Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Attachment Method
2.3. Cyclic Voltammetry and Polarization Resistance Measurements
3. Results and Discussion
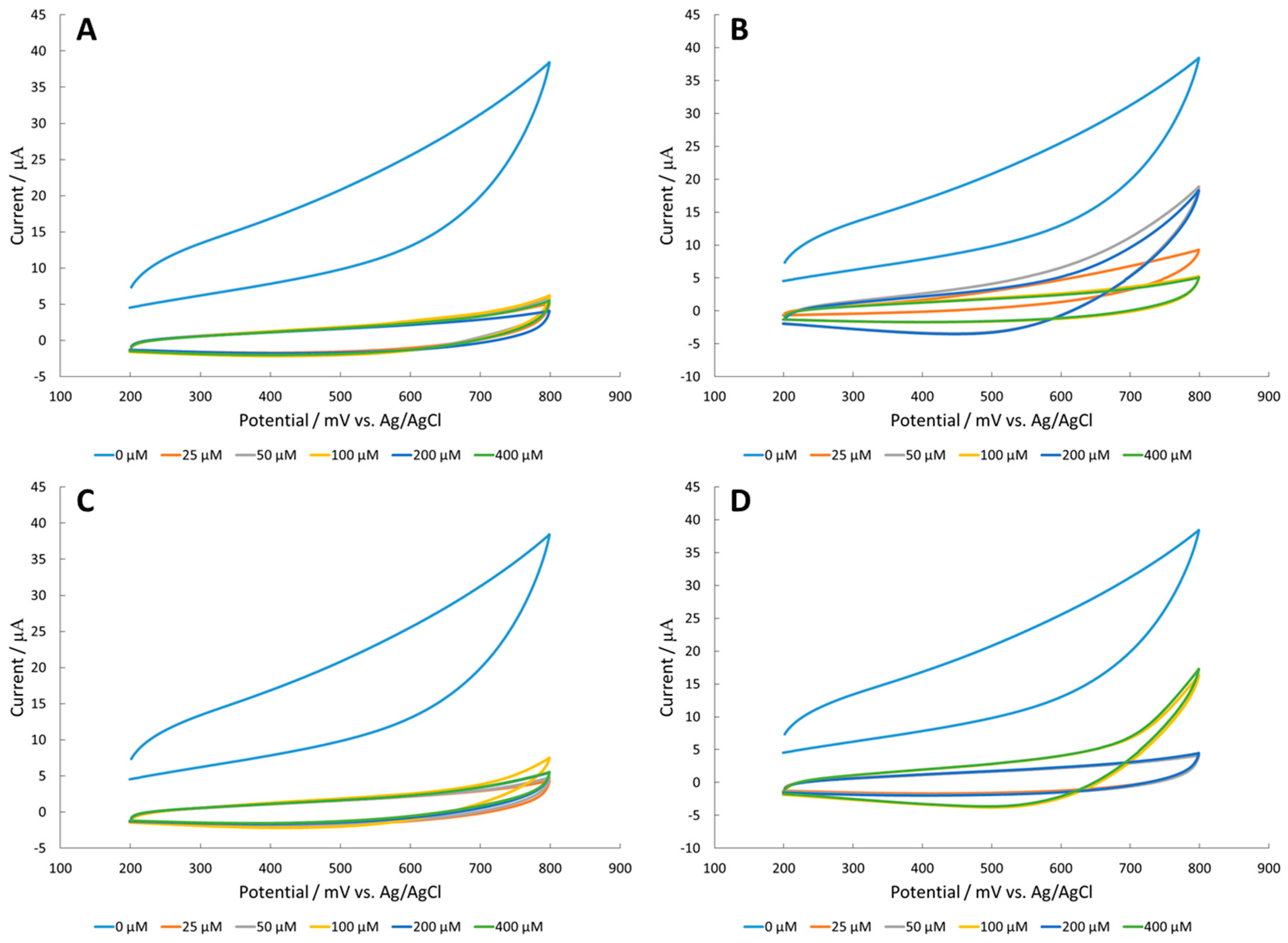
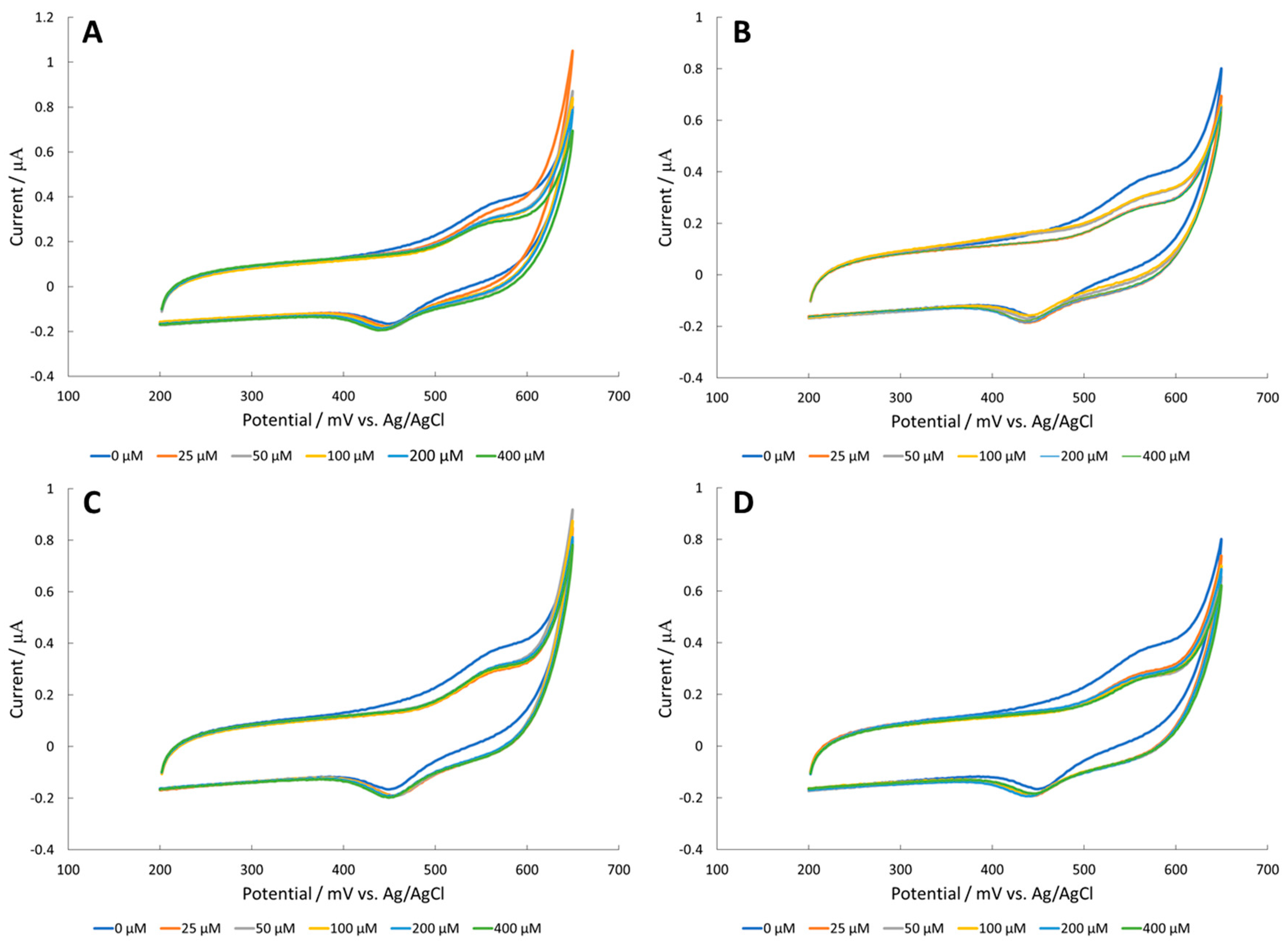
3.1. Amino Acid Detection at a CuO Surface
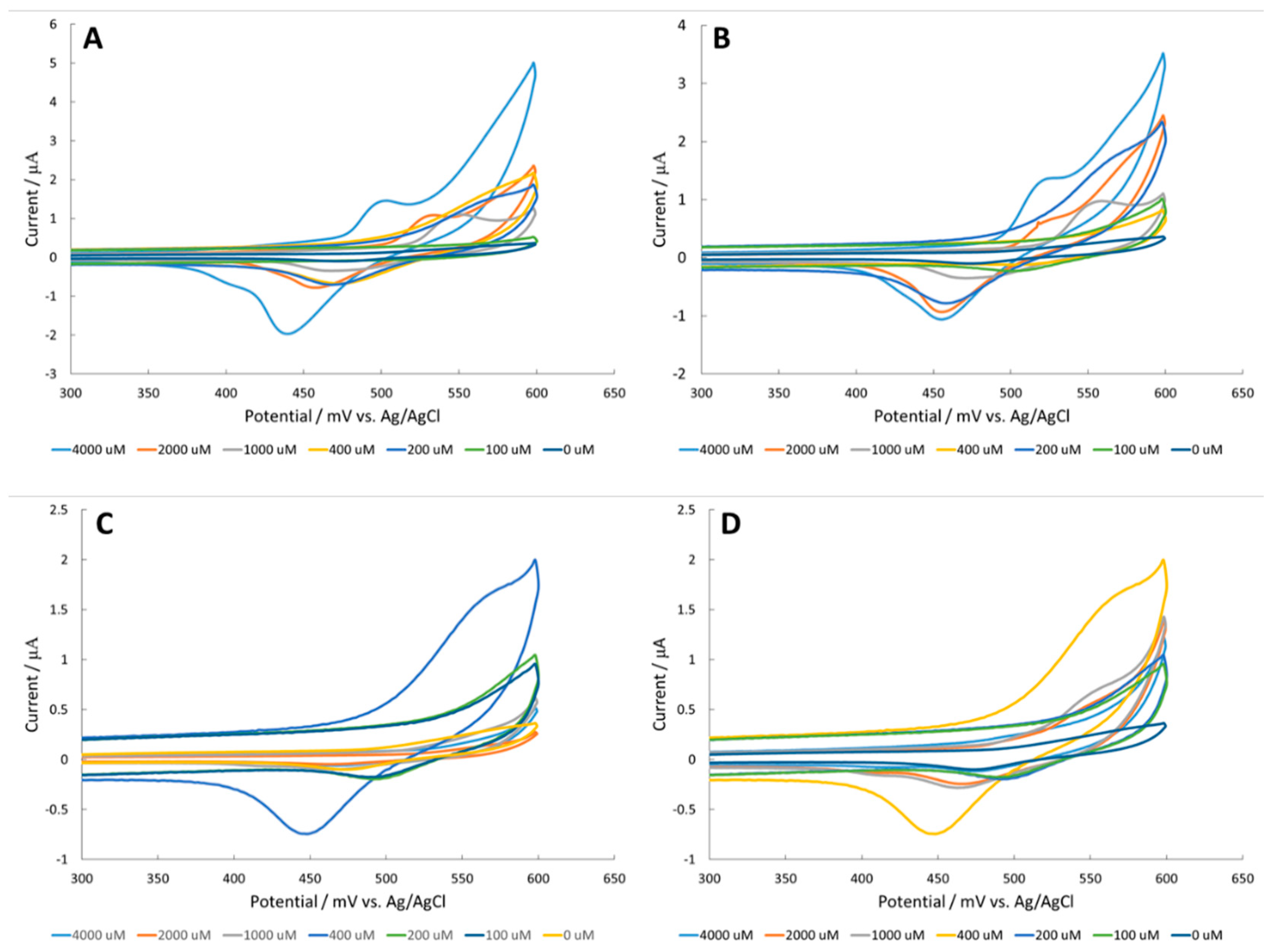
3.2. Amino Acid Detection at a Fe2O3 Surface
3.3. Amino Acid Detection at a NiO Surface
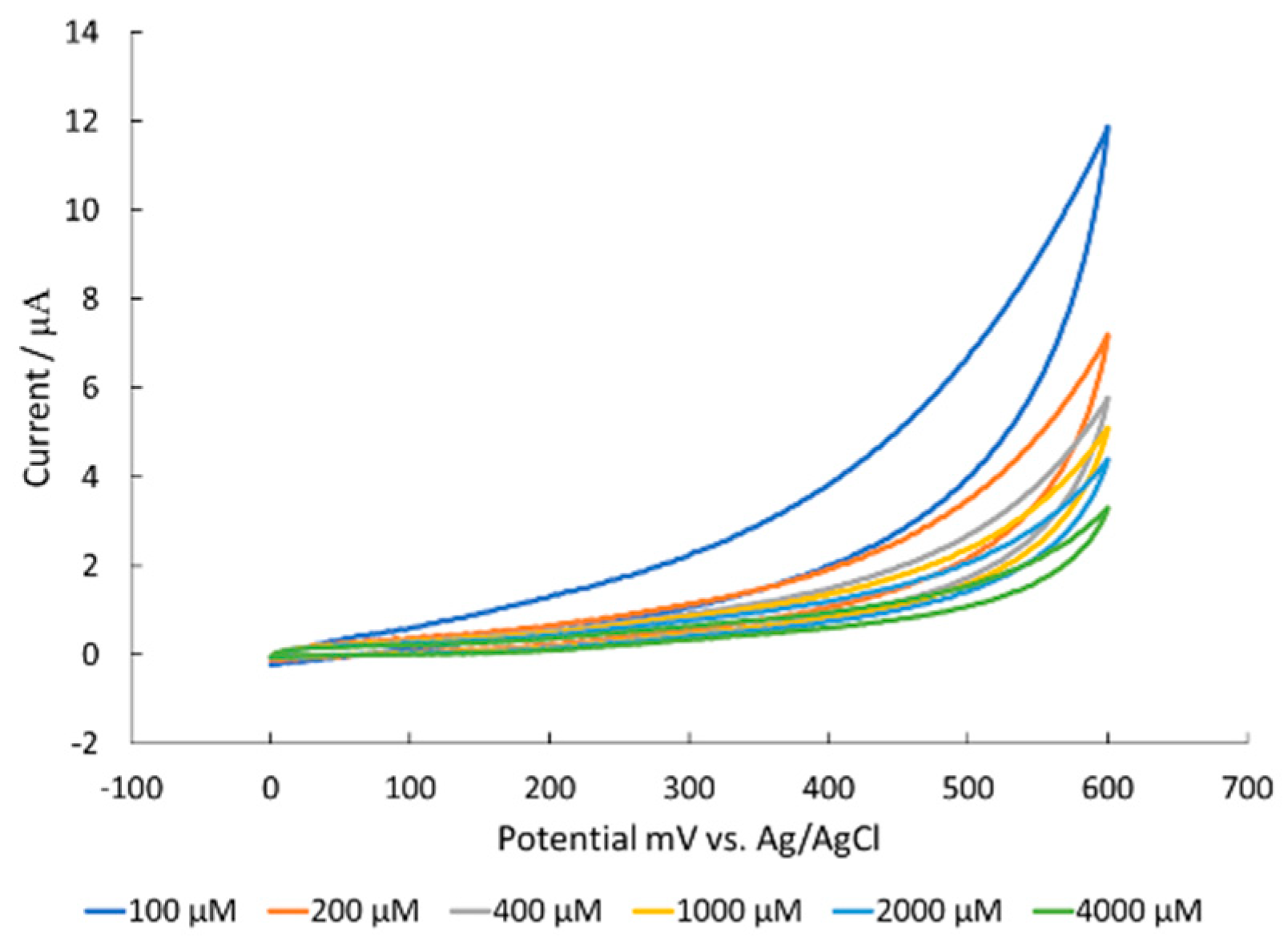
3.4. Corrosion of Nickel Oxide Surface Measured Through Polarization Resistance and Proposed Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-I.; Chun, M.-R.; Yang, J.-S.; Lim, S.-W.; Kim, M.-J.; Kim, S.-W.; Myung, W.-J.; Kim, D.-K.; Lee, S.-Y. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci. Ther. 2015, 21, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Gao, H.-C.; Qi, Z.-G.; Jia, J.-M.; Li, F.F.; Chen, J.J.; Wang, Y.; Guo, J.; Melgiri, N.D.; Xie, P. Peripheral metabolic abnormalities of lipids and amino acids implicated in increased risk of suicidal behavior in major depressive disorder. Metabolomics 2013, 9, 688–696. [Google Scholar] [CrossRef]
