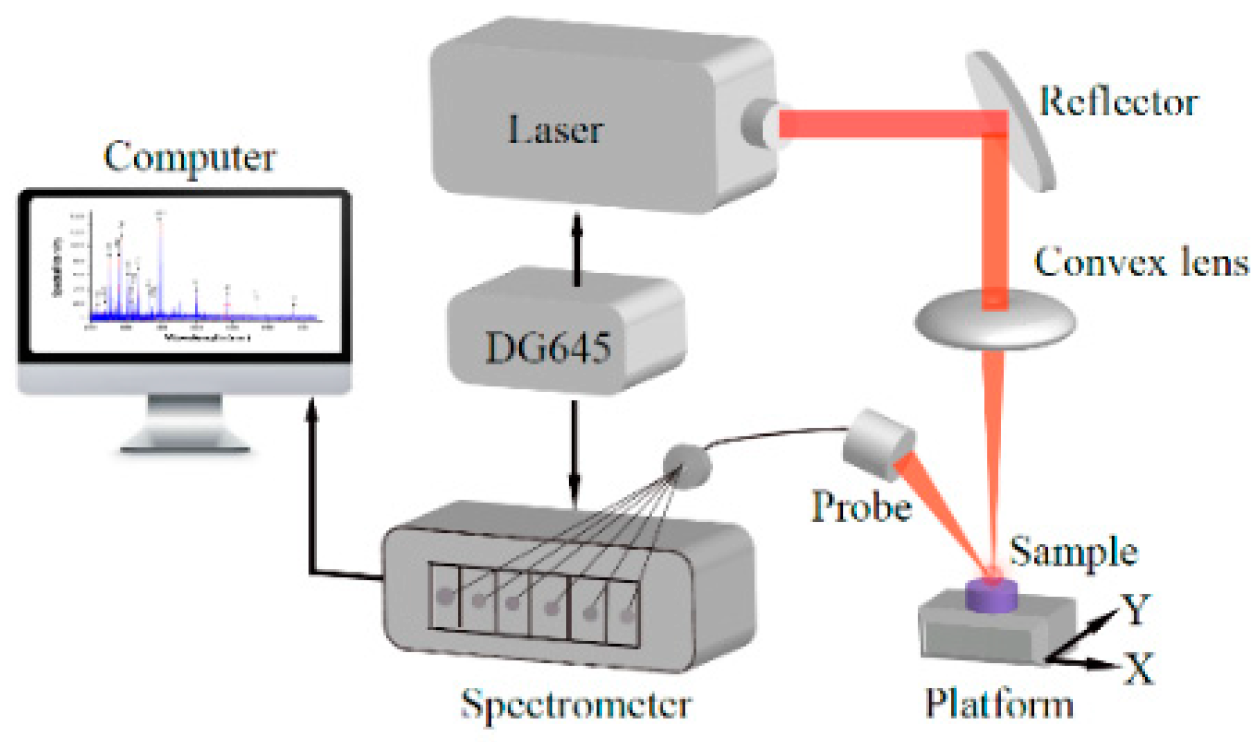
1. Introduction
Transmission line insulators are often contaminated with dust, bird droppings, soluble salts and metal particulates from Nature or surrounding factories. Therefore, pollution flashover more easily occurs, causing large-scale power cuts under wet conditions [
1,
2,
3,
4]. The contamination of insulators is profoundly complex, encompassing species such as NaCl, KNO
3, NaNO
3, SiO
2, C, Al
2O
3, etc. In particular, insulators located near chemical plants, mines, etc. are more likely to attract electrically conductive metal particles, such as Cu, Mn, Ni, etc., which even in micro amounts may cause power frequency flashover accidents [
5].
Serious partial discharge activity and flashovers on insulator strings occurred in 2010 and 2013 at the Ningxia Fuxiang 220 kV substation in China. Surrounding this were densely distributed thermal power plants, cement plants, chemical plants, etc. A variety of Na, Mg, Al, Fe, Mn, Zn, C, CaSO
4 and semiconductors were adhered on the insulators, which distorted local electric fields by stacking them on a transformer sheathed coating, ultimately resulting in corona discharge [
6].
Several researchers have explored the flashover performance of diverse types of contaminants on insulator surfaces. The flashover characteristics of sugar as a contaminant on glass insulators and RTV-coated insulators were worse than those of non-sugary contamination, and compared with non-RTV-coated insulators with non-sugary contamination, the flashover voltage of RTV-coated insulators covered with sugary contamination was higher [
7]. The influence of salt mixtures on the DC flashover voltage discharge characteristics was studied in [
8]. Mixing NaCl and other salts together as an artificial pollutant showed that different mixtures of salts had different flashover voltages and currents. The serious issue of flashover in outdoor insulators due to various environmental stresses and severe saline contaminant accumulation near shorelines was discussed in [
9]. The model showed that the wetting and contamination deposition rate resulted in different surface discharge current characteristics of insulators in rain compared with those in cold fog, leading to different surface flashover voltages. Yang et al. [
10] studied the electrical performance of aluminum phosphate as a contaminant that accumulated on the surface of insulators due to heavy use of aluminum phosphate fertilizer in farmland. However, these types of contamination needed to be collected and sent to the laboratory after an accident has occurred. Then, the contamination components are obtained by taking into account the surrounding environment and the test results, which made it difficult to detect the cause of accidents at the time, resulting in large-scale economic losses. Therefore, having an early warning of the possible evolution of a flashover incident via rapid and accurate detection of contaminant components and contents is particularly important.
