1. Introduction
Accurate measurement of human joint angles is central to the study of human biomechanics. Better biomechanical models and measurement systems enable more robust tools for interacting with and understanding human kinematics. For example, in rehabilitation, understanding human motion informs the plan of care. For sports applications, understanding motion can lead to improved strategy development. To this end, an accurate measurement of human joint angles is desired. This measurement is complicated, as human motion is characteristically nonlinear, non-smooth, and uncorrelated in time [
1]. Among measurement methods, optical motion capture represents the current gold standard [
2], although other computer vision approaches [
3] do exist. Optical motion capture is accurate in triangulating reflective marker position in space, but interpretation of these data as human joint angles requires an assumed human model. An often-used model is OpenSim [
4]. From markers set on major anatomical landmarks of the body, a least-squares optimization to fit a model may be performed to estimate the joint angles of interest.
This approach is financially expensive due to the equipment required to collect the data, and is cumbersome to post-process. The subject must be fitted in non-reflective clothing with markers placed precisely at anatomical landmarks. Any misplacement would manifest itself as an error in the estimation of joint angles. The infrared cameras are expensive, must be calibrated and oriented properly to view a necessarily-large capture volume, and suffer from marker dropout when the markers are occluded by the subject or another object. This approach is a valuable research tool, but can be inappropriate for the clinician, sports performance expert, or engineer when measurements need to be made at low cost and in the environment of interest.
Small, wearable accelerometer, gyroscope, and magnetometer packages, in contrast, offer an inexpensive way to make robust measurements. Decades of research in state estimation and filtering have enabled the appropriate mathematical models and techniques to estimate orientation [
5] in three-dimensional space. The modern inertial measurement unit (IMU) is a common tool of motion measurement in non-deforming bodies. Inertial sensors have become common as navigation components for ballistic missiles, rockets, and aircraft, and are widely used today [
6]. IMUs have become smaller [
7], although magnetometers still, by nature, can become inaccurate in the presence of a disturbing magnetic field [
8]. Applications of IMUs to human motion have also considered navigation through the environment [
9]. Luinge et al. [
10] first applied IMUs to the measurement of human limb orientation, albeit with limitations of only estimating tilt and requiring sensor calibration to a known position every 30 s. For normal upright gait, Mayagoitia et al. [
11] used accelerometers and gyroscopes, precisely aligned to the body segment, to measure knee angle to a root-mean-square-error (RMSE) accuracy of at most 2.73
(5.2% of gait task range of motion). The optical motion capture truth datum was assumed to be a rigid body angle between markers.
The knee’s primary degree of freedom is flexion/extension with a range of motion (ROM) of approximately 142.5
[
12]. Minor degrees of freedom (DOF) of the knee include varus-valgus with ±5
ROM [
13] and internal-external rotation with ROM of 10
[
14]. Whether all three knee angles are needed or only the major flexion/extension DOF is application-dependent. Some clinical applications, such as understanding knee stability [
15], require estimation of the degrees of freedom with lower range of motion. Inertial methods exist to estimate 3D knee angles [
16,
17], but evaluation of these methods in the minor degrees of freedom is difficult due to high measurement error relative to the ROM of the minor DOFs. In Favre et al. [
16], for example, the RMSE of estimation of the knee varus-valgus angle was 35% of the joint’s ROM. Markers on the thigh and shank are subject to millimeters of soft tissue motion [
18], which may confound calculation of joint angles. These ”truth” measurement errors, combined with the low range of the knee’s minor degrees of freedom, lead to a low signal-to-noise ratio when estimating vargus/valgus and internal-external rotation. Thus, caution must be taken when estimating these lower-ROM DOF via either inertial or optical methods. Estimation of flexion/extension is inherently more robust due to a higher range of motion to measurement error ratio.
Inertial measurement techniques inherently require an understanding of the relationship between the coordinate systems of the IMUs and the coordinate system of the joint in question. This relationship is established through a process of calibration, which primarily serves to align or measure relative orientation between the local frames of the IMUs and the joint’s coordinate system (JCS) [
19,
20,
21]—such that computed angles may be interpretable as the anatomical joint angle. One method of calibration is through precise manual alignment of the sensors on the leg segment. Favre et al. [
16,
22] aligns the IMUs such that one of the axes of each IMU is aligned with the knee’s hinge axis while also employing a high-pass filter on the gyroscope data to minimize drift of the angle estimate. This mounting assumption is also seen in other research [
10,
11].
