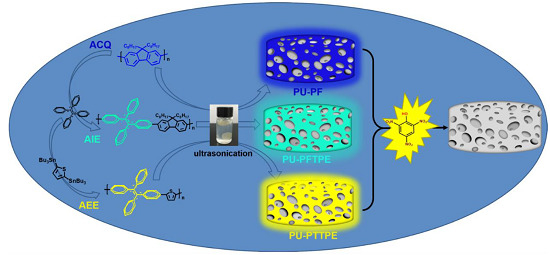
A Comparison of ACQ, AIE and AEE-Based Polymers Loaded on Polyurethane Foams as Sensors for Explosives Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements and Characterization
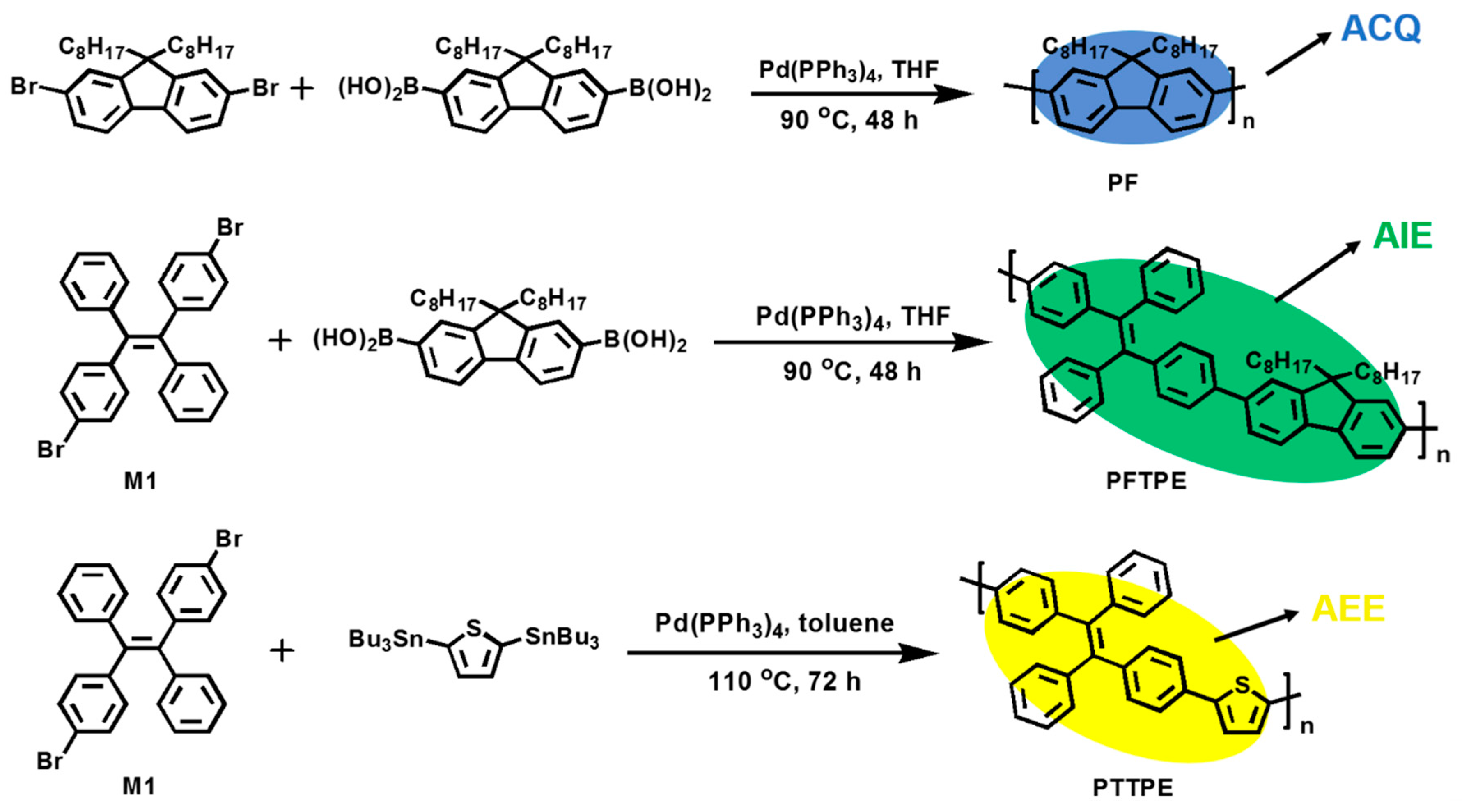
