, 254–261. [Google Scholar] [CrossRef]
- Rojas, R.F.; Huang, X.; Ou, K.L. Toward a functional near-infrared spectroscopy-based monitoring of pain assessment for nonverbal patients. J. Biomed. Opt. 2017, 22, 1–12. [Google Scholar]
- Rojas, R.F.; Huang, X.; Romero, J.; Ou, K.L. fNIRS Approach to Pain Assessment for Non-verbal Patients. In Proceedings of the International Conference on Neural Information Processing, Guangzhou, China, 14–18 November 2017; Springer: Cham Switzerland, 2017; pp. 778–787. [Google Scholar]
- Araki, T.; Wake, R.; Miyaoka, T.; Kawakami, K.; Nagahama, M.; Furuya, M.; Limoa, E.; Liaury, K.; Hashioka, S.; Murotani, K.; et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s Disease patients and its relationship with cerebral blood flow in the prefrontal area. Int. J. Geriatr. Psychiatry 2014, 29, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Inamoto, K.; Higuchi, N.; Ariji, Y.; Nakayama, M.; Izumi, M. Experimental pain in the gingiva and its impact on prefrontal cortical hemodynamics: A functional near-infrared spectroscopy study. Neurosci. Lett. 2014, 575, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kussman, B.D.; Aasted, C.M.; Yücel, M.A.; Steele, S.C.; Alexander, M.E.; Boas, D.A.; Borsook, D.; Becerra, L. Capturing pain in the cortex during general anaesthesia: Near infrared spectroscopy measures in patients undergoing catheter ablation of arrhythmias. PLoS ONE 2016, 11, e0158975. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef]
- Rojas, R.F.; Huang, X.; Ou, K.L.; Lopez-Aparicio, J. Cross correlation analysis of multi-channel near infrared spectroscopy. Comput. Sci. Inf. Technol. 2016, 23–33. [Google Scholar] [CrossRef]
- Balconi, M.; Molteni, E. Past and future of near-infrared spectroscopy in studies of emotion and social neuroscience. J. Cogn. Psychol. 2016, 28, 129–146. [Google Scholar] [CrossRef]
- Crivelli, D.; Balconi, M. Near-infrared spectroscopy applied to complex systems and human hyperscanning networking. Appl. Sci. 2017, 7, 922. [Google Scholar] [CrossRef]
- Scholkmann, F.; Holper, L.; Wolf, U.; Wolf, M. A new methodical approach in neuroscience: Assessing inter-personal brain coupling using functional near-infrared imaging (fNIRI) hyperscanning. Front. Hum. Neurosci. 2013, 7, 813. [Google Scholar] [CrossRef]
- Cao, X. Scientific bases of acupuncture analgesia. Acupunct. Electro-Ther. Res. 2002, 27, 1–14. [Google Scholar] [CrossRef]
- Han, J.; Tang, J.; Ren, M.; Zhou, Z.; Fan, S.; Qiu, X. Central neurotransmitters and acupuncture analgesia. Am. J. Chin. Med. 1980, 8, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ernst, E. Acupuncture analgesia during surgery: A systematic review. Pain 2005, 114, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef]
- Rojas, R.F.; Huang, X.; Ou, K.L. Spatiotemporal Analysis of Brain Activity Response Using Near Infrared Spectroscopy. Int. J. Pharma Med. Biol. Sci. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Xie, Y.F.; Huo, F.Q.; Tang, J.S. Cerebral cortex modulation of pain. Acta Pharmacol. Sin. 2009, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Vierck, C.J.; Whitsel, B.L.; Favorov, O.V.; Brown, A.W.; Tommerdahl, M. Role of primary somatosensory cortex in the coding of pain. PAIN® 2013, 154, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Kiguchi, M.; Kawaguchi, F.; Maki, A. Practicality of wavelength selection to improve signal-to-noise ratio in near-infrared spectroscopy. Neuroimage 2004, 21, 1554–1562. [Google Scholar] [CrossRef]