Alternate methods seek to use a functional calibration procedure to estimate the relationship between the body-mounted IMUs and the anatomical axes of the joint. Zhu et al. [
23] decouples the degrees of freedom of arm motion on a human subject in order to excite the degrees of freedom separately, which allows a novel Kalman filter to estimate limb segment orientation. Luinge et al. [
24] prescribes a pre-defined arm rotation procedure to determine the definitions of local rotation axes in the arm. Cutti et al. [
25] and Favre et al. [
17,
26] each prescribe a functional calibration procedure for determination of leg segment-to-sensor orientation. Vitali et al. [
27] uses two functional alignment motions to set the anatomical coordinate system of the thigh and shank. Cooper et al. [
28] employs similar kinematic constraints of the knee hinge axis as above, but requires the knee hinge axis be provided by optical motion capture. These biomechanically-inspired methods offer powerful estimation tools over human-aligned techniques, especially in the presence of noisy sensors.
As IMU estimation of the knee joint angle has become more accurate, attention has shifted towards methods which require fewer functional calibrations and assumptions of alignments. Seel et al. [
29,
30] presents a novel method for estimating the knee axis through a formulated optimization problem, leveraging a kinematic argument of hinge joint motion. From this axis, the angle can be computed. Müller [
31] extends this concept to the elbow, arguing that the relative angular velocity between two IMUs can be decomposed about the two major degrees of freedom of the elbow, and an optimization method is formulated to calculate those axes. These methods allow for less precise placement of the IMU on the body segment.
The relative angular velocity vector, as Müller implies, is a noteworthy quantity because it represents all relative motion between two IMUs. In the case of the knee, we simplify the knee to one degree of freedom and examine a method for estimating the primary flexion/extension axis. The relative angular velocity between an IMU on the shank and IMU on the thigh should generally point parallel to the direction of the knee hinge axis. This hinge axis should be well approximated by the principal component of the set of aforementioned relative angular velocity vectors. Principal Component Analysis (PCA) [
32] is a general statistical technique to simplify high-dimensional data to a descriptive lower-dimensional structure. In biomechanics, it has been used to study coordination between time-series data [
33]. Landry et al. [
34] used PCA to study similarities in gait waveforms of knee angle between patients with osteoarthritis. Dillman et al. [
35] used PCA in a similar way to study patients with Parkinson’s disease. In the case of physical data, like angular velocities, PCA offers a computationally-efficient method to find a primary directionality of the data.
In this paper, we examine the decomposition of relative angular velocity between a shank and thigh IMU into its primary component: rotation about the knee flexion/extension axis. PCA is used as a robust method to estimate this knee axis. Once the knee axis is estimated, a leg segment coordinate system may be defined for both the thigh and shank. The Euler angles between these two leg segment coordinate frames are then interpreted as the anatomical knee joint angles. For estimation of the knee’s hinge axis, PCA is more computationally-efficient than previously-proposed nonlinear least-squares optimization methods and is robust to the dual-direction representation of an axis. The method requires no assumption of mounting orientation on the body of the IMUs and requires no calibration to estimate the knee flexion/extension axis, any motion of the knee will suffice. A subset of this analysis, IMU-estimated knee angle for subjects 1 and 2, was previously presented [
36]. This paper extends the previous work through analysis of additional subjects, developing an explanatory model of the error, and comparison of the method with ideal simulated IMU data and the method of Seet el al. [
30].
To evaluate the proposed method, the knee flexion/extension axis was estimated during a timed-up-and-go task, which is commonly associated with increased fall risk in older adults [
37]. Truth data were estimated using OpenSim modeling of optical motion capture data. We hypothesize (1) that the estimated knee joint angle from the proposed IMU method will be of similar error performance to other IMU-based measurement systems. (2) Accuracy of the method may be influenced by placement of the IMUs on the leg and soft-tissue noise; these factors are formalized and investigated further.
2. Problem Formulation
The human knee joint is commonly simplified as a single degree-of-freedom (DOF) hinge joint. We refer to this major rotation of the knee as flexion/extension. Other rotations of the knee include internal-external rotation and varus-valgus. These rotations are generally considered small, enabling the simplification of the knee as a hinge joint.
An IMU can be placed both distal and proximal to the knee joint, such that all relative motions between the IMUs are assumed to be due to knee flexion/extension at the joint. Small errors due to other motion of the knee or skin tissue artifacts are not modeled in this work. No assumption of alignment of the IMUs are made; the IMUs are free to be placed in any orientation on the thigh and shank, simply provided that one is distal and one is proximal to the knee joint.
