Simple and Label-Free Fluorescent Detection of Melamine Based on Melamine–Thymine Recognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluorometric Analysis
2.3. Detection of Melamine in Milk Sample
3. Results
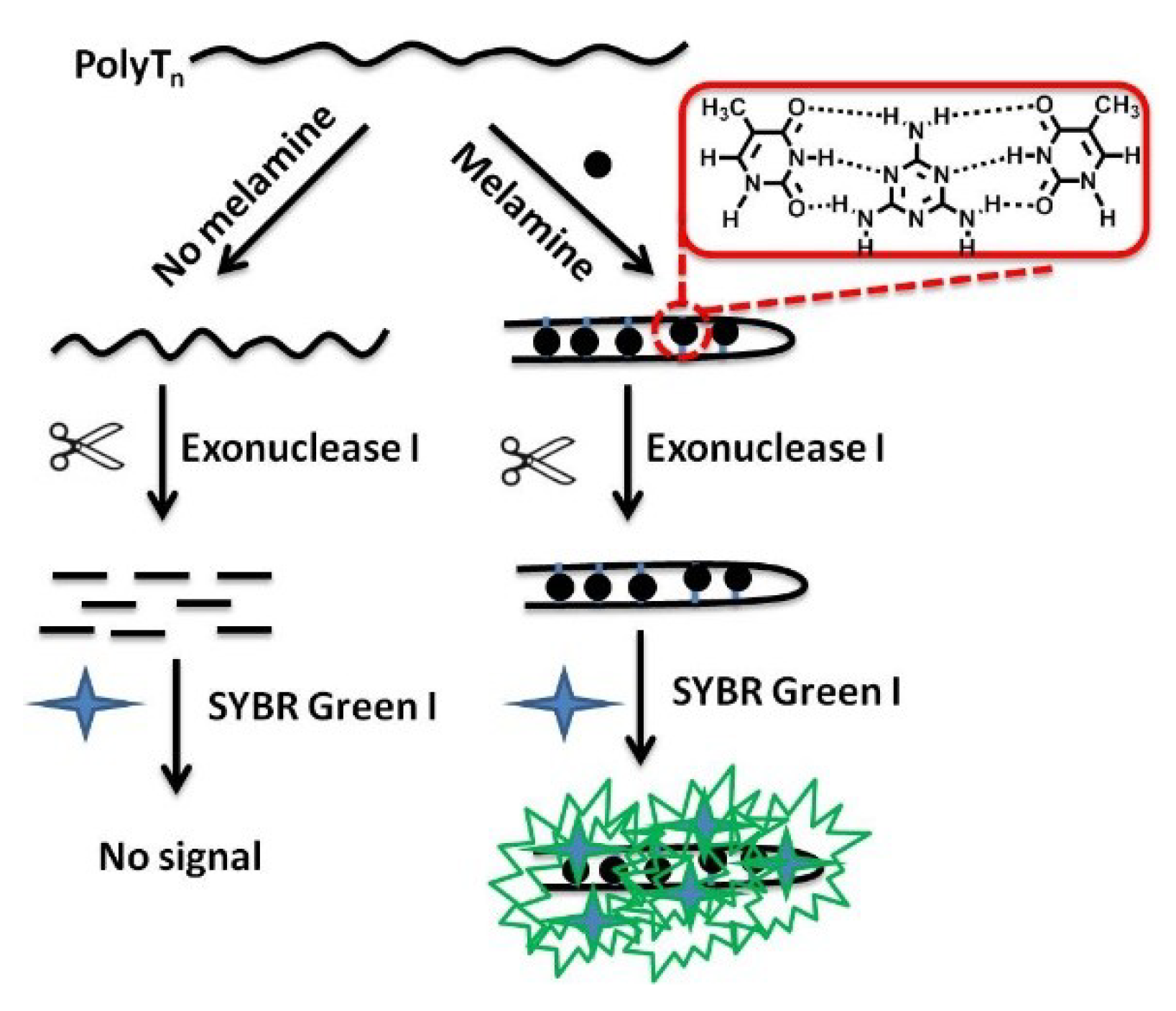
3.1. Detection Principle
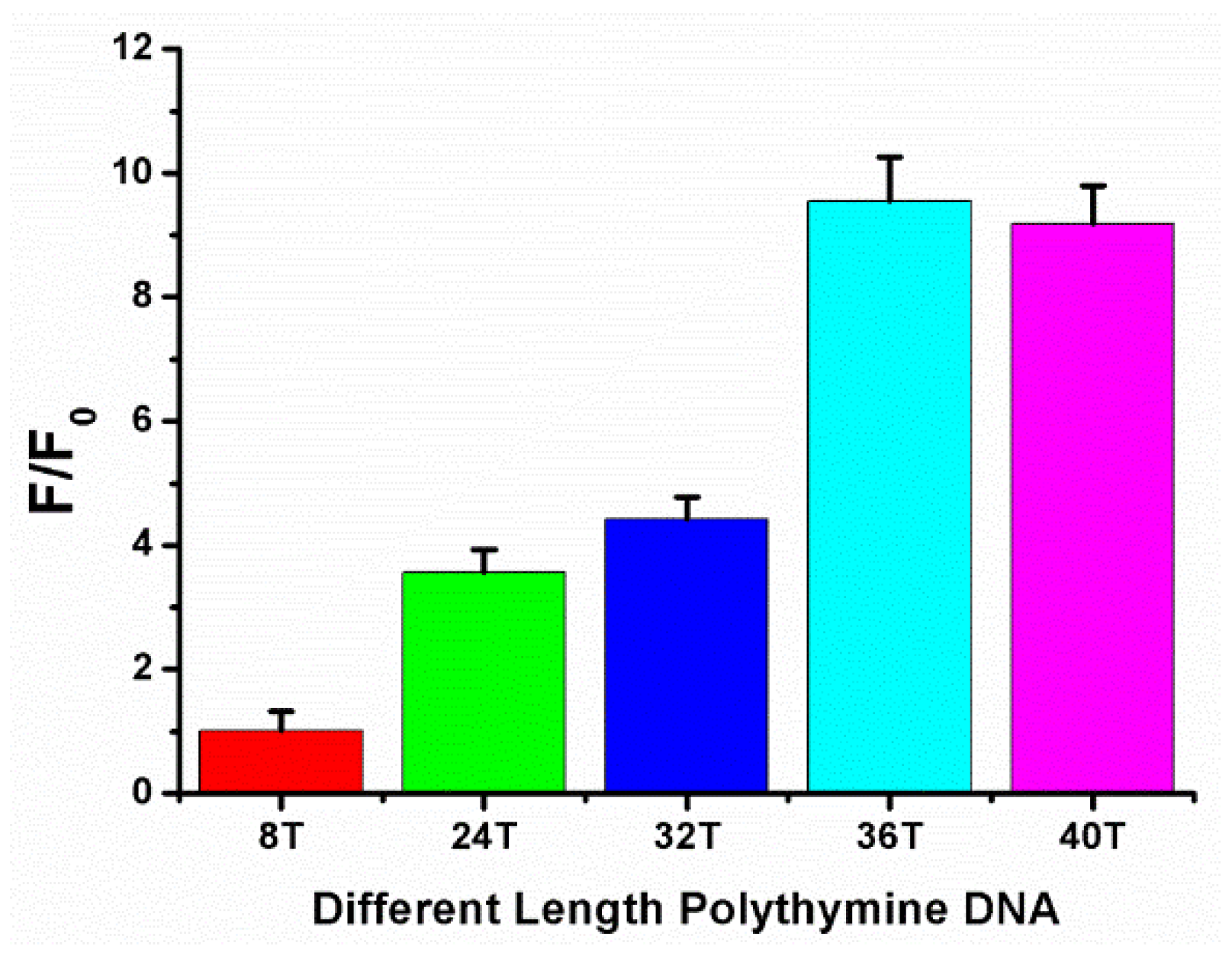
3.2. Optimize the Length of Polythymine DNA
3.3. The Sensitivity of Our Proposed Strategy
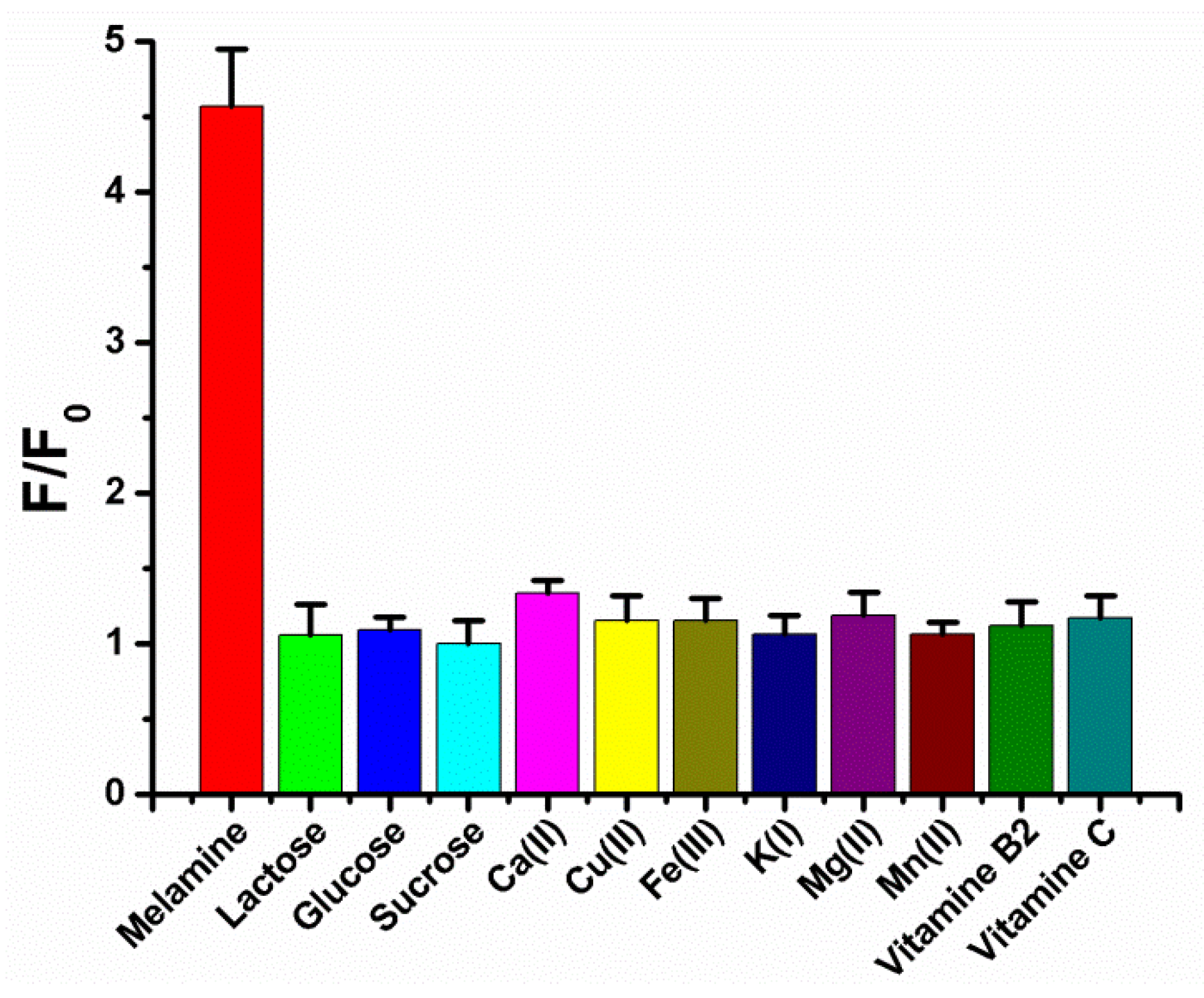
3.4. The Selectivity of Our Proposed Strategy
3.5. The Real Sample Analysis in Milk Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chan, E.Y.; Griffiths, S.M.; Chan, C.W. Public-health risks of melamine in milk products. Lancet 2008, 372, 1444–1445. [Google Scholar] [CrossRef]
- Fodey, T.L.; Thompson, C.S.; Traynor, I.M.; Haughey, S.A.; Kennedy, D.G.; Crooks, S.R.H. Development of an Optical Biosensor Based Immunoassay to Screen Infant Formula Milk Samples for Adulteration with Melamine. Anal. Chem. 2011, 83, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.R.; Zhang, X.C.; Cao, Y.N.; Zhao, Q.L.; Bao, E.D.; Lv, Y.J. Ovarian Toxicity in Female Rats after Oral Administration of Melamine or Melamine and Cyanuric Acid. PLoS ONE 2016, 11, e0149063. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.P.; Goldfarb, D.S. Melamine-related kidney stones and renal toxicity. Nat. Rev. Nephrol. 2011, 7, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Liao, C.W.; Cheng, F.P.; Chou, C.C.; Chang, S.C.; Wu, J.H.; Zen, J.M.; Chen, Y.T.; Liao, J.W. Evaluation of Subchronic Toxicity of Pet Food Contaminated with Melamine and Cyanuric Acid in Rats. Toxicol. Pathol. 