Functionalized Silver Nano-Sensor for Colorimetric Detection of Hg2+ Ions: Facile Synthesis and Docking Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of AgNPs and APD-AgNPs
2.2. In Silico 3D Structure Generation
3. Results and Discussion
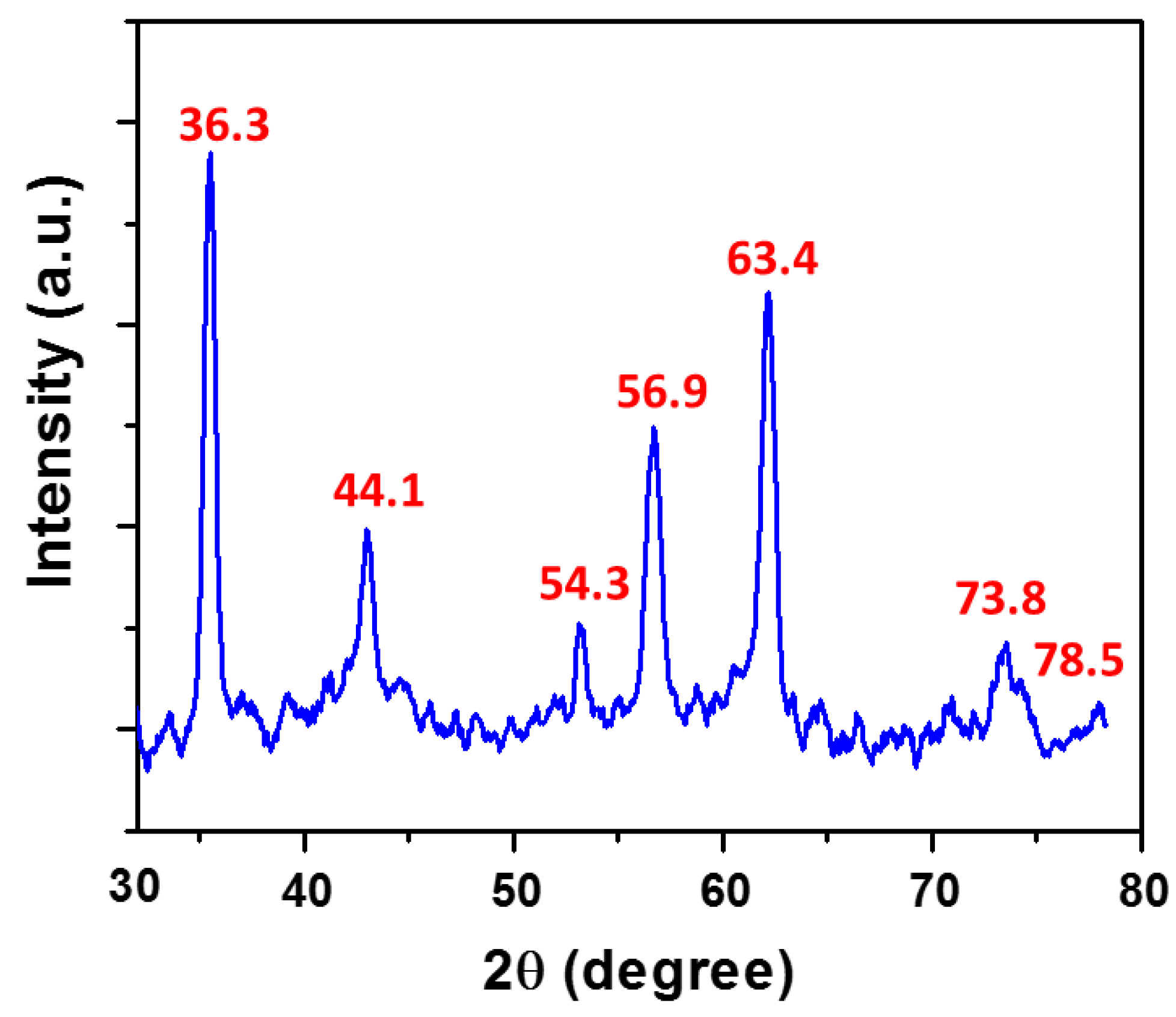
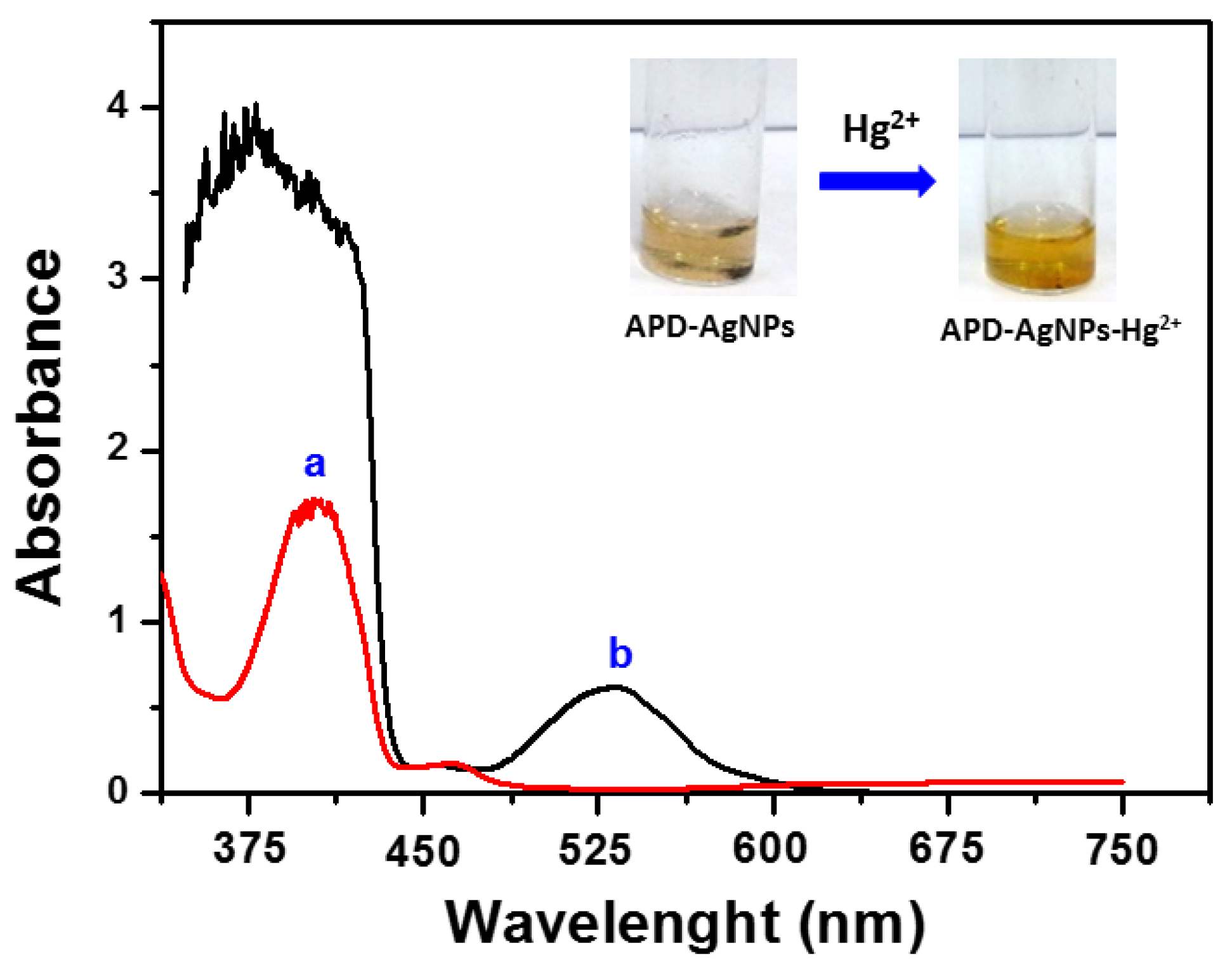
3.1. Characterization of APD-AgNPs-Hg2+ Complex
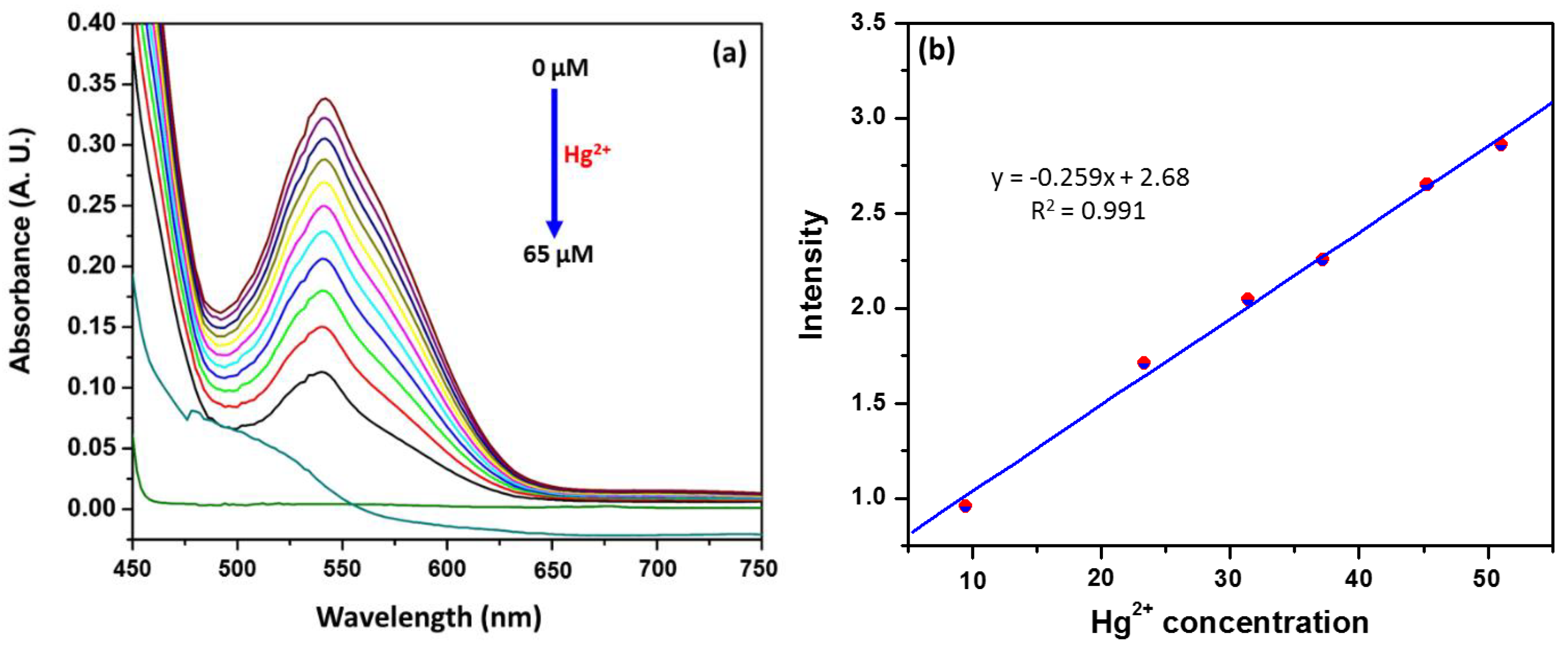
3.2. Colorimetric and Sensitivity Studies of Hg2+ by APD-AgNPs
3.3. Selectivity Studies
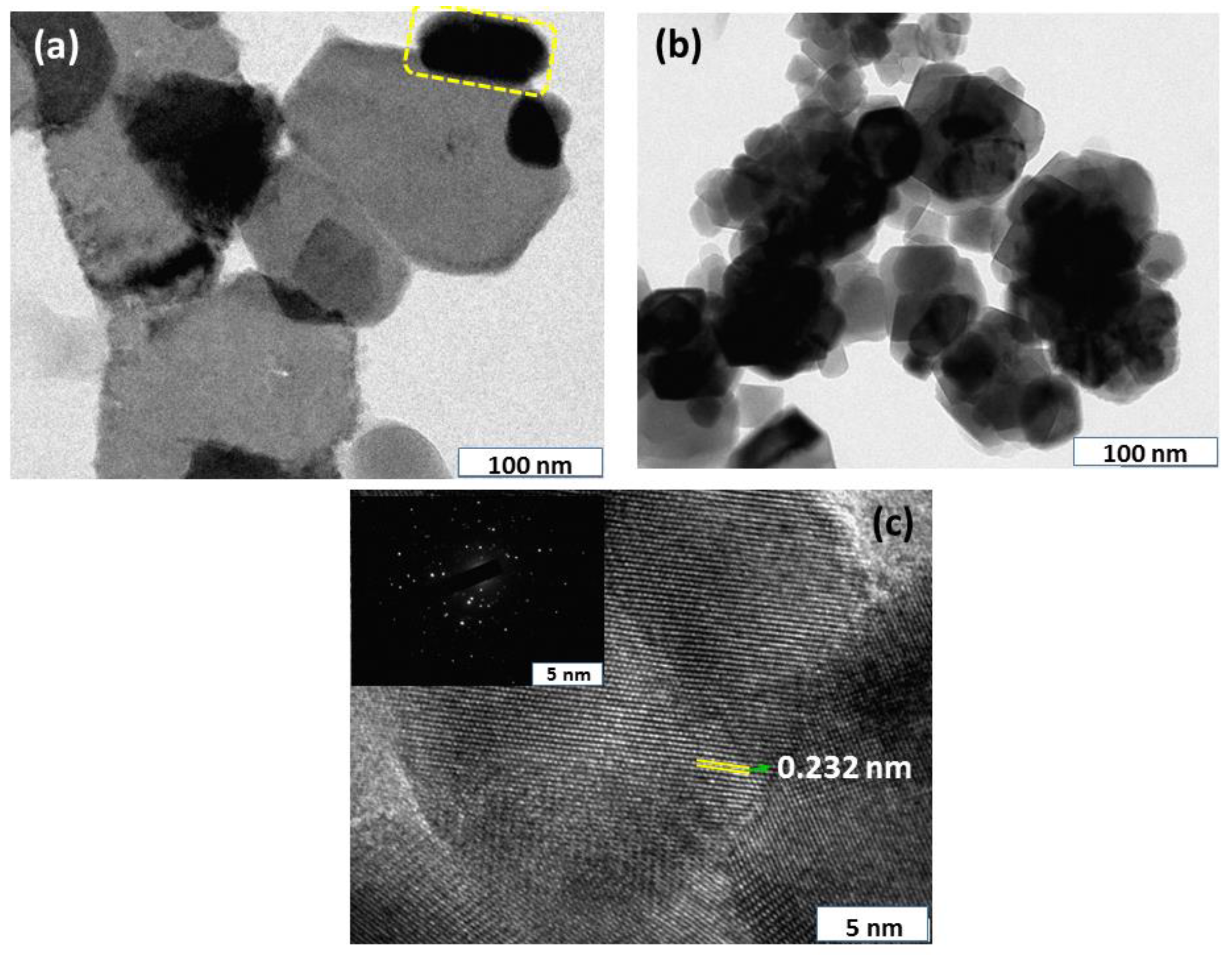
3.4. TEM Investigations
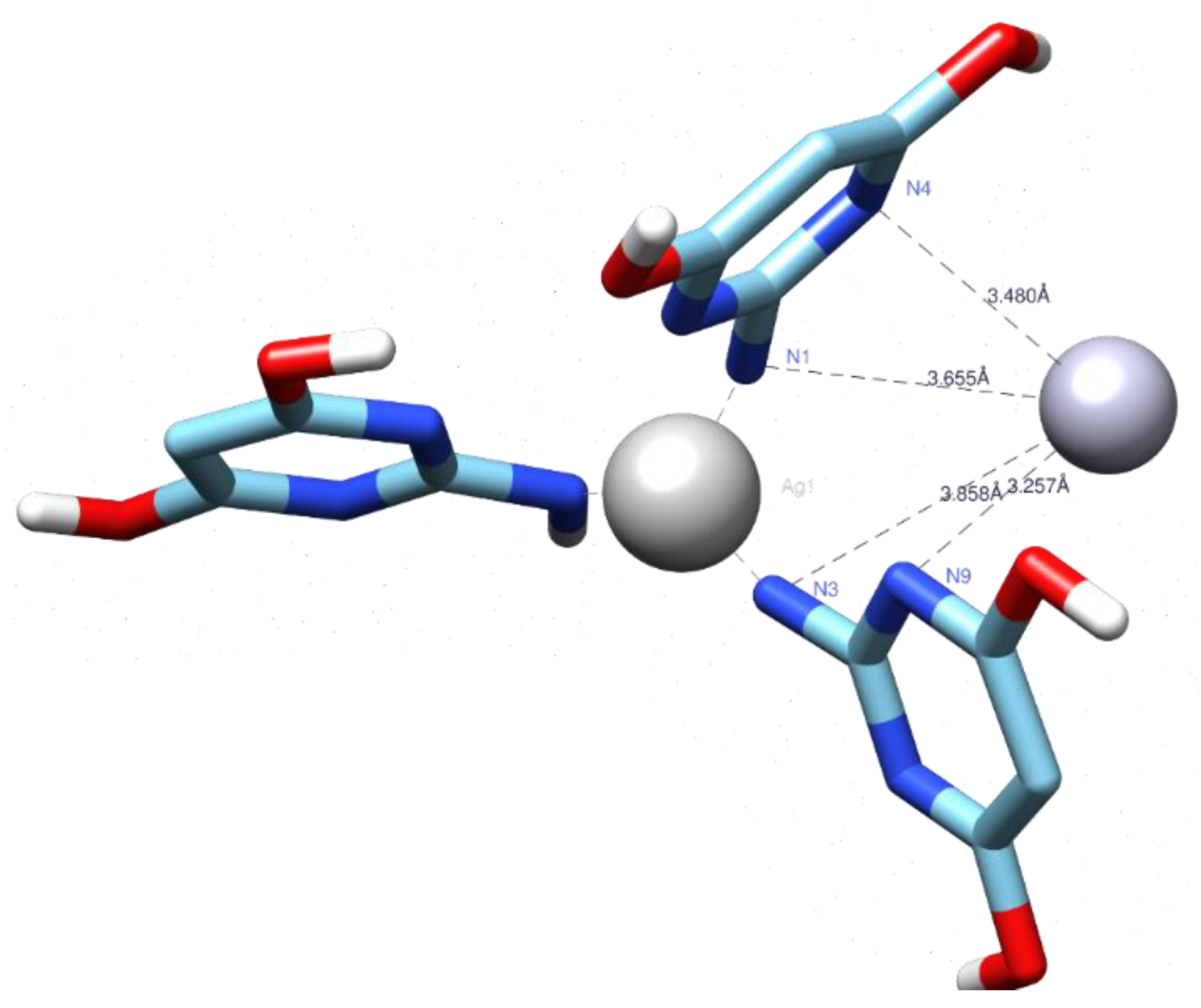
3.5. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vinod Kumar, V.; Anbarasan, S.; Christena, L.R.; Sai Subramanian, N.; Anthony, S.P. Bio functionalized silver nanoparticles for selective colorimetric sensing of toxic metal ions and antimicrobial studies. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 129, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Hutchinson, T.C.; Meena, K.M. Lead, Mercury, Cadmium and Arsenic in the Environment. J. Appl. Toxicol. John. Wiley. Sons. 1987, 366. [Google Scholar] [CrossRef]