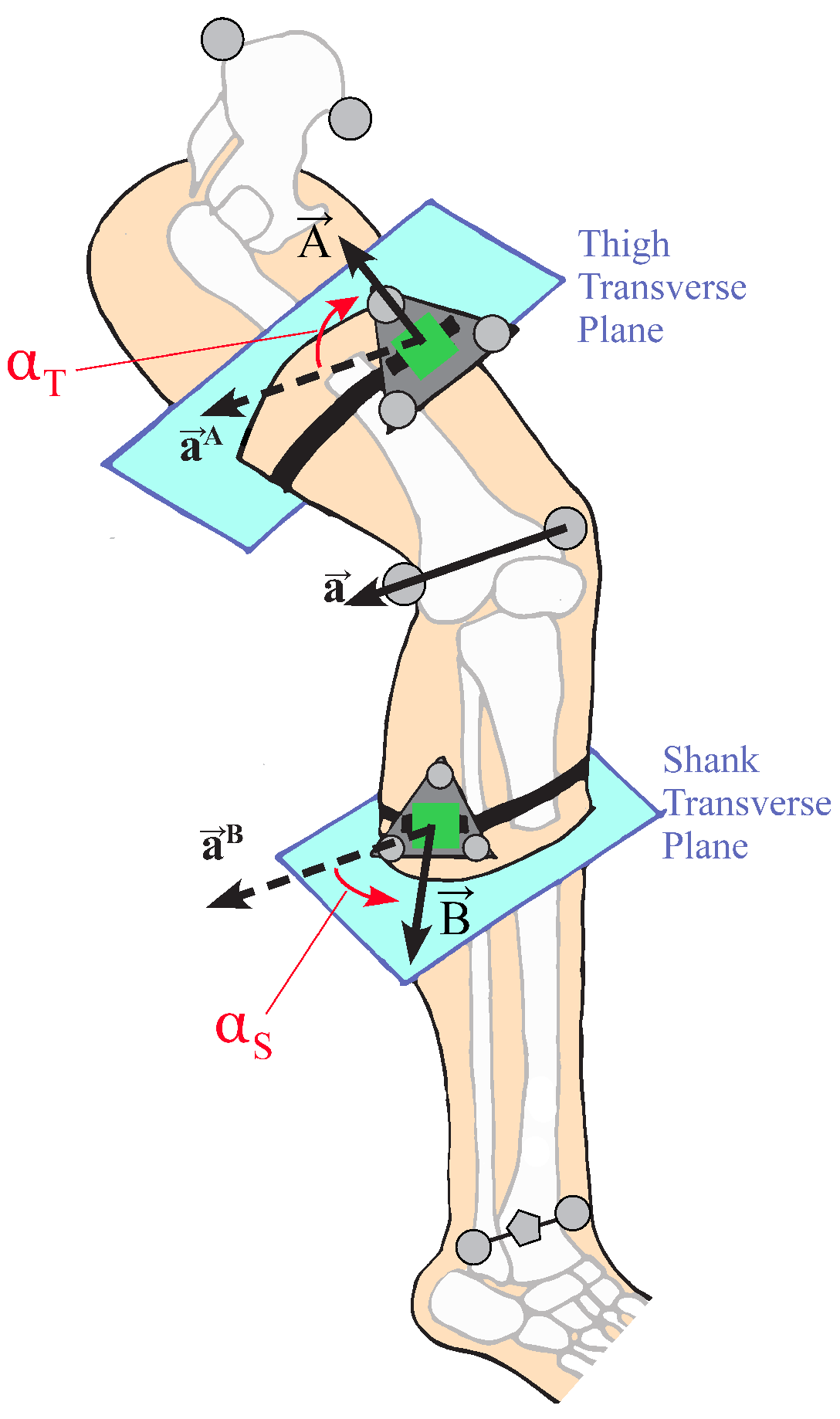
The coordinate frame of the IMU on the thigh will be referred to as frame , and, likewise, the frame of the IMU on the shank will be referred to as frame . The flexion/extension axis of the knee will be referred to as axis . As such, the anatomical axis expressed in frame will be noted as . It can likewise be expressed in frame as .
The relative angular velocity between the two IMUs can be expressed in frame
as:
The rotation matrix
can be computed for every sample measurement
k via simple rotation mathematics from the filtered orientation estimates of the individual IMUs. Multiple state estimators exist for the IMU sensor fusion for orientation problem [
5,
38,
39,
40]. In this work, an unscented Kalman filter, as implemented by the manufacturer of the IMU, was used to estimate IMU orientation.
In the case of a simple 1DOF hinge system with IMUs on either side of the joint, and a low-error measurement system (e.g., low-error gyroscopes and low-error state estimates of the IMU orientations, and ), the relative angular velocity vector between two IMUs, , will point in the direction of the hinge axis of rotation. In practice, this remains true, with some (ideally small) errors in the direction of due to imperfect IMU orientation estimations and small perturbations of the IMUs on the skin surface due to deformable skin tissue artifacts. We assume that the knee anatomical axis is time-invariant, being fixed in time in both IMU coordinate systems. This assumption is consistent with biomechanical modeling techniques such as OpenSim. This assumption can be violated by off-axis rotations. In practice, these off-axis rotations can be considered a combination of unmodeled knee rotations (varus-valgus and internal-external rotation) and errors, namely IMU perturbations due to skin tissue artifacts.
A robust and computationally-efficient method to produce a best single estimate of the axis of rotation, , is through PCA. Operationally, the principle component of a PCA on a set of vectors will find the axis that minimizes the sum of the square errors of the orthogonal distances of individual vector measurements to the estimated principle axis. Since the set of vectors will generally point in the direction of the knee hinge axis (with some noise), PCA may yield a robust estimate of the anatomical knee hinge axis .