2009, 37, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingelfinger, J.R. Melamine and the global implications of food contamination. N. Engl. J. Med. 2008, 359, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Mauer, L.J.; Chernyshova, A.A.; Hiatt, A.; Deering, A.; Davis, R. Melamine detection in infant formula powder using near- and mid-infrared spectroscopy. J. Agric. Food Chem. 2009, 57, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.M.; Lan, T.; Shi, H.C.; Lu, Y. Portable Detection of Melamine in Milk Using a Personal Glucose Meter Based on an in Vitro Selected Structure-Switching Aptamer. Anal. Chem. 2015, 87, 7676–7682. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.M.; Xiang, Y.; Guo, H.L.; Shi, H.C. Label-free fluorescence detection of melamine with a truncated aptamer. Analyst 2016, 141, 4511–4517. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Siddiquee, S. A review of recent advances in melamine detection techniques. J. Food Compos. Anal. 2015, 43, 25–38. [Google Scholar] [CrossRef]
- Ehling, S.; Tefera, S.; Ho, I.P. High-performance liquid chromatographic method for the simultaneous detection of the adulteration of cereal flours with melamine and related triazine by-products ammeline, ammelide, and cyanuric acid. Food Addit. Contam. 2007, 24, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Fan, S.; Wu, Y.N.; Zhang, L.; Zhou, P.P.; Li, J.G.; Chen, H.J.; Zhao, Y.F. Simultaneous determination of melamine, ammelide, ammeline, and cyanuric acid in milk and milk products by gas chromatography-tandem mass spectrometry. Biomed. Environ. Sci. 2009, 22, 87–94. [Google Scholar] [CrossRef]