At present, several methods, such as the equivalent salt deposit density (ESDD), non-soluble deposit density (NSSD), leakage current, and laser-induced fluorescence (LIF) methods are used to measure the level of actual contamination on insulators for decision making before flashovers occur [
11,
12,
13,
14,
15]. However, the widely used ESDD and NSSD methods must be carried out after the samples are taken back to the lab and cannot effectively account for basic considerations, such as the solubility of components [
16]. In addition, metallic particles cannot contribute to the ESDD because the insolubility still cause local flashovers. Additionally, the ESDD and NSDD methods cannot accurately reflect the species and concentration of pollutants. This makes it difficult to effectively judge the actual pollution level. The leakage current can be used to monitor the pollution levels of insulators online but can only predict the risk of a forthcoming flashover, not provide early warnings for operators [
17]. In addition, the magnitude of the leakage current of the insulators would decrease with the decrease of the non-uniform pollution distribution in insulators [
18]. Thus, the prediction accuracy of the leakage current and LIF methods remains to be studied. In addition, effectively characterizing the non-uniform distribution characteristics of contamination with these detection parameters is difficult. Therefore, a new method for the direct and accurate analysis of the types, concentration and distribution of pollution in insulators that could be performed on site without blackouts is of urgent need.
As a popular analytical method for qualitative and quantitative analysis of elements in materials, laser-induced breakdown spectroscopy (LIBS) allows rapid multi-elemental in situ sample analysis in different environments regardless of the presence of gas or water with a wide range of pressure and temperature regimes without sample preparation and has fast analysis speeds, online application, high determination sensitivity to ppm concentrations and non-contact non-destructive operation [
19,
20]. The NASA Mars Science Laboratory (MSL) launched the ‘Curiosity’ to Mars, which consisted of a LIBS spectrometer and a remote microimager (RMI) as part of ChemCam in late 2011 [
21,
22].
Many studies have been performed using LIBS. Rauschenbach et al. [
20] studied the influence of the temperature, moisture and roughness of rocks with LIBS to maximize the sensibility and accuracy of results. Lanza et al. [
23] studied weathered rocks in a Martian atmosphere using LIBS, scanning electron microscopy (SEM) and electron probe microanalysis (EPMA). Principal component analysis (PCA) was used to classify the profile data. Compared to SEM analyses, elemental information of larger areas of steel samples can be monitored due to the increased speed of LIBS measurements, while SEM analysis provides more precise morphological information, such as the shape and size [
24]. The combination of energy dispersive X-ray fluorescence spectrometry (EDXRF) and LIBS was used to detect P, K, Ca, Mg, S, Fe, Cu, Mn and Zn in wheat flour [
25]. LIBS coupled with multivariate analysis (PCA, PLSR and PCA-SVMR) was applied for the rapid detection of heavy metals in rice [
26]. The methods to analyze rock or mineral composition could be used to analyze natural contaminants in insulators because the compositions mainly come from organic matter, dust, and mineral particles, which are similar to those of rocks or minerals. The composition of contaminants with mixed inorganic substances and organic matter causes photothermal ablation and photochemical ablation processes to occur simultaneously when the laser is applied, but the former process occurs mainly when the metal or rock is ablated, which makes little difference.
However, the application of LIBS in high voltage engineering is rare. Regarding the evaluation of aging states for external insulation composite materials in power systems, Wang et al. [
27,
28,
29] studied the elemental compositions of newly prepared samples of room temperature-vulcanized and high temperature-vulcanized silicone rubbers using LIBS. C, O, Fe and Si were detected via LIBS. The results showed that LIBS did not affect the hydrophobicity of the ablated area and that the depth of the ablated craters had a linear relationship with the laser pulse number, which could be used to detect the aging layer depth and element distribution of the silicone rubber, as verified with the EDS results.
With LIBS, metal contaminant samples consisting of conductive metal particles on the surface of insulators with an expanded elemental coverage were studied for the first time in this paper. The characteristic spectral lines were selected to analyze the element composition of artificial samples. Measurement parameters for LIBS were systematically optimized, while calibration curves and multivariate analyses were employed to calibrate the model for quantitative analysis. The elemental distribution of natural pollution in composite insulators was obtained using LIBS for the main elements of some areas, with the PLSR model applied for further interpretation. The use of LIBS to detect metal contaminant will give new improved methods for accurately diagnosing contamination components and content online to reduce the probability of flashover accidents.
3. Results
3.1. System Parameter Optimization
To optimize the measurement process for multi-elemental analysis, several parameters need to be taken into consideration. In the experiment, the pulsed laser generates high-density energy that ablates and excites the materials in the surface of the sample. This creates a near-neutral, bright plasma composed of atoms, molecules and a large number of free electrons. At the beginning of the plasma cooling process, a strong continuous background spectrum was formed by the ionization of each element from bremsstrahlung and complex radiation, lasting hundreds of ns. Then, the electrons in the atoms and molecules in the excited state transition between different discrete bound energy levels, forming a linear spectrum that represents the characteristics of the atom, which is the atomic emission spectrum. The characteristic wavelength and corresponding intensity of the spectral lines are associated with the type and content of the elements measured, which is the basis for the qualitative or quantitative analysis of elements lasting several μs [
31,
32].