Note that this method could also be expressed in terms of frame
. Equation (
1) could be written in terms of frame
to yield
, and PCA performed on
to yield an estimate of the knee hinge axis in terms of frame
,
. This approach is equivalent—the axes are related by the relative orientation as
. This paper will estimate the knee axis and angle in frame
.
A further advantage of PCA to solve this axis estimation problem is the robustness to sign ambiguity of relative angular velocity, which yields a sign ambiguity of the knee hinge axis. In flexion, the relative angular velocity will point in one direction, and, in extension, will point in the opposite direction. Optimization or axis search methods may be confounded by this axis sign ambiguity. Since PCA only seeks to minimize the orthogonal distances of the measurements to a line estimate, it does not suffer from sign ambiguity.
Once the knee hinge axis
has been estimated via PCA, the knee’s anatomical angles must be computed. This work implements an angle calculation method functionally similar to Laidig et al. [
41] and Seel et al. [
30]. A local leg segment coordinate system is defined as
and
for the thigh and shank leg segments. These coordinate systems are the IMU frames
and
rotated such that the
z-axis aligns with the estimated knee hinge axis
or
. For the purposes of flexion/extension calculation only, this segment frame can be related to the local IMU frame as a function of only estimated knee hinge axis as:
for the thigh segment, and similarly for the shank segment. This relationship can be deduced by setting the
z-axis to
, the initial
x-axis to [1 0 0], and then constructing an orthonormal
y and
x-axis. The relative orientation between these new frames
and
can then be calculated as:
where
is the common global frame. Finally, the relative orientation
is then decomposed into
z–
x–
y Euler angles. The first Euler angle, about the
z-axis, is the knee flexion/extension angle, consistent with International Society of Biomechanics (ISB) JCS recommendations [
19].
3. Materials and Methods
3.1. Participants
Fifteen subjects (7 male, 8 female, age = 20.7 ± 1.79 years) participated in the study. The protocol was approved by the Committee on the Use of Humans as Experimental Subjects at MIT (Protocol 1702862119). Exclusion criteria included (1) atypical neurological, heart, lung, or blood conditions; (2) surgeries performed within the previous six months; and (3) physical limitations which would require an assistive device.
3.2. Study Protocol
Each participant in the study completed three motion tasks: a 10-meter-walking task (10MWT), a Standing Balance task (SBT), and a Timed-Up-And-Go test (TUGT). Only the data from the TUGT was considered for this analysis. The TUGT began with the subject sitting on a stool. Then, the subject walked 3 m to a marker, turning around it, walking back to and sitting on the stool. Each participant completed 15 TUGT trials. The first five were practice trials, meant to teach the task, and the results were not processed for this analysis. The data from the final 10 TUGT trials that each participant completed were used for analysis. Each of the 15 subjects completed these 10 full trials, for 150 trials total. Four of the recorded trials were excluded due to missing or incomplete IMU data. Three trials were excluded due to poor/diverging IMU orientation estimates from the manufacturer’s onboard filter. This left 143 trials for analysis. The existence of poor IMU orientation estimates motivated the additional creation of a “simulated” set of IMU data from marker triads that had been placed on the IMUs.
Each subject was outfitted with a set of reflective motion capture markers and strap-on IMUs (Opal IMU, APDM, Inc., Portland, OR, USA). The position of these reflective markers and IMUs can be seen in
Figure 1. As can be seen in the figure, some markers are placed on anatomical landmarks, independent of IMU positioning. These primary markers are placed according to a modified Cleveland Clinic lower body marker set for use in OpenSim inverse kinematic modeling. The position of the reflective markers was captured using a 14-camera Vicon motion capture system (Vicon Motion Systems, Inc., Los Angeles, CA, USA) at a sampling rate of 100 Hz.
3.3. Data Processing
The IMU contains dual 3-axis accelerometers (±16 g, ±200 g), a 3-axis gyroscope (±2000 deg/s), and a 3-axis magnetometer (±8 Gauss). The raw data from those sensors were fused for orientation using an unscented Kalman filter, as implemented by the manufacturer. The orientations from the two respective IMUs to a common global frame were used with the PCA method as described in
Section 2 to estimate the knee hinge axis
in each IMU frame. Then, the segment frames
and
were constructed from Equation (
2). Finally, the relative segment frame orientation
is computed from Equation (
3), which encodes the estimated knee flexion/extension angle.
The optical motion capture marker data were low-pass filtered with a 30 Hz, 6th order Butterworth filter and then processed in OpenSim, with inverse kinematics computed via OpenSim’s gait 2392 model [
42]. This solver minimizes error between the assumed placement of markers relative to an ideal biomechanical model (scaled for each subject) and the measured marker position from optical motion capture. The OpenSim subject model was acquired by scaling the generic OpenSim model for each subject, according to anthropometric measurements derived from the subjects’ marker data while static.
