A Binder Jet Printed, Stainless Steel Preconcentrator as an In-Line Injector of Volatile Organic Compounds
Abstract
:1. Introduction
2. Device Fabrication and Experimental Setup
2.1. Design of 3D-Printed SS PCs and Fluidic Interconnection
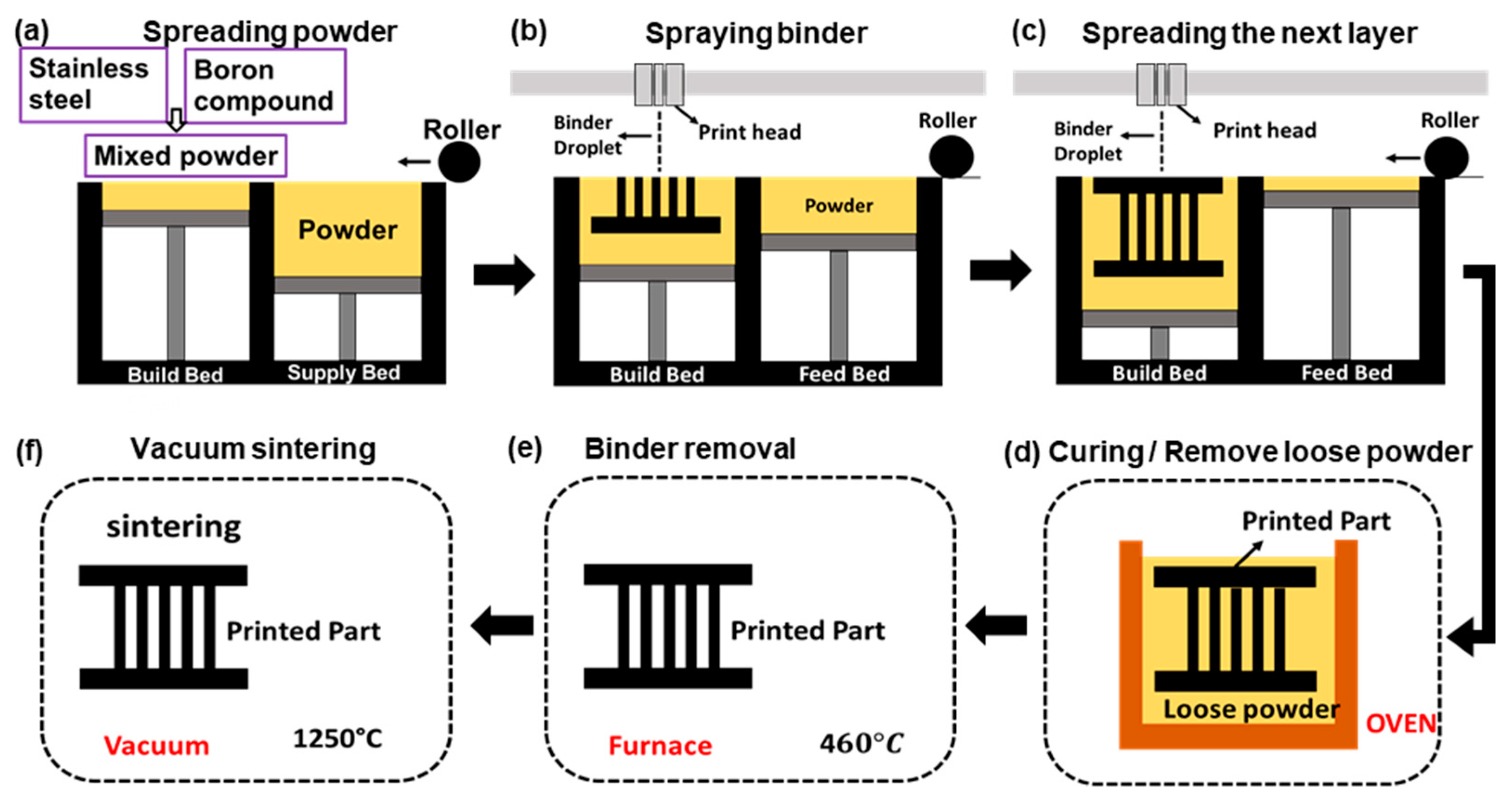
2.2. Fabrication of SS PC via BJP
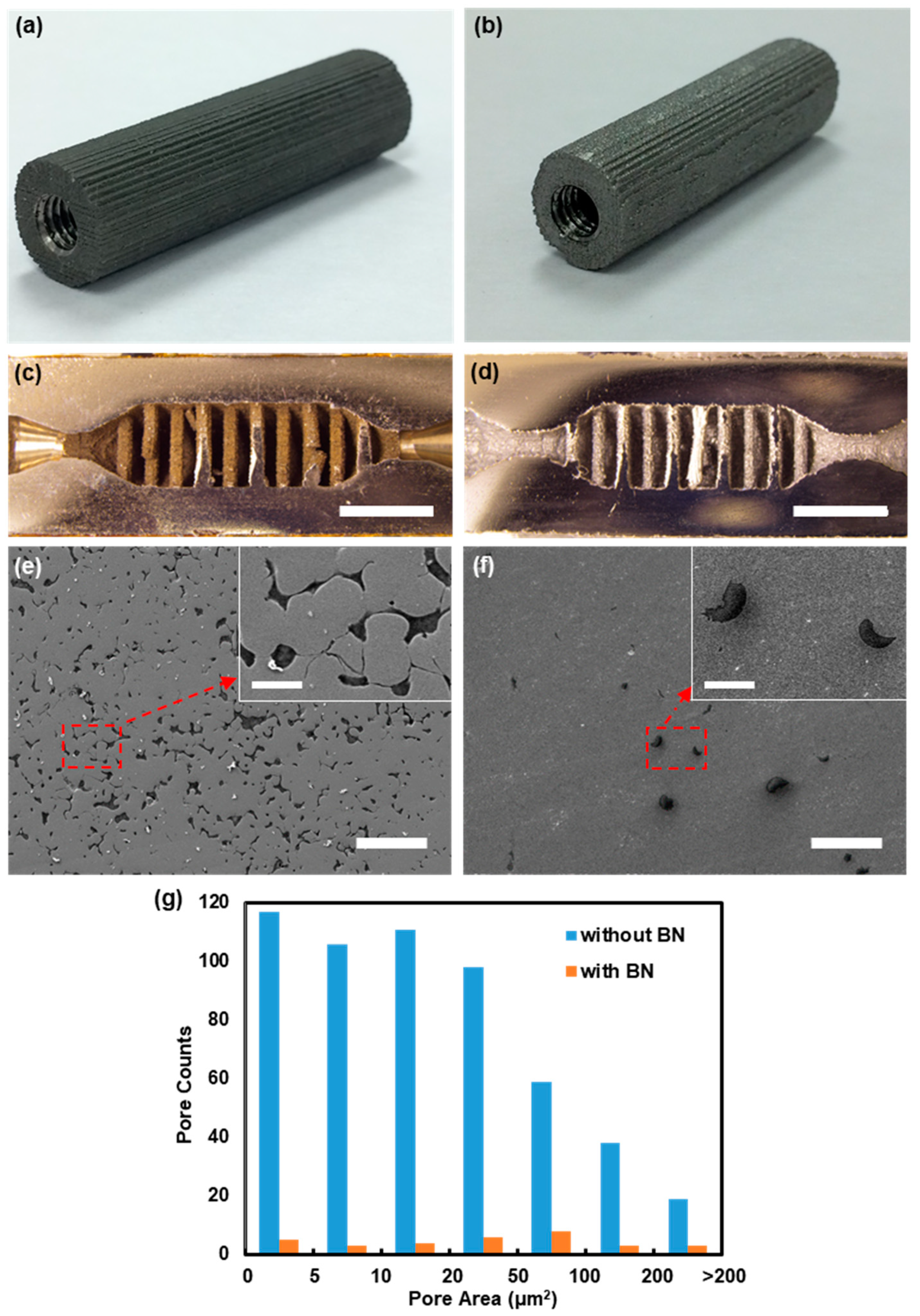
2.3. Materials Characterization
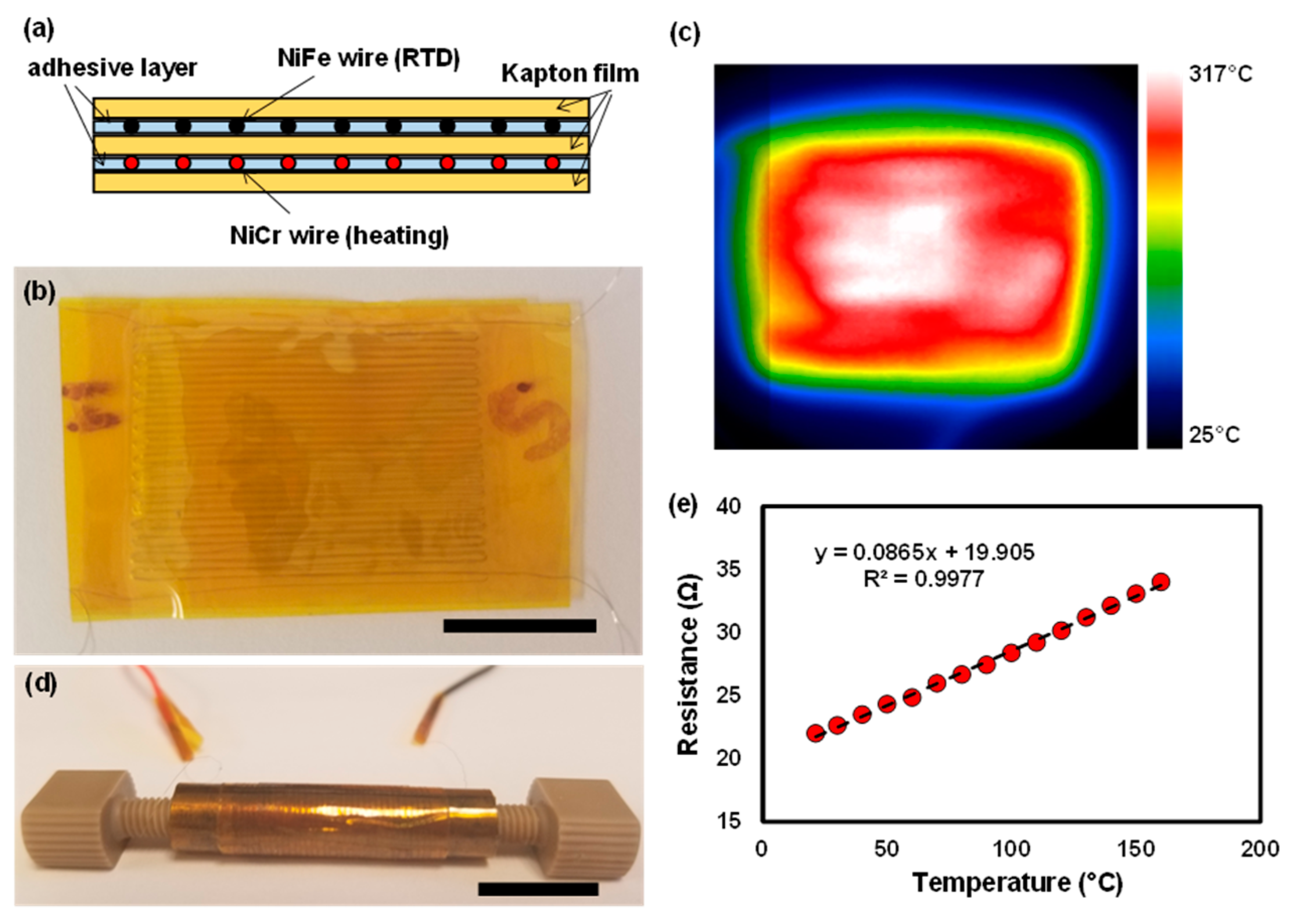
2.4. Membrane Heater Fabrication
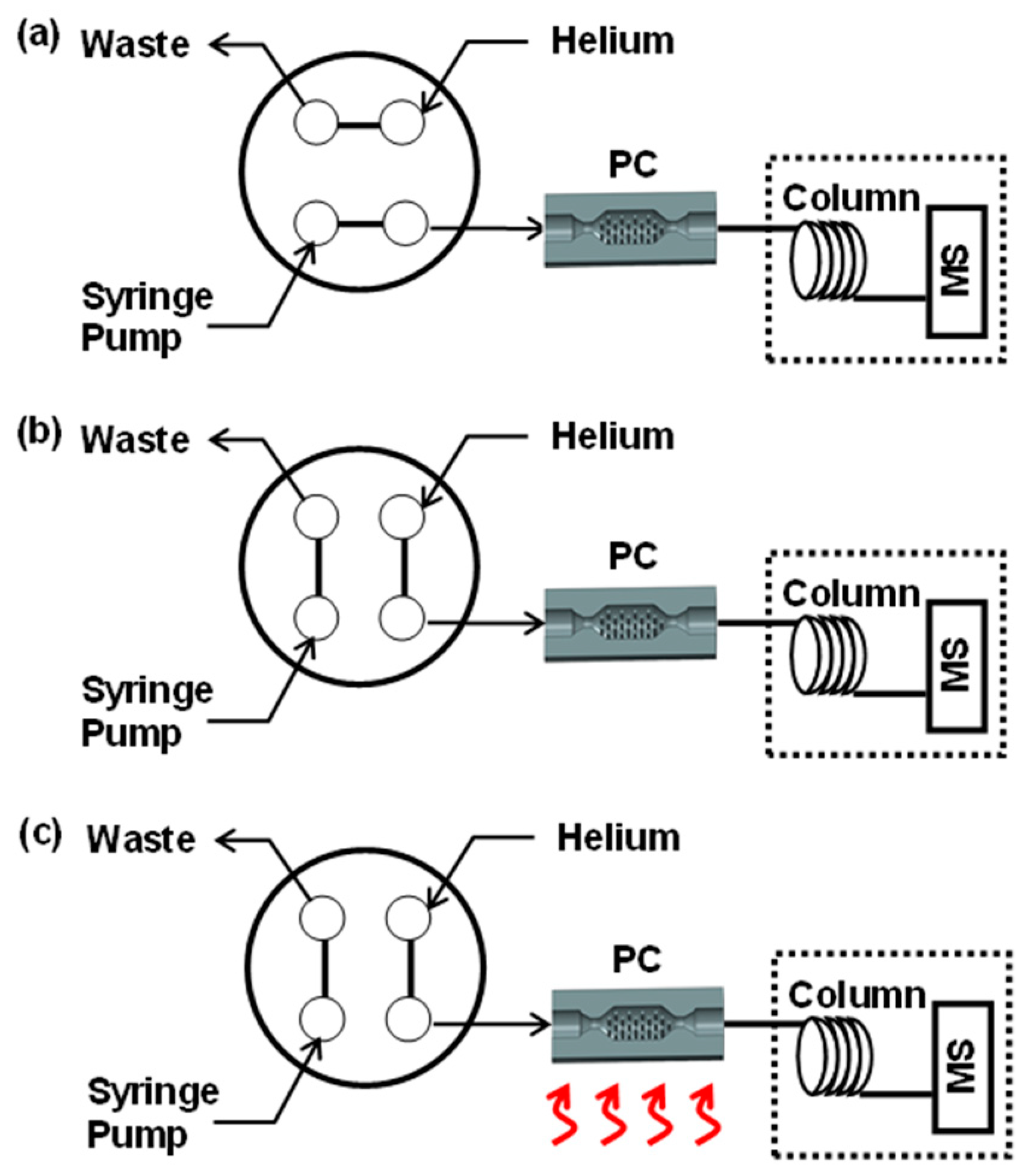
2.5. Preconcentrator Testing
3. Results and Discussion
3.1. Device Design and Fabrication Results
3.2. Fluidic Interconnect and Leak Test
3.3. Membrane Heater and RTD Sensor Characterization
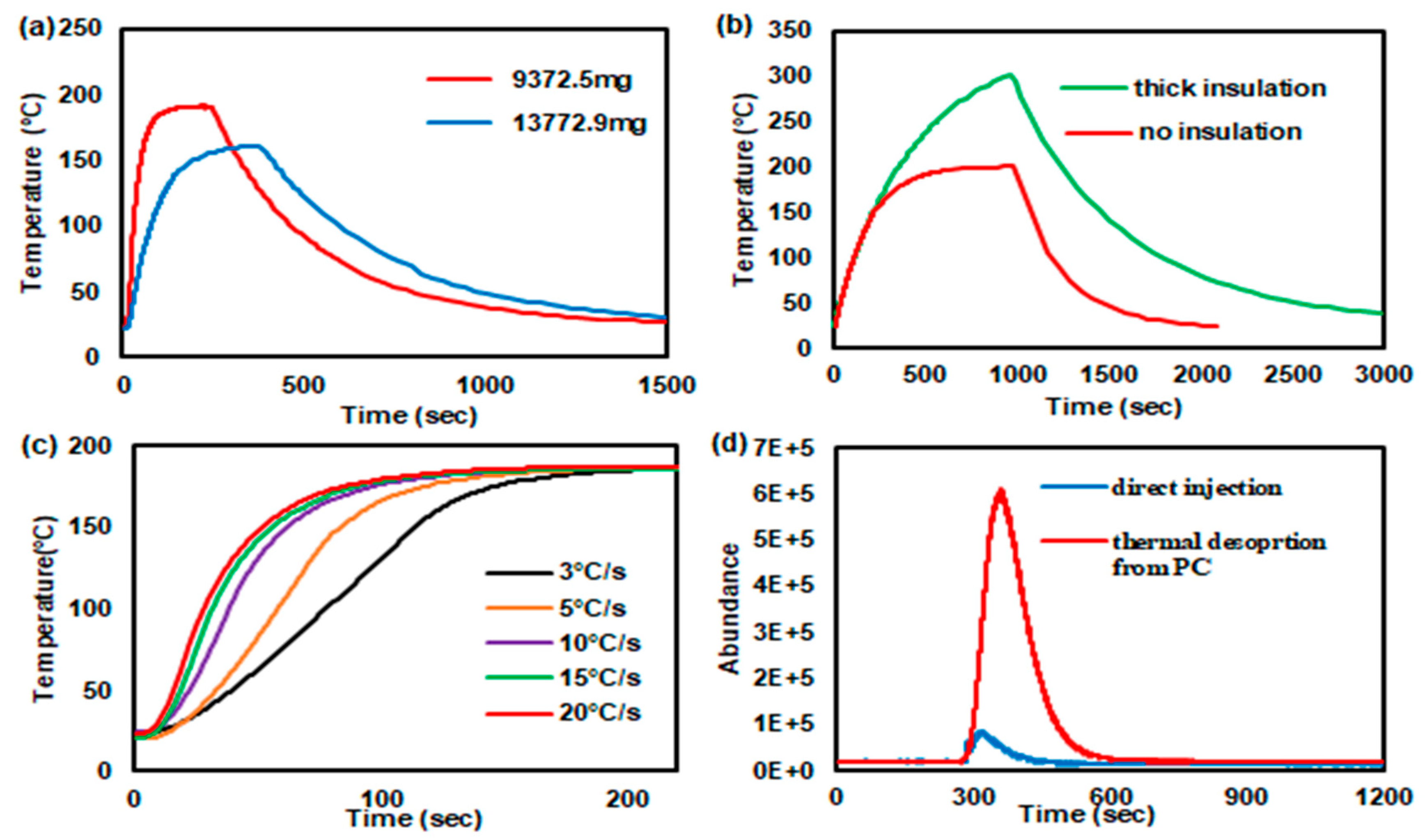
3.4. In-Line Injection Performance of the Printed PCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Rufo, J.C.; Madureira, J.; Fernandes, E.O.; Moreira, A. Volatile organic compounds in asthma diagnosis: A systematic review and meta-analysis. Allergy 2016, 71, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shao, K.; Wang, T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2016, 408, 2759–2780. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Kim, T.; Losic, D.; Tung, T.T. Recent advances in engineered graphene and composites for detection of volatile organic compounds (VOCs) and non-invasive diseases diagnosis. Carbon 2016, 110, 97–129. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Miyake, Y.; Tokumura, M.; Wang, Q.; Wang, Z.; Amagai, T. Comparison of the volatile organic compound recovery rates of commercial active samplers for evaluation of indoor air quality in work environments. Air Qual. Atmos. Health 2017, 10, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of Aldehydes in Beer Using Solid-Phase Microextraction with On-Fiber Derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Bonnassies-Termes, S.; Lavédrine, B. Identification of volatile organic compounds emitted by a naturally aged book using solid-phase microextraction/gas chromatography/mass spectrometry. J. Chromatogr. A 2004, 1026, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Alfeeli, B.; Cho, D.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. MEMS-based multi-inlet/outlet preconcentrator coated by inkjet printing of polymer adsorbents. Sens. Actuators B Chem. 2008, 133, 24–32. [Google Scholar] [CrossRef]
- Ghoshal, A.K.; Manjare, S.D. Selection of appropriate adsorption technique for recovery of VOCs: An analysis. J. Loss Prev. Process Ind. 2002, 15, 413–421. [Google Scholar] [CrossRef]
- Jian, R.-S.; Huang, R.-X.; Lu, C.-J. A micro GC detector array based on chemiresistors employing various surface functionalized monolayer-protected gold nanoparticles. Talanta 2012, 88, 160–167. [Google Scholar] [CrossRef]