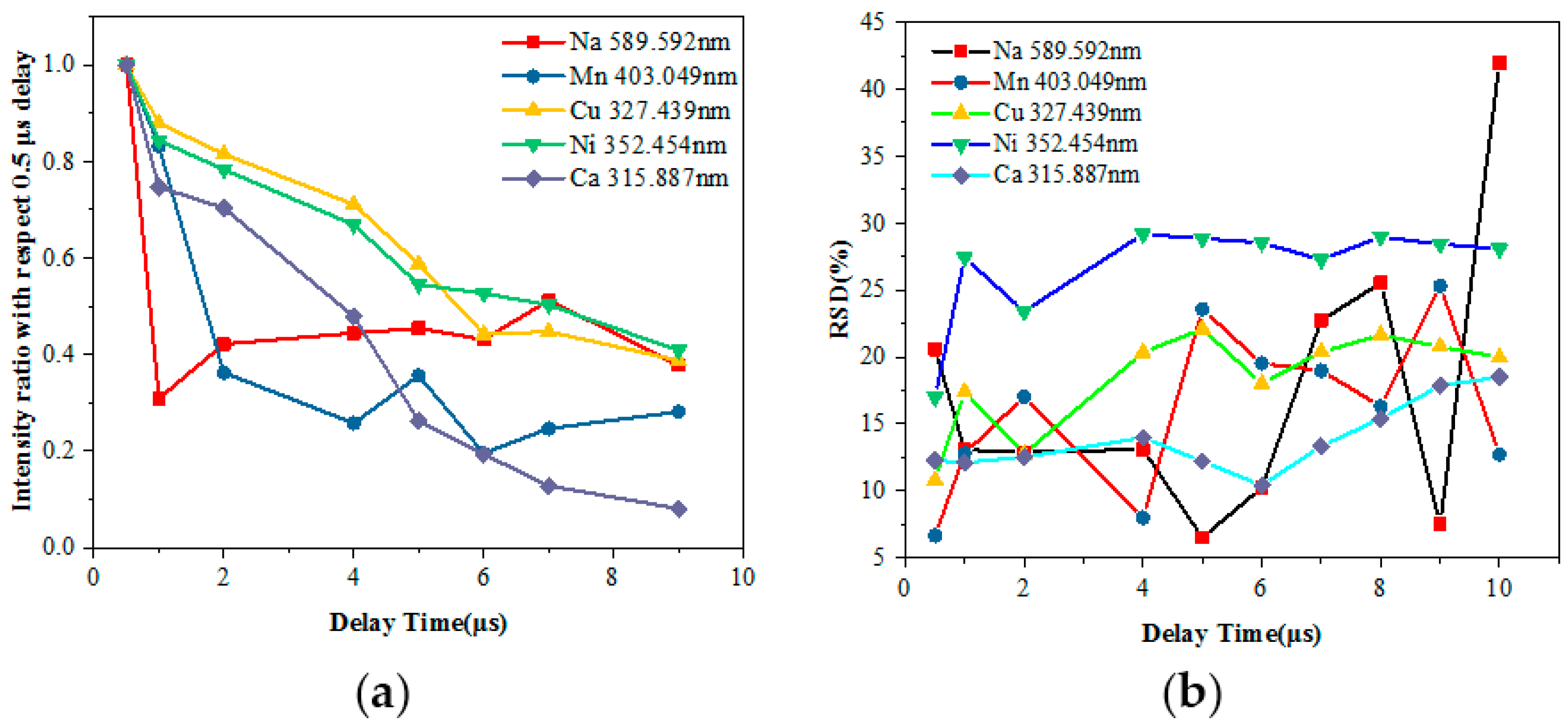
Thus, the LIBS signal for the analyzed main components of the sample (Na, Ni, Cu, Ca and Mn) should be optimized to obtain the highest and most stable signal rather than one affected by the self-absorption. To ensure complete ablation of the sample material across the entire area covered by the laser beam, the gate delays between the laser output and spectrometer were taken into consideration for the optimization. The ratio of each point was calculated using normalization, which divided the spectral intensity of each delay time by the intensity of the delay time at 0.5 μs. The relative standard deviation (RSD) reflecting the precision of the measurement was considered.
Figure 3 shows the influence of the delay time on the spectral intensity and the resulting RSD of different elements measured using LIBS. The RSD value can be expressed as in Equation (1):
where
x1,
x2, and
xn are repeated measurements,
is the average of the repeated results, and
RSD is the relative standard deviation.
There are several characteristic spectral lines for each element, which show nearly the same trend with increasing delay time. Therefore, we selected just one line for each element. When the delay time was 0.5 μs, the continuous background spectrum process was not yet complete; as the time delay increased, the spectral intensity of each element significantly reduced and the RSD of the measured elements slowly increased before gradually stabilizing, except for Na. When the delay time increased from 1 μs to 9 μs, the ratio of Na fluctuated within 0.3 to 0.45, and the RSD values of Na were volatile since it is an alkali metal element and easily ionizable. In addition, the atomic emission spectra occur within 1 μs, which causes the ionization number of Na received by the system to decrease randomly, reducing the accuracy of the measurement as the delay time increases.
Considering the line intensity and RSD with the delay time, the normalized ratio above 0.4 and RSD lower than 20% were selected, as the delay time was optimized from 2 μs to 4 μs. The 3 μs gate delay was used in a later experiment. If a particular element is needed for analysis, a range of delay times with normalized ratios above 0.5 and a minimum of RSD can be selected.
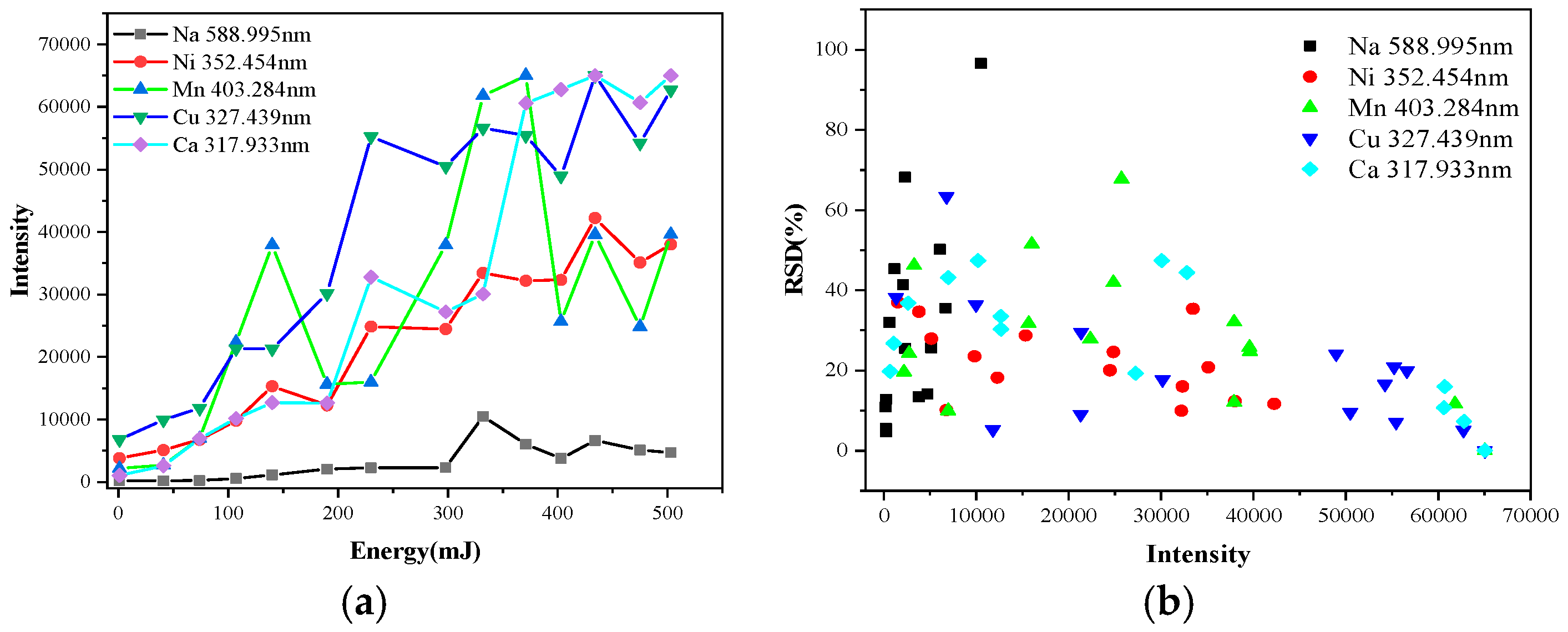
The influence of the laser energy on the spectral intensity and RSD of different elements measured using LIBS is shown in
