A Sensitive Potentiometric Sensor for Isothermal Amplification-Coupled Detection of Nucleic Acids
Abstract
:1. Introduction
2. Materials and Methods
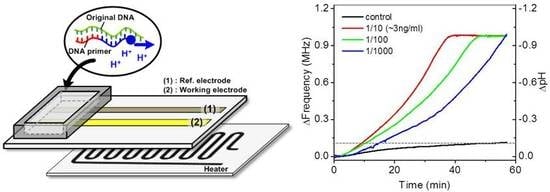
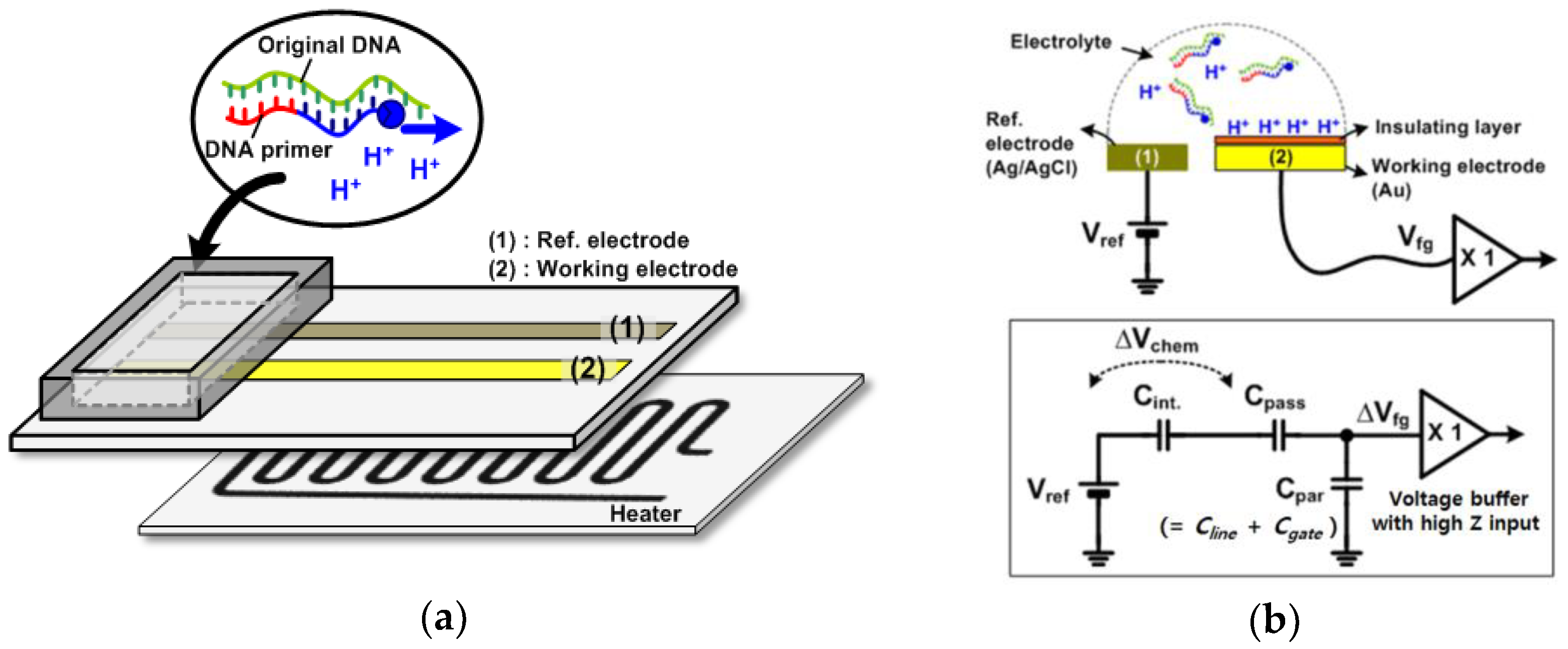
2.1. Device Structure and First-Order Model of the Device
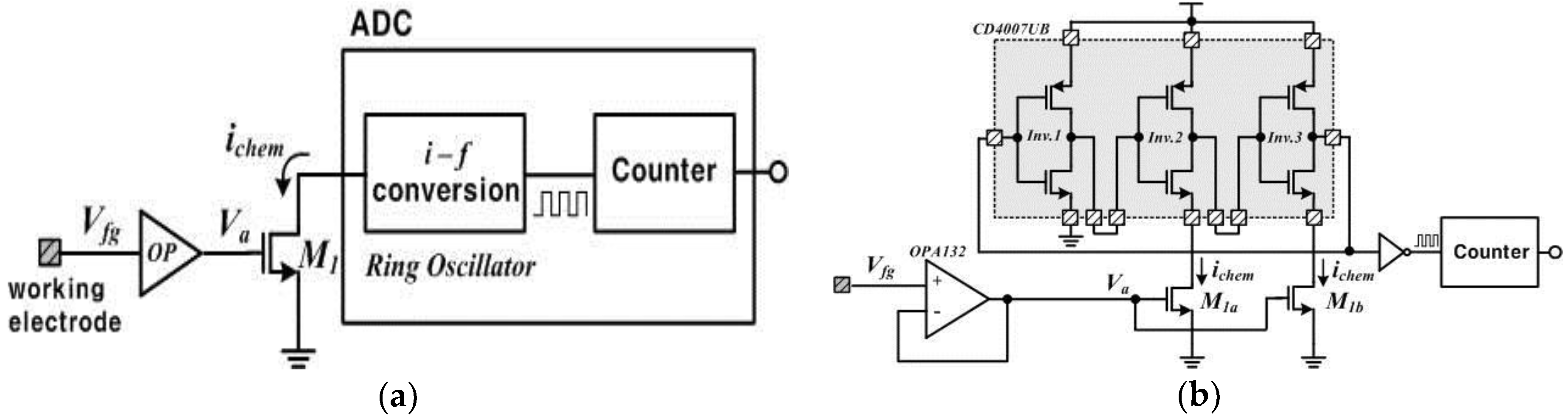
2.2. Design of an FET Sensor with a Ring Oscillator Circuit
2.3. Functionalized Electrodes and Electrical Readout Circuits
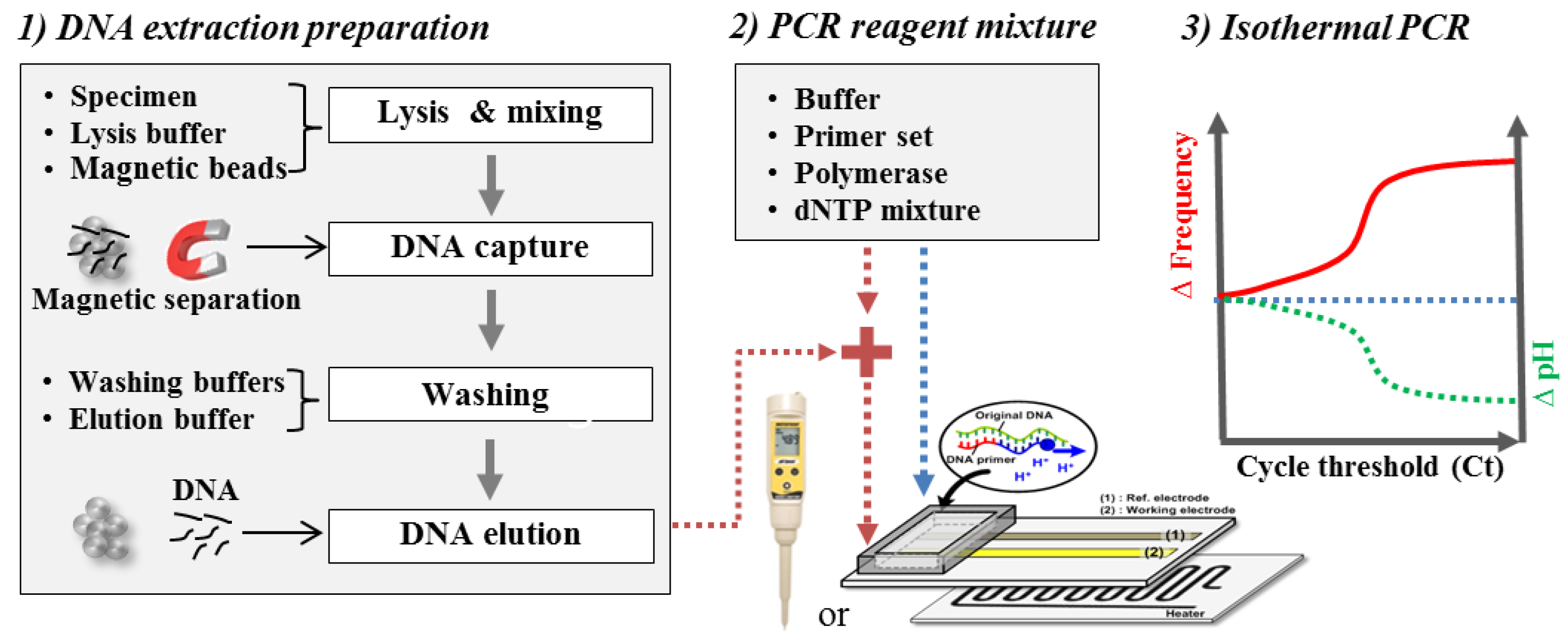
2.4. Extraction and Amplification of Nucleic Acids
3. Results
3.1. Performance of the Designed Sensor
3.2. Isothermal Amplification of Nucleic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanger, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef]
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. Kinetic PCR analysis: Real-time monitoring of DNA amplification. Nat. Biotechnol. 1993, 11, 1026–1030. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Herrmann, M.G.; Moss, A.A.; Rasmussen, R.P. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 1997, 22, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, N.; Yamanaka, K.; Saito, M.; Koketsu, R.; Sasaki, T.; Ikuta, K.; Tamiya, E. Semi-real time electrochemical monitoring for influenza virus RNA by reverse transcription loop-mediated isothermal amplification using a USB powered portable potentiostat. Analyst 2011, 136, 5143–5150. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Nahar, S.; Safavieh, M.; Zourob, M. Real-time electrochemical detection of pathogen DNA using electrostatic interaction of a redox probe. Analyst 2013, 138, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Deféver, T.; Druet, M.; Evrard, D.; Marchal, D.; Limoges, B. Real-time electrochemical PCR with a DNA intercalating redox probe. Anal. Chem. 2011, 83, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Pu, X.; Jiang, D.; Liu, L.; Liu, C.; Liu, X. Development of a real-time resistance measurement for vibrio