- Fan, Y.; Long, Y.F.; Li, Y.F. A sensitive resonance light scattering spectrometry of trace Hg2+ with sulfur ion modified gold nanoparticles. Anal. Chim. Acta. 2009, 653, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Yang, X.R. Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron. 2010, 25, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental exposureto mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tan, Q.; Zhou, K.; Xu, K.; Hou, X. Direct detection of mercury in vapor and aerosol from chemical atomization and nebulization at ambient temperature: exploiting the flame atomic absorption spectrometer. J. Anal. At. Spectrom. 2005, 20, 760–792. [Google Scholar] [CrossRef]
- Fong, B.M.W.; Siu, T.S.; Tee, J.S.K.; Tam, S. Determination of mercury in whole blood and urine by inductively coupled plasma mass spectrometry. J. Anal. Toxicol. 2007, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ichinoki, S.; Kitahata, N.; Fujii, Y. Selective determination of mercury (II) ion in water by solvent extraction followed by reversed-phase HPLC. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 1785–1798. [Google Scholar] [CrossRef]
- Kuswandi, B.; Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Development of a disposable mercury ion-selective optode based on trityl-picolinamide as ionophore. Anal. Chim. Acta. 2007, 591, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sun, Q.Y.; Li, H.B.; Sun, F.; Hu, N.; Wang, P. Screen-printed gold electrode with gold nanoparticles modificationfor simultaneous electrochemical determination of lead and copper. Sens. Actuators B Chem. 2015, 209, 336–342. [Google Scholar] [CrossRef]
- Tu, J.W.; Gan, Y.; Liang, T.; Wan, H.; Wang, P. A miniaturized electrochemical system for high sensitive determination of chromium (VI) by screen-printed carbon electrode with gold nanoparticles modification. Sens. Actuators B Chem. 2018, 272, 582–588. [Google Scholar] [CrossRef]
- Hong, M.Q.; Wang, M.Y.; Wang, J.; Xu, X.Q.; Lin, Z.Y. Ultrasensitive and selective electrochemical biosensor for detection of mercury (II) ions by nicking endonuclease-assisted target recycling and hybridization chain reaction signal amplification. Biosens. Bioelectron. 