Figure 4. The intensity of the spectral lines increased as the laser energy per pulse increased. The intensity of Mn fluctuated greatly because the particle size of Mn was greater than 250 μm at 50% distribution, while those of Ni, NaCl, Cu, and CaSO
4 were between 31.35 μm and 48.385 μm by Laser Particle Size Analyzer (Mastersizer 2000, Malvern Panalytical, Malvern, UK). This leads to an uneven distribution of Mn compared with the other special contaminants of the same nature, causing insufficient material ablation while testing different points. The RSD is related to the sample concentration and spectral line intensity and is influenced by the spectral analysis conditions and instrument performance. From the RSD distribution in
Figure 4b, with the increase of the intensity, the RSD of almost all elements descended slowly, which indicated that increasing the energy can effectively improve the accuracy of the results. However, the energy of 110 mJ could only remove contamination that was within hundreds μm of the surface of the insulators in our experiment at once. Then, laser energy per pulse beyond 110 mJ may damage the surface of the insulator. Furthermore, increasing the laser energy gave some elements enough excitation energy to cause intensity saturation or self-absorption, which would have produced a negative effect. Thus, the laser energy was adjusted to 80 mJ in this study.
3.2. Calibration Curve Method
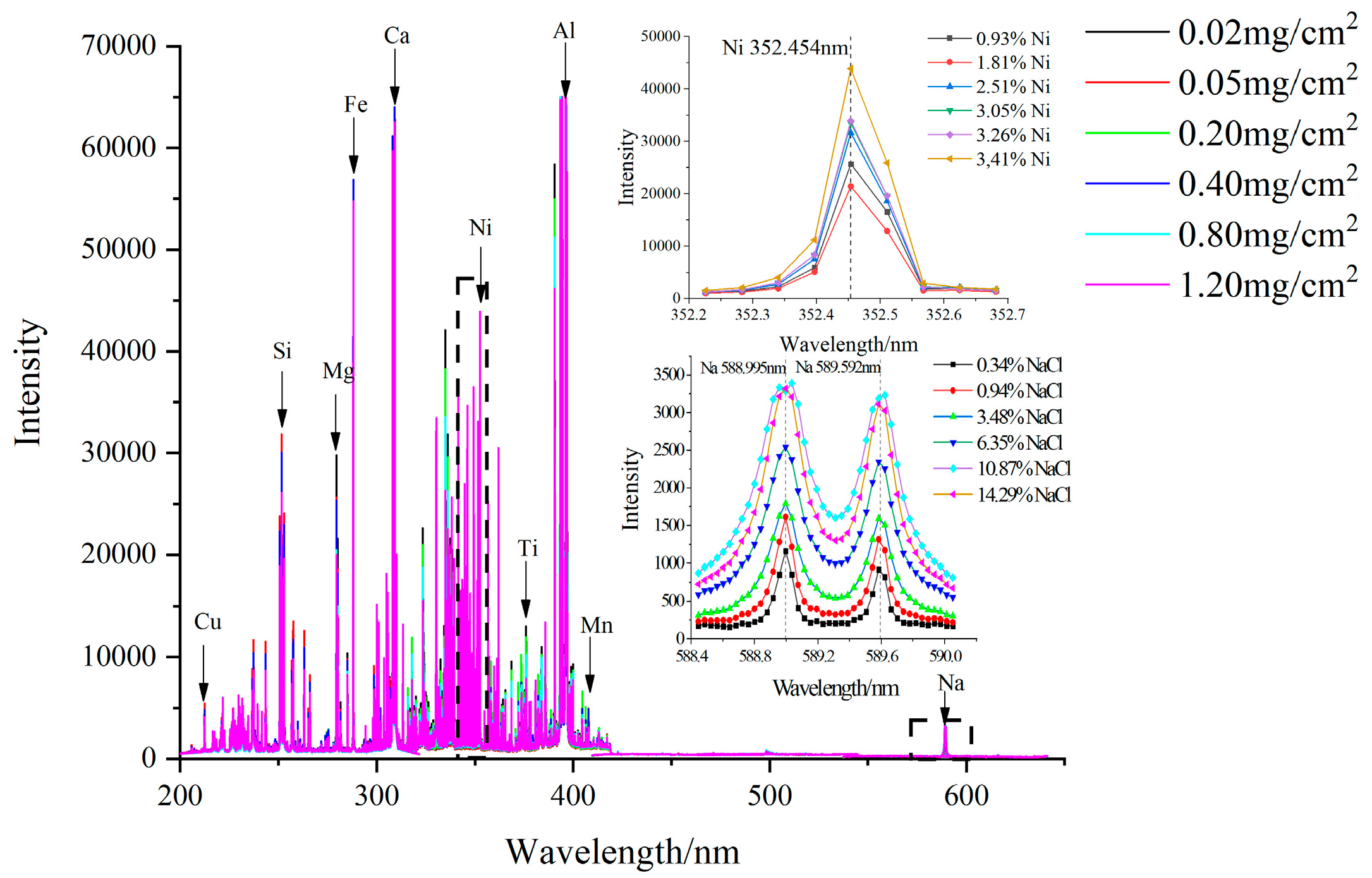
The average spectral lines of the studied Cu, Ca, Ni, Na and Mn intensity of six different artificial contaminants from Group 1 were illustrated in
Figure 5. Also, NaCl and Ni as key values in Group 1 are illustrated in the upper left and lower left corner of
Figure 5. The figure showed that the intensity of the spectral lines would vary with changes in the elemental weight percent. Most of the emission lines were clustered below 400 nm, and increasing the Na and Ni concentration could lead to an increase in the Na emission at 589.592 nm and 588.995 nm and the Ni wavelength at 352.454 nm. According to the NIST database [
33], other main spectral emission lines that correspond to Cu (216.509 nm and 217.894 nm), Mn (403.049 nm and 403.307 nm) and Ca (315.887 nm and 317.933 nm) were also acquired. These lines are generally resonance lines of the elements, and there was no overlap among them.