- Shao, W.; Chen, J.; Fan, S.; Lei, Y.; Xu, H.; Zhou, J.; Cheng, P.; Yang, Y.; Rao, C.; Wu, B.; et al. Combined metabolomics and proteomics analysis of major depression in an animal model: Perturbed energy metabolism in the chronic mild stressed rat cerebellum. OMICS 2015, 19, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Shushan, B. A review of clinical diagnostic applications of liquid chromatography–tandem mass spectrometry. Mass Spectrom. Rev. 2010, 29, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.N.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, G.; Arrigan, D.W.M. Electrochemical strategies for the label-free detection of amino acids, peptides and proteins. Analyst 2007, 132, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Mass spectrometric quantification of L-arginine and its pathway related substances in biofluids: The road to maturity. J. Chromatogr. B 2014, 964, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Krumpochova, P.; Bruyneel, B.; Molenaar, D.; Koukou, A.; Wuhrer, M.; Niessen, W.M.A.; Giera, M. Amino acid analysis using chromatography–mass spectrometry: An inter platform comparison study. J. Pharm. Biomed. Anal. 2015, 114, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Saurina, J.; Hernández-Cassou, S. Liquid chromatographic determination of aniline in table-top sweeteners based on pre-column derivatization with 1,2-naphthoquinone-4-sulfonate. J. Chromatogr. A 1999, 859, 227–233. [Google Scholar] [CrossRef]