- Bae, B.; Kim, J.; Yeom, J.; Chen, Q.; Ray, C.; Shannon, M. Development of a portable gas analyzer using a micro-Gas Chromatograph/Flame Ionization Detector (micro-GC/FID) for NASA’s environmental missions. In Proceedings of the 42nd International Conference on Environmental Systems, San Diego, CA, USA, 15–19 July 2012. [Google Scholar] [CrossRef]
- Azzouz, I.; Marty, F.; Bourouina, T. Recent advances in micro-gas chromatography—The opportunities and the challenges. In Proceedings of the 2017 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Bordeaux, France, 29 May–1 June 2017. [Google Scholar] [CrossRef]
- Kawamura, Y.; Konishi, S.; Nishi, M. Development of a micro gas chromatograph for the analysis of hydrogen isotope gas mixtures in the fusion fuel cycle. Fusion Eng. Des. 2001, 58–59, 389–394. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Lu, C.-J.; Steinecker, W.-H.; Tian, W.-C.; Oborny, M.-C.; Nichols, J.-M.; Agah, M.; Potkay, J.-A.; Chan, H.-K.; Driscoll, J.; Sacks, R.-D.; et al. First-generation hybrid MEMS gas chromatograph. Lab Chip 2005, 5, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- James, F.; Breuil, P.; Pijolat, C.; Camara, M.; Briand, D.; Bart, A.; Cozic, R. Development of a MEMS Preconcentrator for Micro-gas Chromatography Analyses. Procedia Eng. 2014, 87, 500–503. [Google Scholar] [CrossRef]
- Wong, M.-Y.; Cheng, W.-R.; Liu, M.-H.; Tian, W.-C.; Lu, C.-J. A preconcentrator chip employing μ-SPME array coated with in-situ-synthesized carbon adsorbent film for VOCs analysis. Talanta 2012, 101, 307–313. [Google Scholar] [CrossRef]