2017, 94, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Annadhasan, M.; Muthukumarasamyvel, T.; Babu, V.R.S.; Rajendiran, N.; Campus, M. Green Synthesized Silver and Gold Nanoparticles for Colorimetric Detection of Hg2+, Pb2+ and Mn2+ in Aqueous Medium. ACS Sustain. Chem. Eng. 2014, 2, 887–896. [Google Scholar] [CrossRef]
- Ravi, S.S.; Christena, L.R.; Sai Subramanian, N.; Anthony, S.P. Green synthesized silver nanoparticles for selective colorimetric sensing of Hg2+ in aqueous solution at wide pH range. Analyst 2013, 138, 4370–4377. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Chen, W.Y.; Yen, Y.C.; Wang, C.W.; Chang, H.T.; Chen, C.F. Detection of mercury (II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Morin, J.F.; Sasaki, T.; Guerrero, J.M.; Tour, J.M. Recent progress on nanovehicles. Chem. Soc. Rev. 2006, 35, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.X.; Qi, Y.Y.; Jin, Y. Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal. Bioanal. Chem. 2010, 393, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yanli, L.; Xu, L.; Li, J.; Liu, X.; Junshen, L.; Guiying, L. Highly selective, colorimetric detection of Hg2+ based on three color changes of AuNPs solution from red through sandy beige to celandine green. Sens. Actuators B Chem. 2017, 249, 331–338. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Z.; Zhang, C.; Wei, X.; Lou, T.; Zhao, Y. Colorimetric Detection of Hg2+ Based on the Growth of Aptamer-Coated AuNPs: The Effect of Prolonging Aptamer Strands. Small 2017, 13, 1603370–1603377. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lytton-Jean, A.K.R.; Hurst, S.J.; Mirkin, C.A. Silver Nanoparticle Oligonucleotide Conjugates Based on DNA with Triple Cyclic Disulfide Moieties. Nano. Lett. 2007, 7, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.R.; Han, W.S.; Lim, J.M.; Kang, S.W.; Lee, J.Y.; Kang, D.M.; Jung, J.H. Lysine-functionalized silver nanoparticles for visual detection and separation of histidine and histidine-tagged proteins. Langmuir 2010, 26, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, G.; Shiva Prasad, K.; Vinod, T.P.; Balamurugan, V.; Chandan, S. Green Synthesis of Biologically Active Silver Nanoparticles through a Phyto-Mediated Approach Using Areca catechu Leaf Extract. ChemistrySelect 2017, 2, 10354–10359. [Google Scholar] [CrossRef]
- Swarnali, M.; Gadadhar, B.; Jayasree, K.L. Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver Nanoparticles. Appl. Nanosci. 2016, 6, 529–538. [Google Scholar] [CrossRef]