- Schiesel, S.; Lämmerhofer, M.; Lindner, W. Multitarget quantitative metabolic profiling of hydrophilic metabolites in fermentation broths of β-lactam antibiotics production by HILIC–ESI–MS/MS. Anal. Bioanal. Chem. 2010, 396, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC–MS analysis of free amino acids in biological fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.G.; Cataldi, T.R.I.; Guerrieri, A.; Desimoni, E. Copper dispersed into polyaniline films as an amperometric sensor in alkaline solutions of amino acids and polyhydric compounds. Anal. Chim. Acta 1996, 335, 217–225. [Google Scholar] [CrossRef]
- Roushani, M.; Shamsipur, M.; Pourmortazavi, S.M. Amprometric detection of Glycine, l-Serine, and l-Alanine using glassy carbon electrode modified by NiO nanoparticles. J. Appl. Electrochem. 2012, 42, 1005–1011. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Revenga-Parra, M.; Zamora, F.; Pariente, F.; Lorenzo, E. Nanostructured electrochemical detector for the quantification of amino acids related to metabolic diseases. Sens. Actuators B Chem. 2016, 236, 773–780. [Google Scholar] [CrossRef]
- Zen, J.-M.; Hsu, C.-T.; Senthil Kumar, A.; Lyuu, H.-J.; Lin, K.-Y. Amino acid analysis using disposable copper nanoparticle plated electrodes. Analyst 2004, 129, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, Z.; Hou, H.; Wang, X.; Wang, L. Architecture of Fe3O4–graphene oxide nanocomposite and its application as a platform for amino acid biosensing. Electrochim. Acta 2012, 71, 58–65. [Google Scholar] [CrossRef]
- Sigel, H.; Martin, R.B. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Nakamoto, K.; Morimoto, Y.; Martell, A.E. Infrared spectra of aqueous solutions. I. metal chelate compounds of amino acids 1. J. Am. Chem. Soc. 1961, 83, 4528–4532. [Google Scholar] [CrossRef]
- Legin, A.A.; Jakupec, M.A.; Bokach, N.A.; Tyan, M.R.; Kukushkin, V.Y.; Keppler, B.K. Guanidine platinum(II) complexes: Synthesis, in vitro antitumor activity, and DNA interactions. J. Inorg. Biochem. 2014, 133, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Marin-Luna, M.; Sanchez-Sanz, G.; O’Sullivan, P.; Rozas, I. Guanidine complexes of platinum: A theoretical study. J. Phys. Chem. A 2014, 118, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.J.; Pace, S. The coordination chemistry of guanidines and guanidinates. Coord. Chem. Rev. 2001, 214, 91–141. [Google Scholar] [CrossRef]