- Alfeeli, B.; Agah, M. Micro preconcentrator with embedded 3D pillars for breath analysis applications. In Proceedings of the 2008 IEEE SENSORS, Lecce, Italy, 26–29 October 2008. [Google Scholar] [CrossRef]
- Bhushan, A.; Yemane, D.; Overton, E.B.; Goettert, J.; Murphy, M.C. Fabrication and Preliminary Results for LiGA Fabricated Nickel Micro Gas Chromatograph Columns. J. Microelectromech. Syst. 2007, 16, 383–393. [Google Scholar] [CrossRef]
- Bhushan, A.; Yemane, D.; Trudell, D.; Overton, E.B.; Goettert, J. Fabrication of micro-gas chromatograph columns for fast chromatography. Microsyst. Technol. 2007, 13, 361–368. [Google Scholar] [CrossRef]
- Radadia, A.D.; Masel, R.I.; Shannon, M.A.; Jerrell, J.P.; Cadwallader, K.R. Micromachined GC Columns for Fast Separation of Organophosphonate and Organosulfur Compounds. Anal. Chem. 2008, 80, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35–R64. [Google Scholar] [CrossRef]
- Galambos, P.; Lantz, J.; Baker, M.-S.; McClain, J.; Bogart, G.-R.; Simonson, R.-J. Active MEMS Valves for Flow Control in a High-Pressure Micro-Gas-Analyzer. J. Microelectromech. Syst. 2011, 20, 1150–1162. [Google Scholar] [CrossRef]
- Galambos, P.; Lantz, J.W.; James, C.D.; McClain, J.L.; Baker, M.; Anderson, R.; Simonson, R.J. Low leak rate mems valves for micro-gas-analyzer flow control. In Proceedings of the TRANSDUCERS 2009—2009 International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009. [Google Scholar] [CrossRef]