- Rojas, R.F.; Huang, X.; Hernandez-Juarez, J.; Ou, K.L. Physiological fluctuations show frequency-specific networks in fNIRS signals during resting state. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 2550–2553. [Google Scholar]
- Shi, Y.; Liu, Z.; Zhang, S.; Li, Q.; Guo, S.; Yang, J.; Wu, W. Brain network response to acupuncture stimuli in experimental acute low back pain: An fMRI study. Evid. Based Complement. Altern. Med. 2015, 2015, 210120. [Google Scholar] [CrossRef]
- Zhang, Y.; Brooks, D.H.; Franceschini, M.A.; Boas, D.A. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. J. Biomed. Opt. 2005, 10, 011014. [Google Scholar] [CrossRef]
- Cloud, M.A. Reliable Frontal Cortex Activity For An Oral Stroop Task Using Functional Near Infrared Spectroscopy. Master’s Thesis, The University of Texas, Arlington, TX, USA, December 2013. [Google Scholar]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Mallat, S. A Wavelet Tour of Signal Processing; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Farge, M. Wavelet transforms and their applications to turbulence. Annu. Rev. Fluid Mech. 1992, 24, 395–458. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Kirilina, E.; Yu, N.; Jelzow, A.; Wabnitz, H.; Jacobs, A.M.; Tachtsidis, I. Identifying and quantifying main components of physiological noise in functional near infrared spectroscopy on the prefrontal cortex. Front. Hum. Neurosci. 2013, 7, 864. [Google Scholar] [PubMed]
- Rojas, R.F.; Huang, X.; Ou, K.L.; Hernandez-Juarez, J. Exploring the use of optical flow for the study of functional NIRS signals. In Proceedings of the SPIE Medical Imaging, Orlando, FL, USA, 11–16 February 2017; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 101372K. [Google Scholar]
- Honda, Y.; Nakato, E.; Otsuka, Y.; Kanazawa, S.; Kojima, S.; Yamaguchi, M.K.; Kakigi, R. How do infants perceive scrambled face?: A near-infrared spectroscopic study. Brain Res. 2010, 1308, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.; Obrig, H.; Köhncke, C.; Benav, H.; Koch, S.; Steinbrink, J. The oxygenation response to functional stimulation: Is there a physiological meaning to the lag between parameters? Neuroimage 2007, 36, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.F.; Huang, X.; Ou, K.L.; Tran, D.; Islam, S.M. Analysis of pain hemodynamic response using near-infrared spectroscopy (nirs). arXiv, 2015; arXiv:1507.07422. [Google Scholar] [CrossRef]
- Mayers, A. Introduction to Statistics and SPSS in Psychology; Pearson: Harlow, UK, 2013; Volume 28. [Google Scholar]
- Marieb, E.N. Human Anatomy & Physiology; Benjamin-Cummings Publishing Company: San Francisco, CA, USA, 1989. [Google Scholar]
- Rojas, R.F.; Huang, X.; Ou, K.L. Region of interest detection and evaluation in functional near infrared spectroscopy. J. Near Infrared Spectrosc. 2016, 24, 317–326. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture analgesia: Areas of consensus and controversy. Pain 2011, 152, S41–S48. [Google Scholar] [CrossRef]
- Kuo, T.C.; Lin, C.W.; Ho, F.M. The soreness and numbness effect of acupuncture on skin blood flow. Am. J. Chin. Med. 2004, 32, 117–129. [Google Scholar] [CrossRef]
- Zhou, W.; Benharash, P. Significance of “Deqi” response in acupuncture treatment: Myth or reality. J. Acupunct. Meridian Stud. 2014, 7, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Shi, G.X.; Li, Q.Q.; Zhang, Z.H.; Xu, Q.; Liu, C.Z. Characterization of deqi sensation and acupuncture effect. Evid. Based Complement. Altern. Med. 2013, 2013, 319734. [Google Scholar] [CrossRef] [PubMed]