- Chang, C.Y.; Liao, H.K.; Juo, C.G.; Chen, S.H.; Chen, Y.J. Improved analysis of membrane protein by PVDF-aided, matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chim. Acta 2006, 556, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.A.; Klampfl, C.W.; Buchberger, W. Analysis of melamine resins by capillary zone electrophoresis with electrospray ionization-mass spectrometric detection. Electrophoresis 2005, 26, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.X.; Cheng, D.; Mao, L.H. Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem. 2010, 122, 895–900. [Google Scholar] [CrossRef]
- Yun, W.; Li, H.; Chen, S.Q.; Tu, D.W.; Xie, W.Y.; Huang, Y. Aptamer-based rapid visual biosensing of melamine in whole milk. Eur. Food Res. Technol. 2014, 238, 989–995. [Google Scholar] [CrossRef]
- Qi, W.J.; Wu, D.; Ling, J.; Huang, C.Z. Visual and light scattering spectrometric detections of melamine with polythymine-stabilized gold nanoparticles through specific triple hydrogen-bonding recognition. Chem. Commun. 2010, 46, 4893–4895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.R.; Li, Y.; Xu, J.Y.; Bie, J.X.; Liu, X.; Guo, J.J.; Luo, Y.L.; Shen, F.; Sun, C.Y.; Yu, Y.L. Highly Sensitive Aptamer-Based Colorimetric Detection of Melamine in Raw Milk with Cysteamine-Stabilized Gold Nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Qiang, W.; Sheng, J.; Lei, J.; Ju, H. Pretreatment-free fast ultraviolet detection of melamine in milk products with a disposable microfluidic device. J. Chromatogr. A 2010, 1217, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, X.; Hu, C.; Zhang, Y.; Jia, N. A novel solid-state electrochemiluminescence sensor for melamine with Ru(bpy)3(2+)/mesoporous silica nanospheres/Nafion composite modified electrode. Biosens. Bioelectron. 