- Junling, D.; Hongzong, Y.; Ranran, W.; Weiwei, W. Facile colorimetric detection of Hg2+ based on anti-aggregation of silver nanoparticles. Biosens. Bioelectron. 2014, 57, 139–142. [Google Scholar] [CrossRef]
- Jeevika, A.; Shankaran, D.R. Functionalized silver nanoparticle probe for visual colorimetric sensing of mercury. Mater. Res. Bull. 2016, 83, 48–55. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiva Prasad, K.; Shruthi, G.; Shivamallu, C. Functionalized Silver Nano-Sensor for Colorimetric Detection of Hg2+ Ions: Facile Synthesis and Docking Studies. Sensors 2018, 18, 2698. https://doi.org/10.3390/s18082698
Shiva Prasad K, Shruthi G, Shivamallu C. Functionalized Silver Nano-Sensor for Colorimetric Detection of Hg2+ Ions: Facile Synthesis and Docking Studies. Sensors. 2018; 18(8):2698. https://doi.org/10.3390/s18082698
Chicago/Turabian StyleShiva Prasad, Kollur, Govindaraju Shruthi, and Chandan Shivamallu. 2018. "Functionalized Silver Nano-Sensor for Colorimetric Detection of Hg2+ Ions: Facile Synthesis and Docking Studies" Sensors 18, no. 8: 2698. https://doi.org/10.3390/s18082698