The average LIBS spectra of the samples, including 50 shots for each sample (five locations and ten excitations per location), were averaged to weaken the influence of the matrix effect. The peak intensities of the elemental emission lines were calculated by averaging the values based on a normal distribution. Then, the spectral data in different wavelength ranges were preprocessed by normalization to effectively improve the influence of various non-target factors on the spectral signal (energy hunting, resolution difference, exotic environment, etc.). This was done to improve the sensitivity and separation rate of the data to enhance the prediction ability and robustness of the model. The normalization method used was area normalization, which divided the spectrum collected from the spectrometer by the sum of the area of the entire spectral data and considered the difference of baselines for different CCDs. Each channel was normalized separately.
The calibration curve method was obtained with correlation coefficient (R
2) values of 0.86665 and 0.90807 for Na (589.592 nm and 588.995 nm), 0.77334 for Ni, 0.81787 and 0.74674 for Cu (216.509 nm and 217.894 nm), 0.82835 and 0.81174 for Ca (315.887 nm and 317.933 nm) and 0.66915 and 0.61523 for Mn (403.049 nm and 403.307 nm), as shown in
Figure 6. The quantitative analysis of the special contaminants can be done if we have sufficient samples and can find a database of different samples. It is worth noting that the slopes of the calibration curves corresponding to different wavelengths of the same elements, such as Cu, were quite different, which may cause errors in the calibration results when the single variable wavelength is used to characterize the calibration curves.
To evaluate the measurement sensitivity and precision, the limit of detection (LOD) was calculated, as in Equation (2):
where
S.D. is the standard deviation of the response, and
S is the slope of the calibration curve.
The smallest LOD was selected in the calculation results at different wavelengths of the same element. From the known primary slope and background intensity, the remaining surface contamination could be estimated to be in the ppm range, as shown in
Table 3. Particularly, Na (589.592 nm), Ni (352.454 nm), Cu (324.754 nm), Mn (403.049 nm), and Ca (317.933 nm) emissions could be maintained beyond 382.03 ppm, 65.071 ppm, 41.191 ppm, 115.073 ppm and 16.316 ppm. Generally, Ca exists in pollution in the form of compounds, such as CaCO
3 and CaSO
4; assuming that the LOD of Ca in those compounds was 16.316 ppm, the LOD of CaCO
3 and CaSO
4 would be 55.4744 ppm and 40.79 ppm, respectively. In some studies, researchers obtained lower detection limits, as low as 69 ppm for Na [
34] and even 0.1 ppb via dual-pulse and crossed-beam lasers for Na on a water film [
35]. However, assuming that the LOD of Na in NaCl was 382.03 ppm, the weight percent of Na would be 0.0382%, which is within the pollution area division of b, as
Table 2 shows, and is adequate for detecting the contaminant on the surface of insulators in moderately or severely polluted areas. The LOD of other metallic elements was satisfactory for the detection of elements on insulators near factories and mines or near urban roads. In [
36], Gondal studied the LOD of Na, Ca, Mn, etc. in cable via LIBS. They obtained lower LOD of Na (589.59 nm) at 39.56 ppm and Mn (257.61 nm) at 4.2 ppm while obtained higher LOD of Ca (393.36 nm) at 43.2 ppm. The reason for the difference of Ca, Mn might be that the metal ions detected in this paper are all simple materials, while the components in [
36] are compounds. Na, a kind of alkali metal, was greatly influenced by environment and instrument parameters, which resulted in great difference of experimental results. In addition, it was worth noting that the LOD results were different at different wavelengths of the same element.
3.3. Multivariate Methods for Classification and Predication
The types of contaminants in complex samples would interfere with each other, and the analysis of a single element would not be able to provide the relationship between the contents and intensity because the relationship between the LIBS intensity and elemental content is typically complicated by matrix effects, such as laser-to-sample coupling efficiency, self-absorption and trace element abundances [
37]. Quantitative LIBS analysis on special contaminants would present a challenge because of the widely varying elemental compositions. Encouragingly, multivariate methods that use information from the entire spectrum or some observed emission lines rather than a single emission line have been shown to yield more precise results. In this paper, the K-means, PCA and PLSR were employed for qualitative or quantitative analysis as a means of exploiting the matrix effects.