- Xu, H.-B.; Fang, L.; Hu, Z.-C.; Chen, Y.-C.; Chen, J.-J.; Li, F.-F.; Lu, J.; Mu, J.; Xie, P. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res. 2012, 200, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, F.; Baldwin, R.P. Constant-potential amperometric detection of underivatized amino acids and peptides at a copper electrode. Anal. Chem. 1991, 63, 1702–1707. [Google Scholar] [CrossRef]
- Qu, H.; Ma, H.; Zhou, W.; O’Connor, C.J. In situ surface functionalization of magnetic nanoparticles with hydrophilic natural amino acids. Inorg. Chim. Acta 2012, 389, 60–65. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Toprak, M.S.; Baykal, A.; Altınçekiç, T.G.; Aslan, A.; Bozkurt, A.; Coşgun, S. l-lysine coated iron oxide nanoparticles: Synthesis, structural and conductivity characterization. J. Alloys Compd. 2009, 484, 371–376. [Google Scholar] [CrossRef]
- Sousa, M.H.; Rubim, J.C.; Sobrinho, P.G.; Tourinho, F.A. Biocompatible magnetic fluid precursors based on aspartic and glutamic acid modified maghemite nanostructures. J. Magn. Magn. Mater. 2001, 225, 67–72. [Google Scholar] [CrossRef]