- Narayanan, S.; Alfeeli, B.; Agah, M. Two-Port Static Coated Micro Gas Chromatography Column with an Embedded Thermal Conductivity Detector. IEEE Sens. J. 2012, 12, 1893–1900. [Google Scholar] [CrossRef]
- Kaanta, B.C.; Chen, H.; Lambertus, G.; Steinecker, W.H.; Zhdaneev, O.; Zhang, X. High Sensitivity Micro-Thermal Conductivity Detector for Gas Chromatography. In Proceedings of the 2009 IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009. [Google Scholar] [CrossRef]
- Shopova, S.I.; White, I.M.; Sun, Y.; Zhu, H.; Fan, X.; Frye-Mason, G.; Thompson, A.; Ja, S.-J. On-Column Micro Gas Chromatography Detection with Capillary-Based Optical Ring Resonators. Anal. Chem. 2008, 80, 2232–2238. [Google Scholar] [CrossRef]
- Yeom, J.; Field, C.R.; Bae, B.; Masel, R.I.; Shannon, M.A. The design, fabrication and characterization of a silicon microheater for an integrated MEMS gas preconcentrator. J. Micromech. Microeng. 2008, 18, 125001. [Google Scholar] [CrossRef]
- Yeom, J.; Oh, I.; Field, C.; Radadia, A.; Ni, Z.; Bae, B.; Han, J.; Masel, R.I.; Shannon, M.A. Enhanced toxic gas detection using a MEMS preconcentrator coated with the metal organic framework absorber. In Proceedings of the 2008 IEEE 21st International Conference on Micro Electro Mechanical Systems, Tucson, AZ, USA, 13–17 January 2008; pp. 232–235. [Google Scholar] [CrossRef]