K-means, an unsupervised method, works iteratively to group samples by means that reveals their similarity with measured variables [
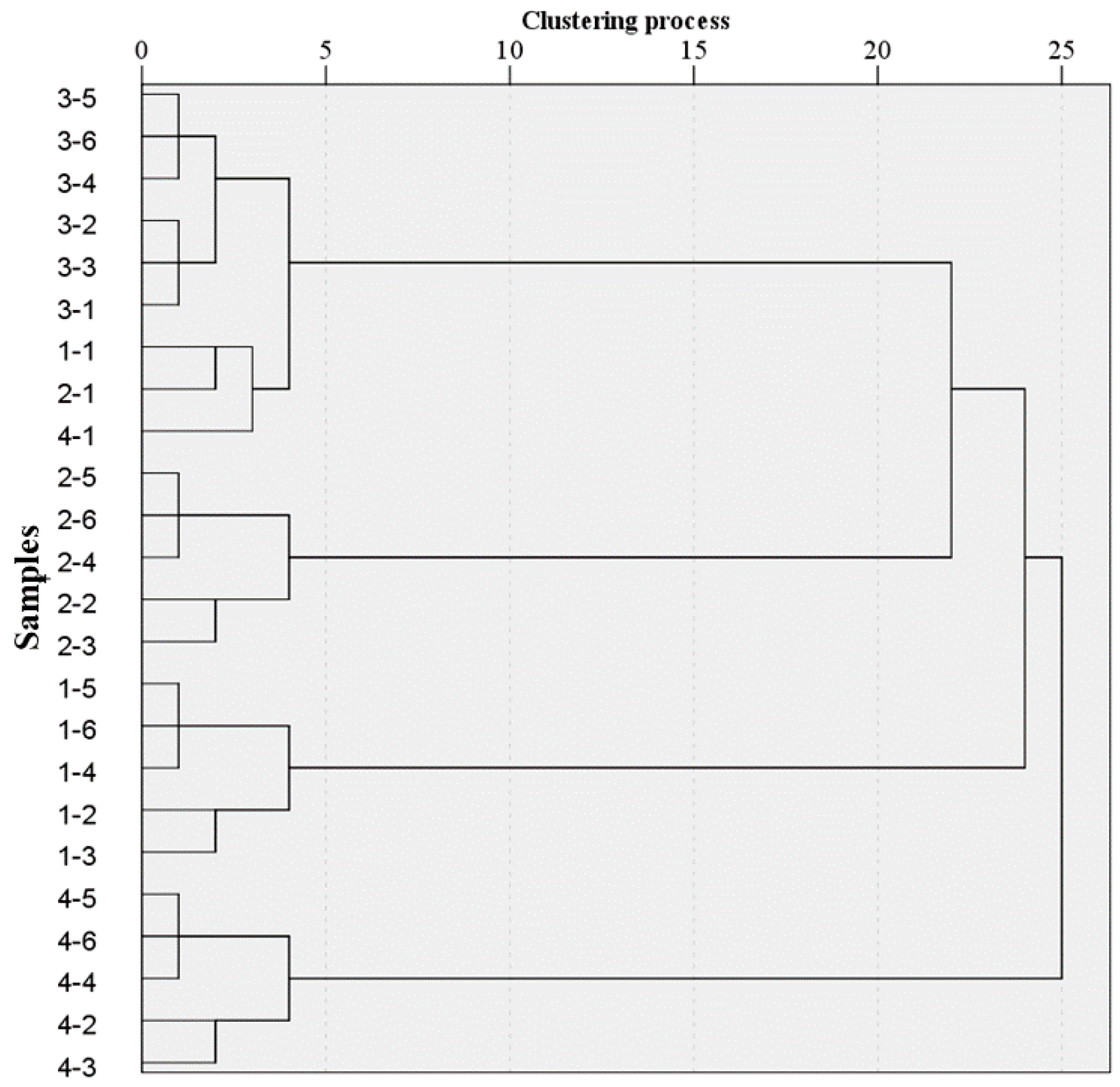
38]. Classification based on observed emissions can obtain acceptable results. According to
Table 2, the total number of test samples of 24 and the k number of 4 were set previously. At the beginning of the algorithm, four samples were randomly selected as the initial mean vector, then the Squared Euclidean Distances between other samples and the four initial vectors respectively was calculated. Compared with the calculated four distances of each sample, the shortest distance was chosen and assigned to the clustering group. For example, compared with the four distances (A1, A2, A3, A4) of sample 4-6 which correspond to four vectors (1-3, 2-3, 3-3, 4-3), the shortest distance is A4. Then, the sample 4-6 was clustered to 4-3. Similarly, the current clustering groups could be obtained by calculating the above process for all the samples of the dataset after the first iteration. Repeat the iterative procedures above until the clustering groups didn’t change. Seeing to the left from the right side of the graph in
Figure 7, two clustering groups were formed provisionally after the first iteration, three clustering grouped together after the second iteration and finally iterative procedures ended until four clustering groups were invariant. In
Figure 7, the classification accuracy was 87.5%, where 1-1, 2-1 and 4-1 and all samples of Group 3 (3-1 to 3-6) clustered together, and other categories besides X-1 of each were classified as shown in
Figure 7.