The IMU data were collected at 128 Hz, while the Vicon data were collected at 100 Hz. For discrete comparison of the datasets, the IMU-based knee angle estimates were downsampled to 100 Hz. The RMSE of the knee angle was calculated between the OpenSim-estimated truth data and the proposed method as:
where
N is the number of data points included in the calculation. The absolute RMSE, Equation (
4b), is the traditional calculation of RMSE. Any static offsets between the data being compared would manifest itself in absolute RMSE. However, it was found in the present study that there were static offsets between the OpenSim knee angle and the estimated knee angle from the IMUs. There are two primary sources of this static offset error: (1) human error in marker placement on anatomical landmarks and (2) the thigh and shank IMUs not lying in a plane perfectly parallel to the underlying OpenSim model bone segments. For the former, error in placement of the anterior/posterior superior iliac spine (ASIS/PSIS) markers, knee markers, and ankle markers would set a different angle datum when the rigid body angle between the markers is calculated and could influence the optimized OpenSim inverse kinematics solution. The latter source of error is due to the IMU not lying “flat” on the leg segment. If the thigh IMU is canted, for example, due to the shape of the quadriceps, the rigid body angle datum between the IMUs would be affected and would create a static offset from the assumed biomechanics model. In order to better understand the error of the proposed method without being confounded by the two aforementioned sources of static offset error, we also present zero-mean RMSE in Equation (
4c). This equation is similar to normal RMSE, but the static offset between signals has been removed by subtracting the mean of the residuals from the residual data. This formulation permits a better comparison between the underlying waveforms.
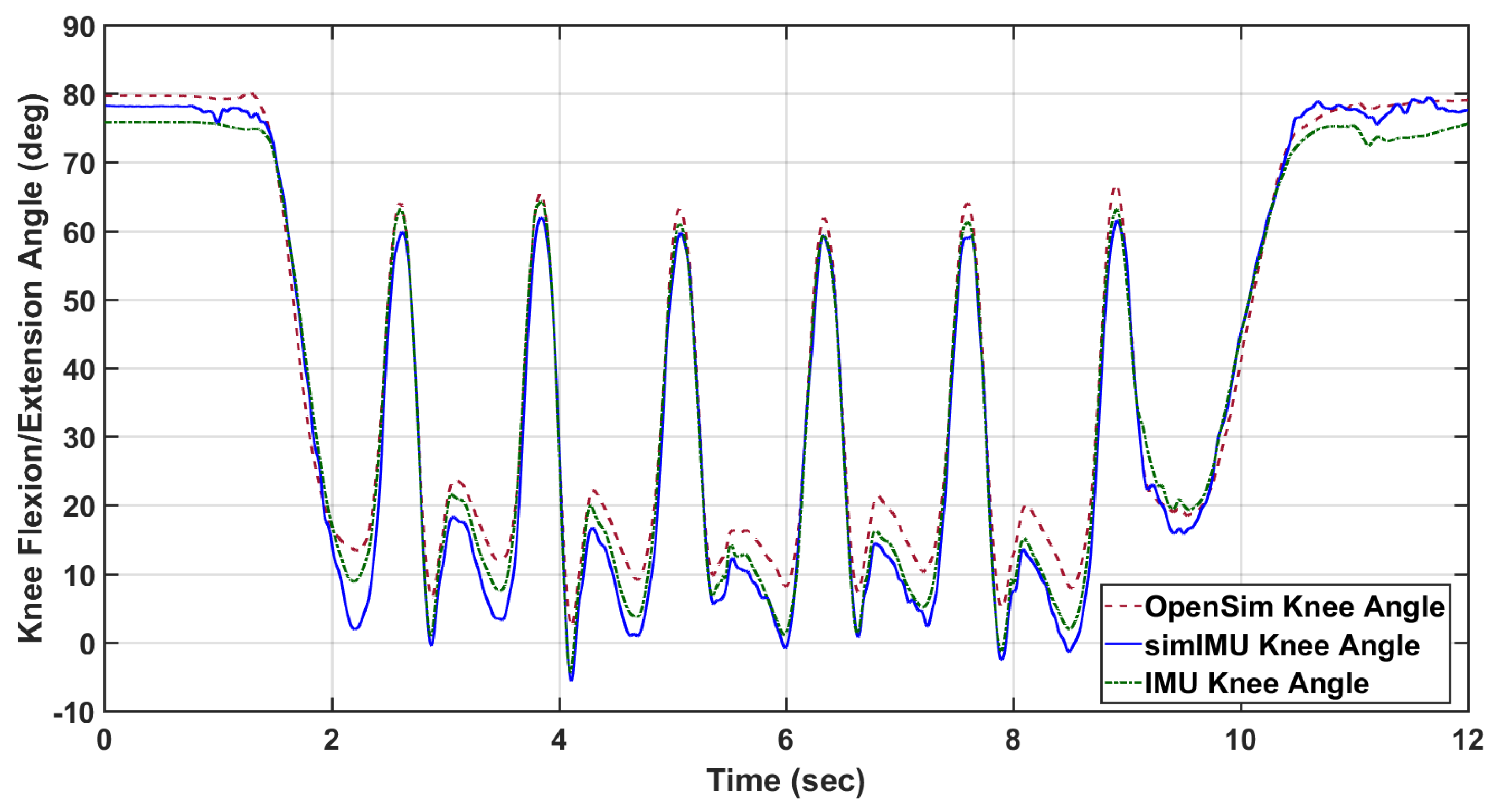
For each trial, time-series measurements were compared: the ground-truth knee flexion/extension angle according to OpenSim inverse kinematics and the proposed method formulated in frame
. An example of these data can be seen in
Figure 2. The IMUs and Vicon system were synchronized in time using an external trigger for subjects 9 and 11–15. For all other subjects, the IMU-estimated knee angle and OpenSim-modeled knee angle were synchronized in time via cross-correlation. For purposes of bounding the beginning and end of the trial for IMU data, the trial was defined to begin when the marker on top of the subject’s head first reached 300 mm/s vertical velocity, indicating that the subject was in the process of standing. Likewise, the trial was defined to end when the subject finally attained 300 mm/s of downward velocity for the same marker, indicating that the subject was in the process of sitting. This parameter was found from empirical tuning to work for all subjects, and included very minimal non-gait motion.