- Pušnik, K.; Peterlin, M.; Cigić, I.K.; Marolt, G.; Kogej, K.; Mertelj, A.; Gyergyek, S.; Makovec, D. Adsorption of amino acids, aspartic acid, and lysine onto iron-oxide nanoparticles. J. Phys. Chem. C 2016, 120, 14372–14381. [Google Scholar] [CrossRef]
- Veisi, H.; Azadbakht, R.; Saeidifar, F.; Abdi, M.R. Schiff based-functionalized multi walled carbon nano tubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for suzuki reaction in aqueous media under mild conditions. Catal. Lett. 2017, 147, 976–986. [Google Scholar] [CrossRef]



| Rate of Corrosion (10−3 Mils per Year) | |
|---|---|
| 1 mM Arginine in 100 mM NaOH | 7.8 ± 0.6 |
| 400 μM Creatine in 100 mM NaOH | 9.5 ± 1.2 |
| 100 mM NaOH (no analyte) | 6.3 ± 0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tooley, C.A.; Gasperoni, C.H.; Marnoto, S.; Halpern, J.M. Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids. Sensors 2018, 18, 3144. https://doi.org/10.3390/s18093144
Tooley CA, Gasperoni CH, Marnoto S, Halpern JM. Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids. Sensors. 2018; 18(9):3144. https://doi.org/10.3390/s18093144
Chicago/Turabian StyleTooley, Christian A., Charles H. Gasperoni, Sabrina Marnoto, and Jeffrey Mark Halpern. 2018. "Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids" Sensors 18, no. 9: 3144. https://doi.org/10.3390/s18093144