2013, 41, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Liu, J.; Zhang, T.; Li, W.; Liu, W.; Meng, M.; He, F.; Wan, Y.; Feng, C.; Wang, S.; et al. Preparation of monoclonal antibody for melamine and development of an indirect competitive ELISA for melamine detection in raw milk, milk powder, and animal feeds. J. Agric. Food Chem. 2010, 58, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.Y.; Li, Y.S.; Ren, H.L.; Lu, S.Y.; Tian, X.L.; Hao, Y.M.; Zhang, Y.Y.; Shen, Q.F.; Liu, Z.S.; et al. Monoclonal antibody based inhibition ELISA as a new tool for the analysis of melamine in milk and pet food samples. Food Chem. 2012, 135, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Zhou, G.H.; Liu, Z.H.; Ma, Q.; Feng, Y.Q.; Zeng, G.P.; Tinnefeld, P.; He, Z.K. Visual detection of melamine in milk samples based on label-free and labeled gold nanoparticles. Talanta 2011, 85, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.L.; Liu, Y.L.; Lu, L.H. Hydrogen-Bonding Recognition-Induced Color Change of Gold Nanoparticles for Visual Detection of Melamine in Raw Milk and Infant Formula. J. Am. Chem. Soc. 2009, 131, 9496–9497. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.Y.; Pratumyot, Y.; Piao, X.J.; Bong, D. Discrete Assembly of Synthetic Peptide-DNA Triplex Structures from Polyvalent Melamine-Thymine Bifacial Recognition. J. Am. Chem. Soc. 2012, 134, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.Q.; Tu, Y.Q.; Wu Y., P.; W, Y.; Liu, S.; Pei, Q.Q.; Cui, X.J.; Huang, J.D. Exonuclease III-aided recycling amplification of proximity ligation assay using thymine-melamine-thymine triplex structure for ultrasensitive fluorometric determination of melamine. Food Control 2018, 92, 325–330. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.Y.; Ding, Y.; Zhang, X.F.; Xu, K.L.; Hou, X.D.; Wu, P. Modulation of the Singlet Oxygen Generation from the Double Strand DNA-SYBR Green I Complex Mediated by T-Melamine-T Mismatch for Visual Detection of Melamine. Anal. Chem. 