parahaemolyticus detection by the lecithin-dependent hemolysin gene. PLoS ONE 2013, 8, e72342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yuan, Y.; Song, Y.; Zhuo, Y.; Li, T.; Chai, Y.; Yuan, R. Using the ubiquitous pH meter combined with a loop mediated isothermal amplification method for facile and sensitive detection of Nosema bombycis genomic DNA PTP1. Chem. Commun. 2014, 50, 15932–15935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, J.; Wang, R.; Wang, L.; Ying, Y. Portable pH-inspired electrochemical detection of DNA amplification. Chem. Commun. 2014, 50, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.J.; Ou, C.P.; Amin-Desai, K.; Athanasiou, P.; et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P.; DeRooij, N.F.; Zeme, J.N. Physical mechanisms for chemically sensitive semiconductor devices. Nature 1978, 273, 438–443. [Google Scholar] [CrossRef]
- Purushothaman, S.; Toumazou, C.; Ou, C.P. Protons and single nucleotide polymorphism detection: A simple use for the Ion Sensitive Field Effect Transistor. Sens. Actuators B Chem. 2006, 114, 964–968. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.P. Electrochemistry of ion-selective electrodes. Sens. Actuators 1981, 1, 197–260. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeong, Y.T.; Park, H.J.; Shin, J.K.; Choi, P.; Lee, J.H.; Lim, G. An FET-type charge sensor for highly sensitive detection of DNA sequence. Biosens. Bioelectron. 2004, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, J.O.; Choi, S.; Yoon, J.B.; Cho, G.H. A CMOS label-free DNA sensor using electrostatic induction of molecular charges. Biosens. Bioelectron. 2012, 31, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.D.; Mulato, M. ZnO extended-gate field-effect transistors as pH sensors. Appl. Phys. Lett. 2005, 87, 143508–143511. [Google Scholar] [CrossRef]
- AlAhdal, A.; Toumazou, C. ISFET-based chemical Schmitt trigger. Electron. Lett. 2012, 48, 549–551. [Google Scholar] [CrossRef]