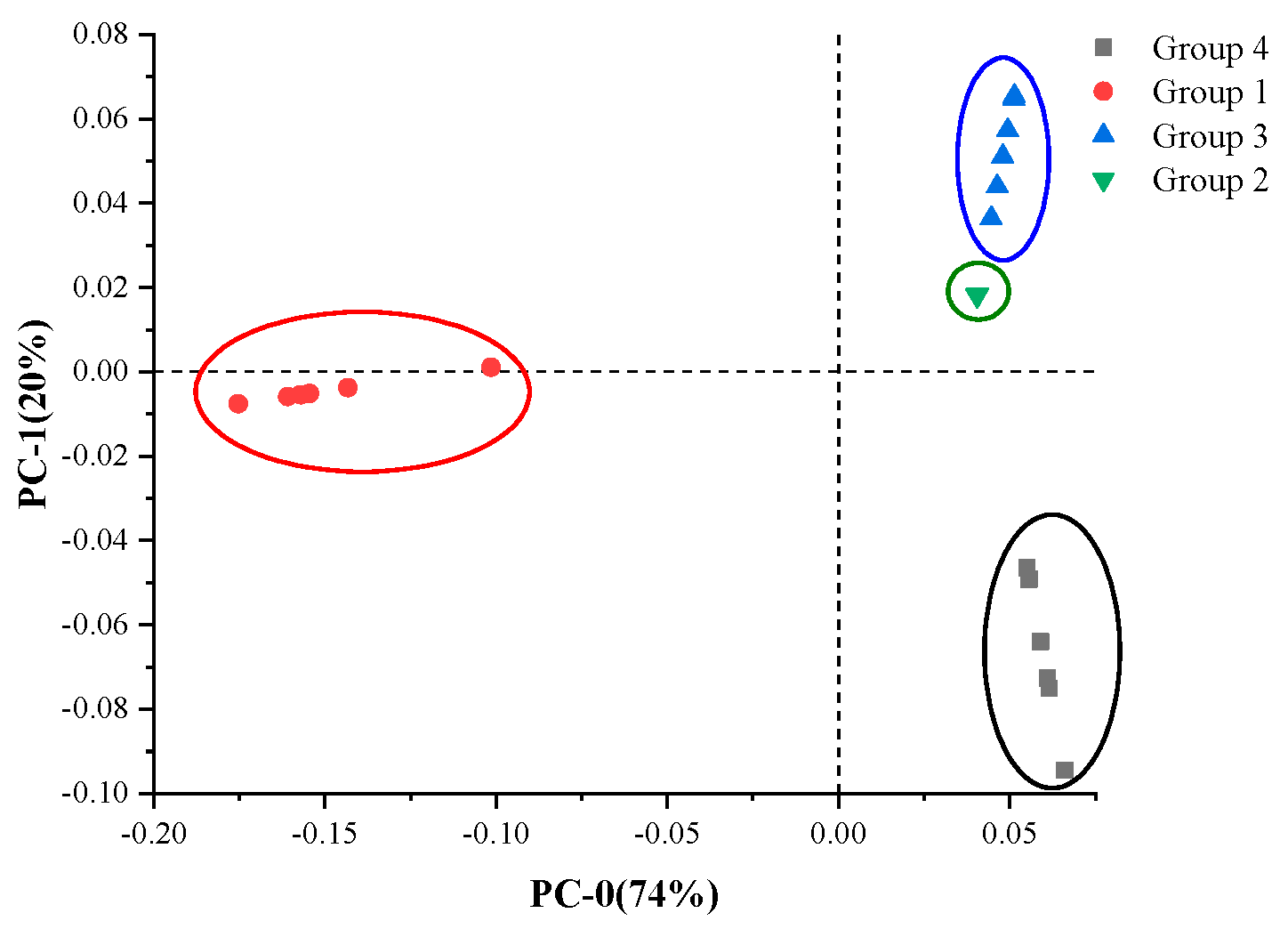
The PCA classification method was used to obtain a more accurate classification result. The pattern recognition method includes both supervised and unsupervised approaches. K-means belongs to the former, while PCA belongs to the latter.
Figure 8 is a two-dimensional scatter plot of scores for two specified components (PC-0 and PC-1) from PCA, where 94% of the variation in the data was described by the two components. Four distinct species of special contaminants were clearly classified, as shown in
Figure 8. Group 1, Group 3 and Group 4 were clearly separated, where the samples from each category had slight differences, leading to a small separation in each cluster, while the cluster of Group 2 gathered at almost one point, indicating that the Group 2 samples had extremely strong similarities in this analysis.
Furthermore, multivariate metrology analysis can make full use of the spectral information to establish a model between contamination content and spectral intensity to perform a quantitative analysis. PLSR was applied in the prediction of the content in metal contamination. PLSR models both the X-matrices and Y-matrices simultaneously to find the latent variables in X to predict the latent variables best in Y. In the model input tab, the matrix in which the normalized intensities of Na, Ni, Cu, Mn and Ca were set as X-matrices and the weight percentages of the observed elements above were set as Y-matrices was analyzed in the data frame where all the weights of the variables were 1 and the Kernel PLS was selected for the model calibration. PLSR uses a full cross-validation in which one can choose the number of segments of a data set, and then, the cross-validation procedure randomly selects the number of samples to take as an unknown dependent variable. This provides a good way to check the quality of the regression model. If the model gives generally good results, the plot will show points close to a straight line through the origin with slope close to 1 (y = x), with R2 close to 1. The four categories (Group 1 to Group 4) were used as the training and cross-validation data sets for the model.
Figure 9 contains the calibration and validation plots for all five elements analyzed. These plots demonstrated highly consistent results regardless of the calibration or validation set curves. The PLSR model based on the observed emissions obtained acceptable results. The R
2 values for Na, Ni, Cu, Ca and Mn were greater than 0.8, and the fitting lines were nearly through the origin. The RMSEV (root mean square error of cross validation) is a measure of the dispersion of the validation samples around the regression line when cross-validation is used.
The closer the RMSEV values of the calibration and validation are, the better the data are modeled. The results of the RMSEV for the calibration and validation of the observed elements were typically close, as shown in
Table 4 for Ni, Mn, Cu and Ca. Additionally, the average values of standard error (SD) for validating Na, Ni, Cu, Ca and Mn in the model were 1.620, 0.250, 0.238, 0.043 and 0.291 which indicated that the results predicted by the PLSR model can be well distributed around the mean value. Also, the regression coefficient in the PLSR could be used to calculate the values, which was convenient for offline numerical predictions.
Overall, some studies show that the PLSR was generally the most accurate method for the analysis of typical geologic materials [
39,
40]. The experimental results in this paper verified the above conclusions. Based on this model, a database possessing the types and contents of observed elemental information can be established, through which the types and concentration contents of the contamination can be predicted, even when the spectral data are collected using only a LIBS analysis on insulators onsite in the future.