2017, 89, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ji, X.H.; He, Z.K. Smart Composite Reagent Composed of Double-Stranded DNA-Templated Copper Nanoparticle and SYBR Green I for Hydrogen Peroxide Related Biosensing. Anal. Chem. 2017, 89, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Li, T.T.; Tu, X.Q.; Luo, L. Label-free fluorescent aptasensor for thrombin detection based on exonuclease I assisted target recycling and SYBR Green I aided signal amplification. Sens. Actuators B Chem. 2018, 265, 98–103. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Ge, J.; Xu, H.H.; Zhang, L.; Hu, Y.L.; Huang, Z.M.; Li, Z.H. A label-free aptasensor for highly sensitive ATP detection by using exonuclease I and oligonucleotide-templated fluorescent copper nanoparticles. Anal. Methods 2017, 9, 2710–2714. [Google Scholar] [CrossRef]

| Sample Number | Melamine Added (μM) | Melamine Detected (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 20 | 21.95 ± 2.21 | 109.75 |
| 2 | 80 | 86.12 ± 5.09 | 107.65 |
| 3 | 150 | 148.76 ± 8.43 | 99.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, J.; Wu, Q.; Wang, Y.; Li, L.; Ding, B. Simple and Label-Free Fluorescent Detection of Melamine Based on Melamine–Thymine Recognition. Sensors 2018, 18, 2968. https://doi.org/10.3390/s18092968
Yang H, Wang J, Wu Q, Wang Y, Li L, Ding B. Simple and Label-Free Fluorescent Detection of Melamine Based on Melamine–Thymine Recognition. Sensors. 2018; 18(9):2968. https://doi.org/10.3390/s18092968
Chicago/Turabian StyleYang, Hualin, Jiujun Wang, Qinghua Wu, Yun Wang, Li Li, and Baomiao Ding. 2018. "Simple and Label-Free Fluorescent Detection of Melamine Based on Melamine–Thymine Recognition" Sensors 18, no. 9: 2968. https://doi.org/10.3390/s18092968