- Stacher, G.; Wancura, I.; Bauer, P.; Lahoda, R.; Schulze, D. Effect of acupuncture on pain threshold and pain tolerance determined by electrical stimulation of the skin: A controlled study. Am. J. Chin. Med. 1975, 3, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Kawakita, K.; Sakita, M. Analgesic effects induced by TENS and electroacupuncture with different types of stimulating electrodes on deep tissues in human subjects. PAIN® 1995, 63, 181–187. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, T.Y.; Lee, M.S.; Lee, H.; Shin, B.C.; Lee, H. Acupuncture for acute low back pain: A systematic review. Clin. J. Pain 2013, 29, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Baeumler, P.I.; Fleckenstein, J.; Takayama, S.; Simang, M.; Seki, T.; Irnich, D. Effects of acupuncture on sensory perception: A systematic review and meta-analysis. PLoS ONE 2014, 9, e113731. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef]
- Fardo, F.; Auksztulewicz, R.; Allen, M.; Dietz, M.J.; Roepstorff, A.; Friston, K.J. Expectation violation and attention to pain jointly modulate neural gain in somatosensory cortex. Neuroimage 2017, 153, 109–121. [Google Scholar] [CrossRef]





| Task | Mean Difference | Standard Error | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Needle in | Twirl 1 | 0.206 | 0.112 | 0.354 | −1.101 | 0.512 |
| Twirl 2 | 0.439 | 0.110 | 0.001 | 0.137 | 0.740 | |
| Twirl 3 | 0.535 | 0.111 | 0.000 | 0.231 | 0.839 | |
| Needle out | 0.208 | 0.113 | 0.347 | −0.100 | 0.517 | |
| Twirl 1 | Twirl 2 | 0.233 | 0.111 | 0.214 | −0.068 | 0.534 |
| Twirl 3 | 0.329 | 0.111 | 0.025 | 0.026 | 0.632 | |
| Needle out | 0.003 | 0.112 | 1.000 | −0.305 | 0.310 | |
| Twirl 2 | Twirl 3 | 0.096 | 0.109 | 0.904 | −0.202 | 0.395 |
| Needle out | −0.230 | 0.111 | 0.231 | −0.533 | 0.073 | |
| Twirl 3 | Needle out | −0.326 | 0.112 | 0.029 | −0.631 | −0.021 |
| Task | Mean Difference | Standard Error | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Needle in | Twirl 1 | 0.278 | 0.110 | 0.086 | −0.023 | 0.580 |
| Twirl 2 | 0.357 | 0.000 | 0.011 | 0.055 | 0.659 | |
| Twirl 3 | 0.353 | 0.111 | 0.013 | 0.050 | 0.656 | |
| Needle out | −0.381 | 0.113 | 0.007 | −0.689 | −0.073 | |
| Twirl 1 | Twirl 2 | 0.079 | 0.111 | 0.955 | −0.225 | 0.383 |
| Twirl 3 | 0.074 | 0.112 | 0.964 | −0.231 | 0.380 | |
| Needle out | −0.659 | 0.114 | 0.000 | −0.970 | −0.349 | |
| Twirl 2 | Twirl 3 | −0.004 | 0.112 | 1.000 | −0.310 | 0.301 |
| Needle out | −0.738 | 0.114 | 0.000 | −1.049 | −0.427 | |
| Twirl 3 | Needle out | −0.734 | 0.114 | 0.000 | −1.046 | −0.422 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez Rojas, R.; Liao, M.; Romero, J.; Huang, X.; Ou, K.-L. Cortical Network Response to Acupuncture and the Effect of the Hegu Point: An fNIRS Study. Sensors 2019, 19, 394. https://doi.org/10.3390/s19020394
Fernandez Rojas R, Liao M, Romero J, Huang X, Ou K-L. Cortical Network Response to Acupuncture and the Effect of the Hegu Point: An fNIRS Study. Sensors. 2019; 19(2):394. https://doi.org/10.3390/s19020394
Chicago/Turabian StyleFernandez Rojas, Raul, Mingyu Liao, Julio Romero, Xu Huang, and Keng-Liang Ou. 2019. "Cortical Network Response to Acupuncture and the Effect of the Hegu Point: An fNIRS Study" Sensors 19, no. 2: 394. https://doi.org/10.3390/s19020394