3.4. Elemental Mapping of a Composite Insulator with Natural Pollution
The distribution of contamination in operating insulators is usually uneven due to the multiple effects of wind, rain, fog, etc., and the non-uniform pollution distribution in insulators has a significant effect on the flashover characteristics. Thus, it is necessary to acquire the distribution and concentration of contaminants in insulators. In this paper, types and contents of the observed elements of some areas were obtained using LIBS, and the elemental mappings of the contamination was performed. The measurement parameters determined during the optimization procedure described above were used for the elemental mapping experiment.
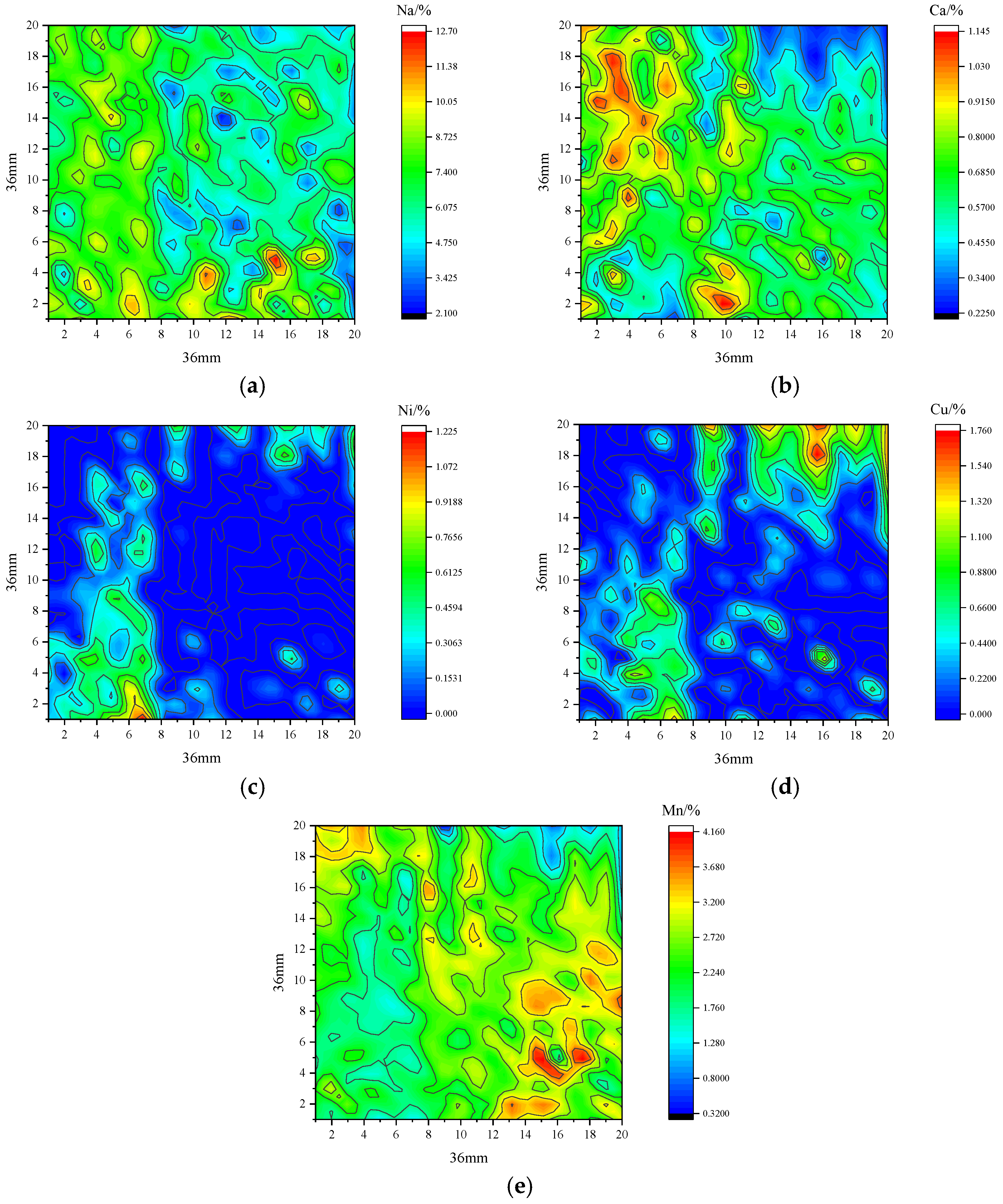
After the intensity of 20 × 20 arrays was obtained with LIBS by summing 400 points in a region of 36 × 36 mm, the content was predicted with the PLSR model above after normalization. The 2D elemental distributions on the surface of the insulator could be obtained to analyze the type and location of the contamination agglomerates, as shown in
Figure 10, where the envelope lines in the graphs are the lines of equal concentration. The results were instructive for the quantitative analysis of contamination using LIBS.
First, the distribution of the observed elements on the sampled surface were different, and the distributions of the same element on the sample surface were also uneven. This may lead to inaccurate measurements of the contamination composition in one sample when only a few points on the surface of the sample are normally sampled. Additionally, Na and Ca, as the main components of natural pollution on insulators, were distributed similarly over the whole area, and the average concentration of Na at 6.8 ± 3.7% was much stronger than that of Ca at 0.65 ± 0.39%. Ni and Cu are only distributed in marginal areas, which can suggest that some Ni and Cu metal particles might attach to the surface. During operation, the clusters of Ni and Cu could distort local electric fields by stacking them on the insulators, which would produce corona discharge or brush discharge to accelerate aging of the insulators. Additionally, the distribution of Mn on the sampling surface was uniform, with the average weight percent of 2.4 ± 1.2%, relatively higher than those of Ni and Cu. There was an obvious accumulation phenomenon in the distribution of metal particles, which might be a homogeneous accumulation of Mn compounds on the sampling surface.
Overall, elemental mapping provided a new method to analyze the distribution of elements online, which can represent the accumulation of elements over the entire surface. Compared to the SEM analysis, elemental information of larger areas of materials of several cm2 could be monitored due to the speed of LIBS measurement, while SEM analysis provides more precise information, such as shape and size, within an area of some μm2. More precise methods of determining element content by LIBS need further study.