- Manginell, R.P.; Charles Frye-Mason, G.C.; Kottenstette, R.; LEWIS, P.R.; Wong, C.C. Microfabricated planar preconcentrator. In Proceedings of the Solid State Sensors and Actuators Workshop, Hilton Head, SC, USA, 4‒8 June2000. [Google Scholar]
- Alamin Dow, A.B.; Lang, W. A micromachined preconcentrator for ethylene monitoring system. Sens. Actuators B Chem. 2010, 151, 304–307. [Google Scholar] [CrossRef]
- Tian, W.-C.; Pang, S.W.; Lu, C.-J.; Zellers, E.T. Microfabricated preconcentrator-focuser for a microscale gas chromatograph. J. Microelectromech. Syst. 2003, 12, 264–272. [Google Scholar] [CrossRef]
- Tian, W.C.; Chan, H.K.L.; Lu, C.J.; Pang, S.W.; Zellers, E.T. Multiple-stage microfabricated preconcentrator-focuser for micro gas chromatography system. J. Microelectromech. Syst. 2005, 14, 498–507. [Google Scholar] [CrossRef]
- Akbar, M.; Wang, D.; Goodman, R.; Hoover, A.; Rice, G.; Heflin, J.R.; Agah, M. Improved performance of micro-fabricated preconcentrators using silica nanoparticles as a surface template. J. Chromatogr. A 2013, 1322, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, S.K.; Zellers, E.T.; Kurabayashi, K. Microfabricated passive vapor preconcentrator/injector designed for microscale gas chromatography. Lab. Chip 2012, 12, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip 2012, 12, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Comina, G.; Suska, A.; Filippini, D. Low cost lab-on-a-chip prototyping with a consumer grade 3D printer. Lab Chip 2014, 14, 2978–2982. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Talebi, M.; Deverell, J.; Sandron, S.; Nesterenko, P.N.; Heery, B.; Thompson, F.; Beirne, S.; Wallace, G.G.; Paull, B. 3D printed titanium micro-bore columns containing polymer monoliths for reversed-phase liquid chromatography. Anal. Chim. Acta 2016, 910, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sandron, S.; Heery, B.; Gupta, V.; Collins, D.A.; Nesterenko, E.P.; Nesterenko, P.N.; Talebi, M.; Beirne, M.; Thompson, F.; Wallace, G.G.; et al. 3D printed metal columns for capillary liquid chromatography. Analyst 2014, 139, 6343–6347. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Bauder, T.J.; Suen, H.; Rego, K.; Yeom, J.; Kwon, P. Additively Manufactured Full-Density Stainless Steel 316L with Binder Jet Printing. In Proceedings of the ASME 2018 13th International Manufacturing Science and Engineering Conference, College Station, TX, USA, 18–22 June 2018. [Google Scholar] [CrossRef]
- Do, T.; Kwon, P.; Shin, C.S. Process development toward full-density stainless steel parts with binder jetting printing. Int. J. Mach. Tools Manuf. 2017, 121, 50–60. [Google Scholar] [CrossRef]
- Regmi, B.P.; Agah, M. Micro Gas Chromatography: An Overview of Critical Components and Their Integration. Anal. Chem. 2018, 90, 13133–13150. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, A.; Yemane, D.; McDaniel, S.; Goettert, J.; Murphy, M.C.; Overton, E.B. Hybrid integration of injector and detector functions for microchip gas chromatography. Analyst 2010, 135, 2730–2736. [Google Scholar] [CrossRef]