Finally, it is noted that IMU orientation estimate is imperfect, especially in indoor settings. Erroneous orientation estimates of the IMU will induce error into the IMU-estimated knee angle—an error which is not due to the proposed method. In order to control for this error, IMU data was also simulated using the marker triads on the IMUs shown in
Figure 1. As the proposed method is agnostic to the alignment of the coordinate systems to the leg segment, the simulated IMU coordinate system can be constructed from the marker triad in any orientation. The relative orientation between the marker triad coordinate system and the Vicon global frame can then be determined. From the simulated IMU orientation and the Vicon sampling rate, the discrete angular velocity was determined. This orientation and angular velocity was then used in the proposed method just as measured IMU angular velocity and estimated orientation would be. An example of the knee angle from this simulated IMU data with the proposed method is also included in
Figure 2.
3.4. Measures Collected
It was previously hypothesized in
Section 1 hypothesis 2 that IMU location and soft tissue noise may affect the accuracy of the method. This is motivated by the amount of soft tissue artifacts—fat tissue and muscle contraction—of the leg seeming to vary across the lateral-to-anterior surface. Due to the contraction of the quadriceps, we postulate that an IMU on the anterior surface would experience more perturbation relative to the underlying bone structure than an IMU on the lateral surface of the thigh. This relative motion violates the rigid body assumption of the method.
In order to analyze this hypothesis, the circumferential placement of the IMU was defined as the angle between the IMU normal vector and the lateral direction of the leg segment, measured in the transverse plane normal to the body segment (
Figure 3). The transverse plane of the body segment is defined as mutually orthogonal to the sagittal and coronal planes. The coronal plane of the thigh is defined as the plane that contains the medial and lateral knee markers and the hip joint center, calculated by relationships to pelvic anthropometry as suggested by Seidel et al. [
43]. The coronal plane of the shank contains the two knee markers and the centerpoint of the medial and lateral ankle markers. The sagittal planes of both leg segments are defined as the plane whose normal runs through both knee markers. The circumferential angle
of the IMU is then defined as the angle between the lateral-pointing vector of the body segment in the transverse plane, and the IMU normal vector projected into the transverse plane. The circumferential angle for the thigh IMU would be calculated as:
and similarly for the shank IMU, where
represents the projection of the thigh IMU normal vector into the transverse plane. For example, an IMU placed perfectly on the lateral surface of a body segment would be at
, whereas an IMU placed perfectly on the anterior surface of the thigh or shank would be at
. Note that, for the calculation of
in this study, the IMU marker triads were used to define the IMU normal vector, and the knee hinge axis
was determined from the medial and lateral knee markers.