- Jovanovic, G.; Stojcev, M.; Stamenkovic, Z. A CMOS voltage controlled ring oscillator with improved frequency stability. Sci. Publ. State Univ. Novi Pazar Ser. A Appl. Math. Inform. Mech. 2010, 2, 1–9. [Google Scholar]
- Dai, C.L. A capacitive humidity sensor integrated with micro heater and ring oscillator circuit fabricated by CMOS–MEMS technique. Sens. Actuators B Chem. 2007, 122, 375–380. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, D.; Yoon, J.; Kwon, O.; Lee, J. Differential pH-sensitive sensor with DC electrode-offset compensation. Electron. Lett. 2017, 53, 251–253. [Google Scholar] [CrossRef]
- Kwon, O.; Yoon, J.; Jeong, K.; Lee, D.; Lee, K.H.; Kwak, B. Development of a self-contained sample preparation cartridge for automated PCR testing. BioChip J. 2015, 9, 300–305. [Google Scholar] [CrossRef]
- Sawada, K.; Shimada, T.; Ohshina, T.; Takao, H.; Ishida, M. Highly sensitive ion sensors using charge transfer technique. Sens. Actuators B Chem. 2004, 98, 69–72. [Google Scholar] [CrossRef]
- Poghossian, A.; Cherstvy, A.; Ingebrandt, S.; Offenhäusser, A.; Schöning, M.J. Possibilities and limitations of label-free detection of DNA hybridization with field-effect-based devices. Sens. Actuators B Chem. 2005, 111–112, 470–480. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-H.; Lee, D.; Yoon, J.; Kwon, O.; Lee, J. A Sensitive Potentiometric Sensor for Isothermal Amplification-Coupled Detection of Nucleic Acids. Sensors 2018, 18, 2277. https://doi.org/10.3390/s18072277
Lee K-H, Lee D, Yoon J, Kwon O, Lee J. A Sensitive Potentiometric Sensor for Isothermal Amplification-Coupled Detection of Nucleic Acids. Sensors. 2018; 18(7):2277. https://doi.org/10.3390/s18072277
Chicago/Turabian StyleLee, Kang-Ho, Dongkyu Lee, Jongsu Yoon, Ohwon Kwon, and Jaejong Lee. 2018. "A Sensitive Potentiometric Sensor for Isothermal Amplification-Coupled Detection of Nucleic Acids" Sensors 18, no. 7: 2277. https://doi.org/10.3390/s18072277