| Fe | Cr | Ni | C | Mo | Mn | Si | |
|---|---|---|---|---|---|---|---|
| Bulk SS316 | bal. | 16~18 | 10~14 | 0.08 max | 2~3 | 2 max | 0.75 max |
| EDS measured (SS316 + BN) | 61.3 | 16 | 11.3 | 6.9 | 2.1 | 1.6 | 0.7 |
| B 1s | C 1s | O 1s | Ca 2p3 | Fe 2p | Cr 2p | Ni 2p | Mo 3d5 | Mn | |
|---|---|---|---|---|---|---|---|---|---|
| Run 1 | 1.6 | 14.1 | 30.4 | 3.1 | 22.0 | 22.0 | 0 | 0 | 6.8 |
| Run 2 | 0 | 3.4 | 5.1 | 0 | 56.2 | 13.0 | 13.5 | 8.9 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Bauder, T.; Do, T.; Suen, H.; Boss, C.; Kwon, P.; Yeom, J. A Binder Jet Printed, Stainless Steel Preconcentrator as an In-Line Injector of Volatile Organic Compounds. Sensors 2019, 19, 2748. https://doi.org/10.3390/s19122748
Huang X, Bauder T, Do T, Suen H, Boss C, Kwon P, Yeom J. A Binder Jet Printed, Stainless Steel Preconcentrator as an In-Line Injector of Volatile Organic Compounds. Sensors. 2019; 19(12):2748. https://doi.org/10.3390/s19122748
Chicago/Turabian StyleHuang, Xiaolu, Tyler Bauder, Truong Do, Hawke Suen, Connor Boss, Patrick Kwon, and Junghoon Yeom. 2019. "A Binder Jet Printed, Stainless Steel Preconcentrator as an In-Line Injector of Volatile Organic Compounds" Sensors 19, no. 12: 2748. https://doi.org/10.3390/s19122748