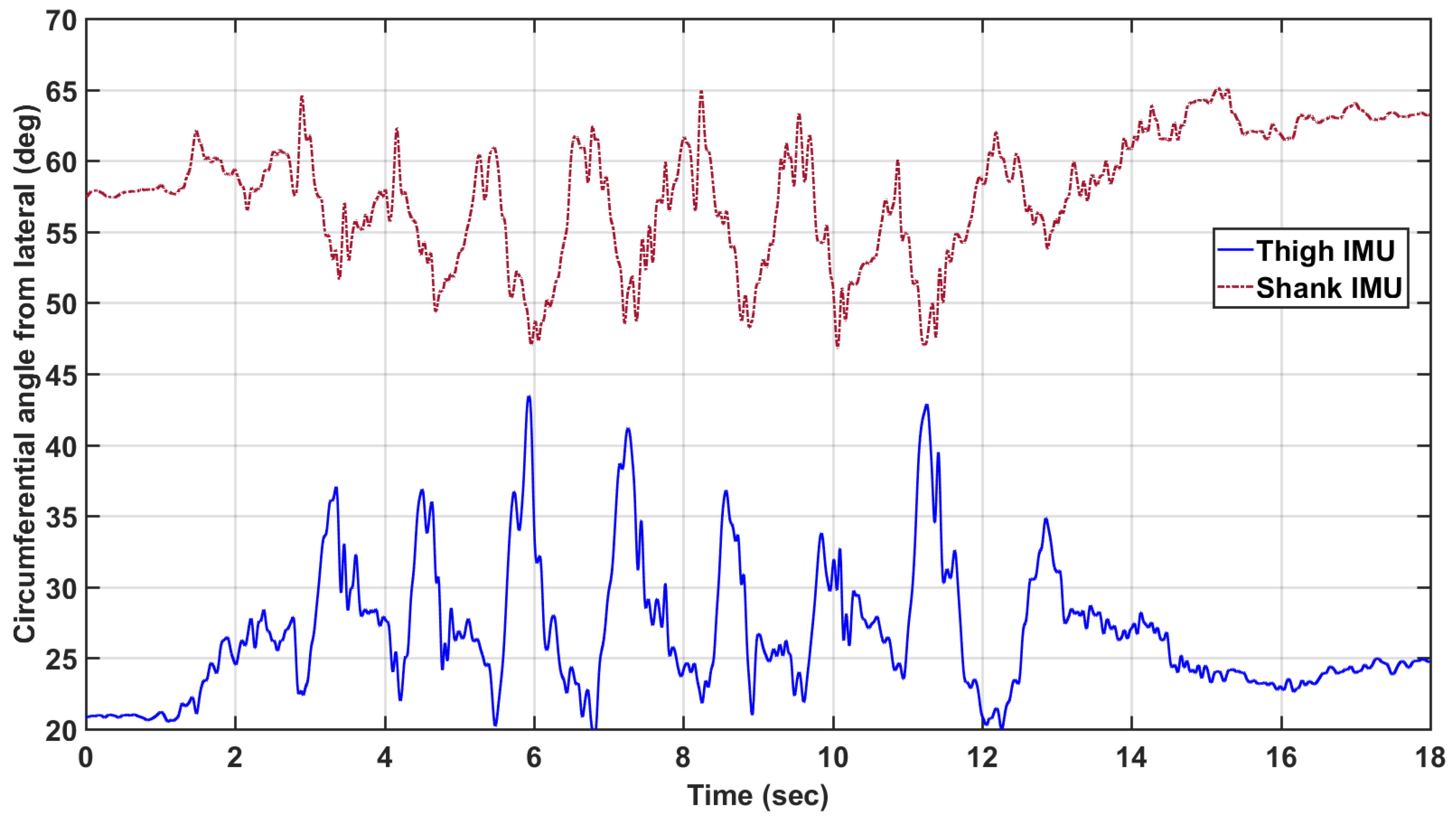
The circumferential angle was computed as a function of time for every trial. It was found that the angle varied significantly over the course of the trial (
Figure 4). The general position of the IMUs on the leg segment were quantified as the mean of the circumferential angle over time, and the standard deviation is used as an analog for the amount of motion relative to the rigid body bone structure that the IMU experienced. The mean and standard deviation of the circumferential angle will be denoted as
and
, respectively, for the thigh. Likewise, the circumferential angle measures for the shank are denoted as
and
.
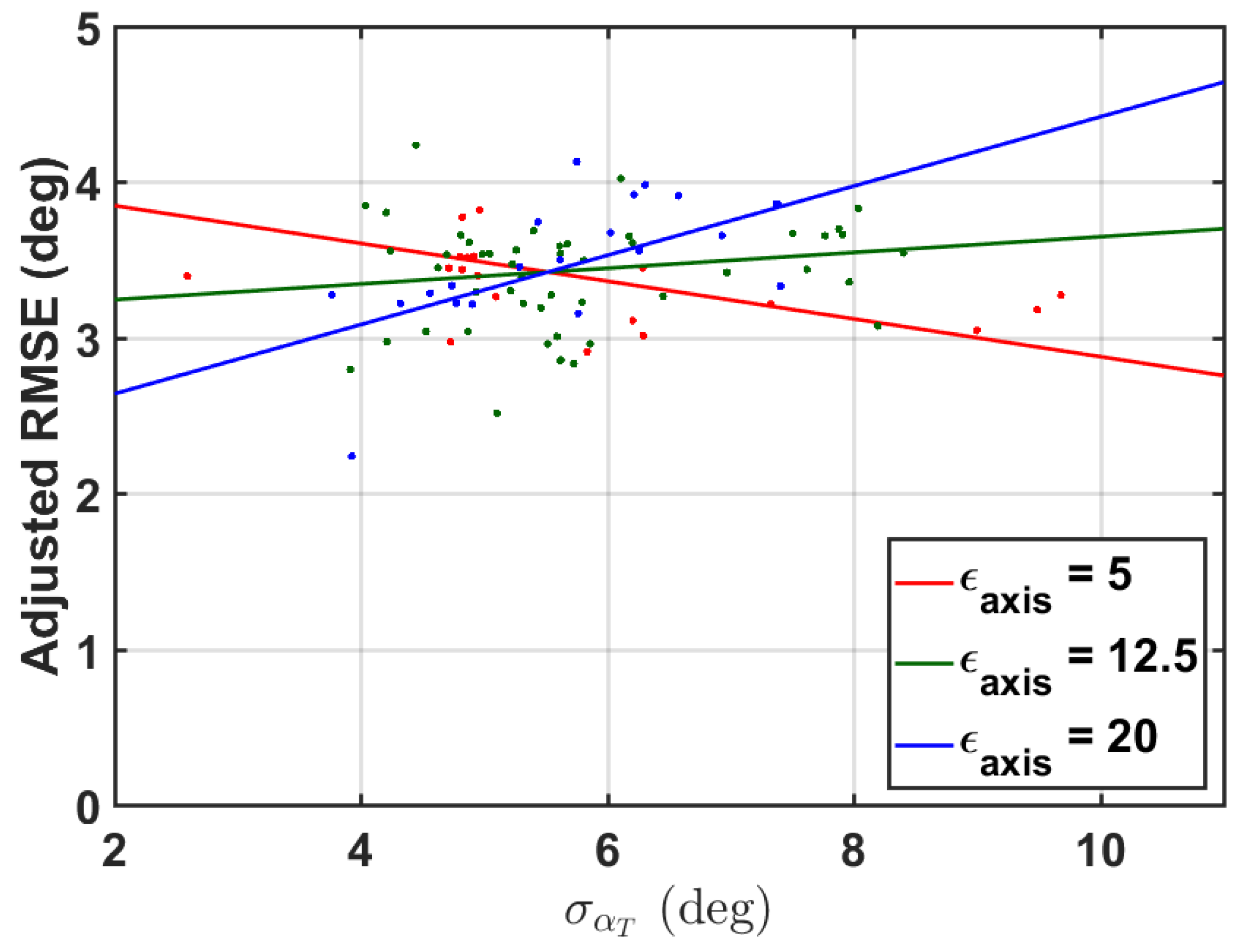
It was further hypothesized that subjects with more fatty tissue would induce more perturbations to the IMU, and thus more error. While measures of subject BMI were not collected in this study, the ratio of a subject’s thigh width-to-length was used as a surrogate measure. For each subject, this measure will be denoted as .
Finally, it was hypothesized that with poor knee axis estimation comes poor knee angle estimation. Within a trial, the simulated IMU-estimated knee axis was compared with the knee axis as defined by the medial and lateral knee markers. The median of this axis estimation error was also used as a predictor for knee angle error, denoted as .
3.5. Statistical Analysis
Data were analyzed for global results via the Bland–Altman method [
44,
45], a statistical method to evaluate the agreement of two multi-sample measurements. Beyond the overall estimation of error RMSE, Bland–Altman analysis can enable inferences about the linearity of the residuals over the measurement domain. Bland–Altman analysis is usually represented in two plots, the first of the two plots will show a linear regression of the simulated IMU-estimated knee angle vs. the OpenSim-modeled motion capture knee angle. These would be ideally linearly correlated with a unity slope. The second plot will show the difference between measurements on the vertical axis and the average of the two measurements on the horizontal axis. These deviations would ideally have zero mean and a low variance.
All of the knee angles according to OpenSim and the proposed method with simulated IMU data were concatenated for error calculation and Bland–Altman analysis to create a composite assessment of all subjects. Measurement error over the entire study will be reported in terms of overall linearity coefficient, static bias of the measurement error with probability of that bias being non-zero, and 95% confidence interval of the error.
A post hoc linear mixed effects model was constructed to investigate factors that contribute to RMSE, here we specifically consider the zero-mean RMSE of the simulated IMU method as this model would highlight the effects of the factors on the PCA methodology. RMSE per trial was modeled as a linear function of the aforementioned fixed-effect factors: circumferential angle mean and standard deviation for the thigh and shank, thigh-width-to-length ratio, and estimated axis error, along with relevant interaction effects. As the trials within a subject are not independent, subject is included as a random effect, modeled as a random intercept. Factors which did not have a nearly-significant main effect or interaction effect were then removed from the model. This reduced model is presented in the results section. When fitting the model, fixed factors were removed from the model if the estimated slopes of the interaction and main effect were not significant at ; however, for interpretation, we consider significance as . These factors were removed to prevent overfitting of the model.