2.3. Synthesis of PF, PFTPE and PTTPE
2.3.1. Synthesis of Poly(2,7-9,9′-dioctyl-9H-fluorene) (PF)
2.3.2. Synthesis of Poly((E)-1,2-diphenyl-1,2-di-p-tolylethene-alt-9,9′-dioctyl-fluorene) (PFTPE)
2.3.3. Synthesis of Poly((E)-1,2-diphenyl-1,2-di-p-tolylethene-alt-2,5-thiophene) (PTTPE)
3. Results and Discussion
3.1. Optical Properties of PF, PFTPE and PTTPE
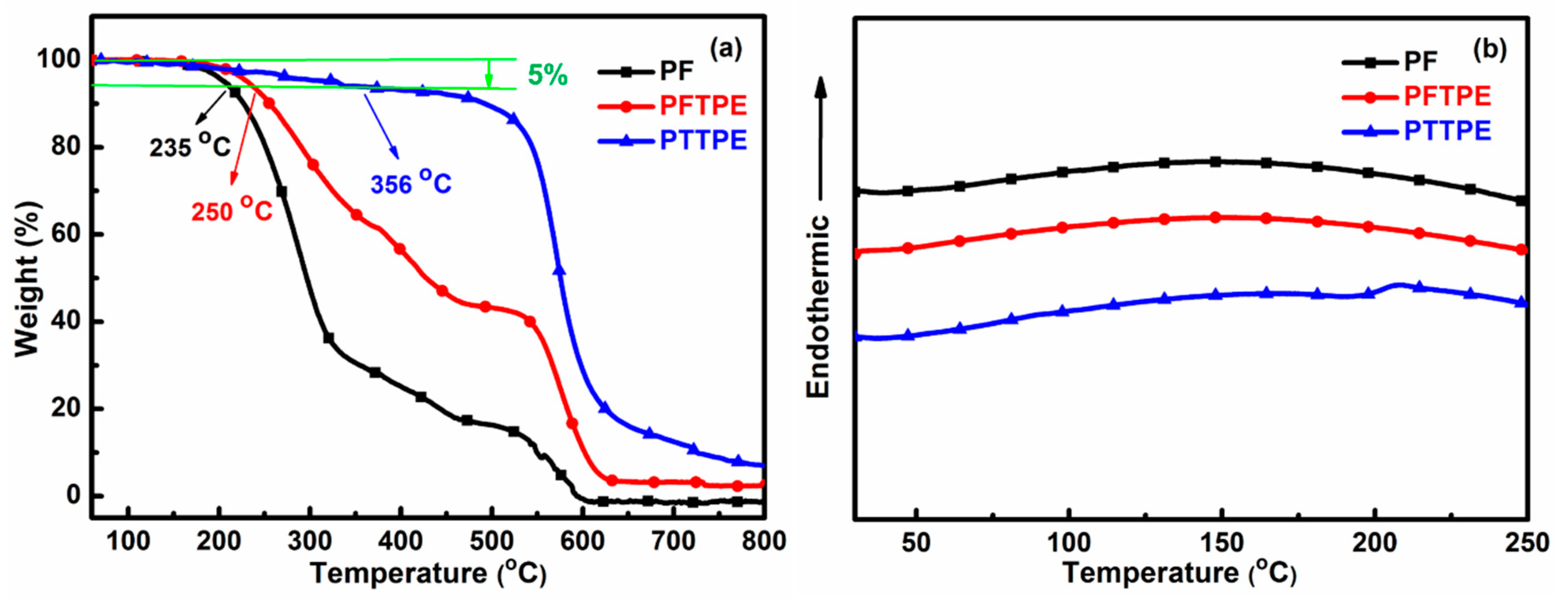
3.2. Thermal Properties of PF, PFTPE and PTTPE
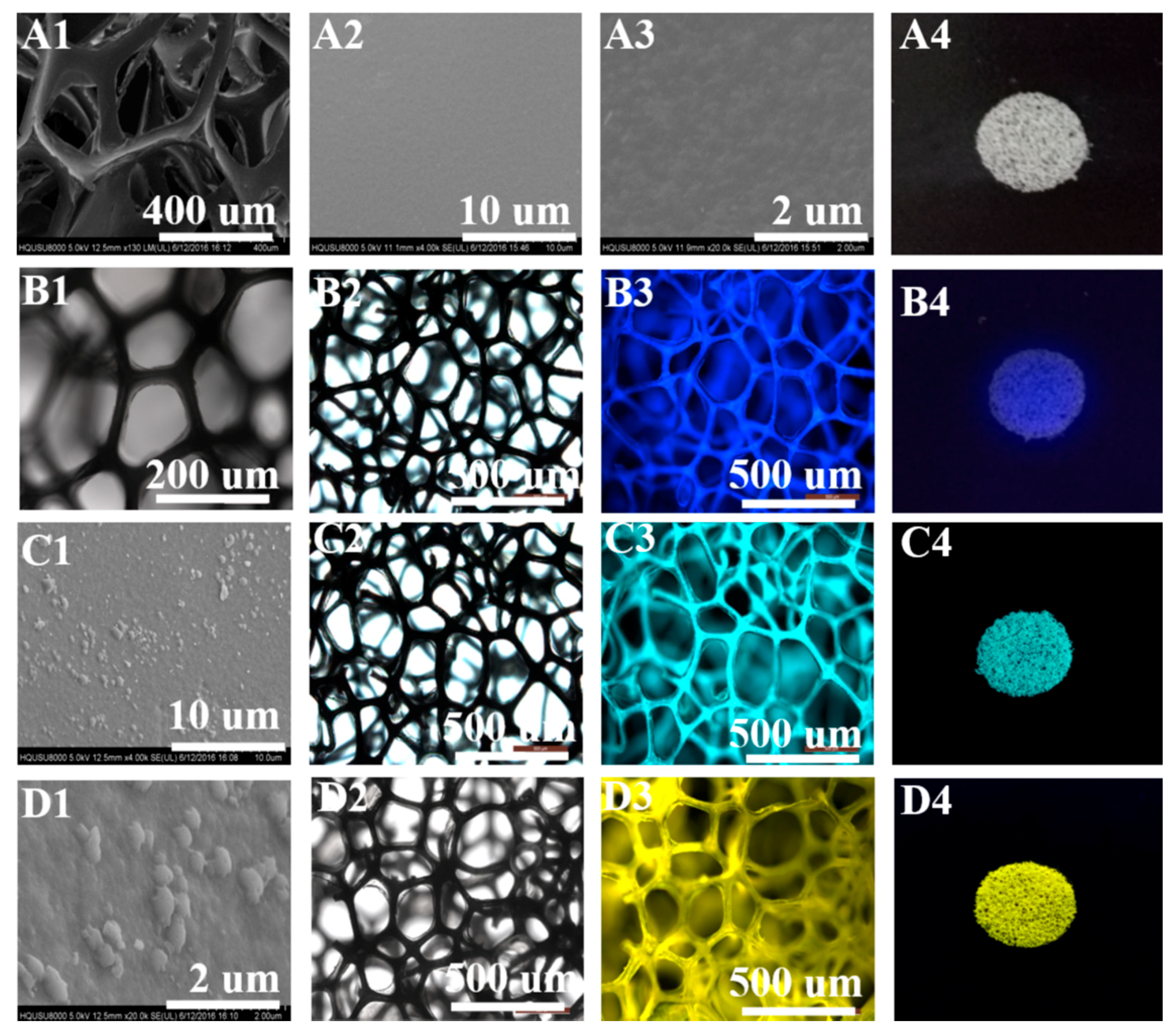
3.3. Preparation of Functional Sensors
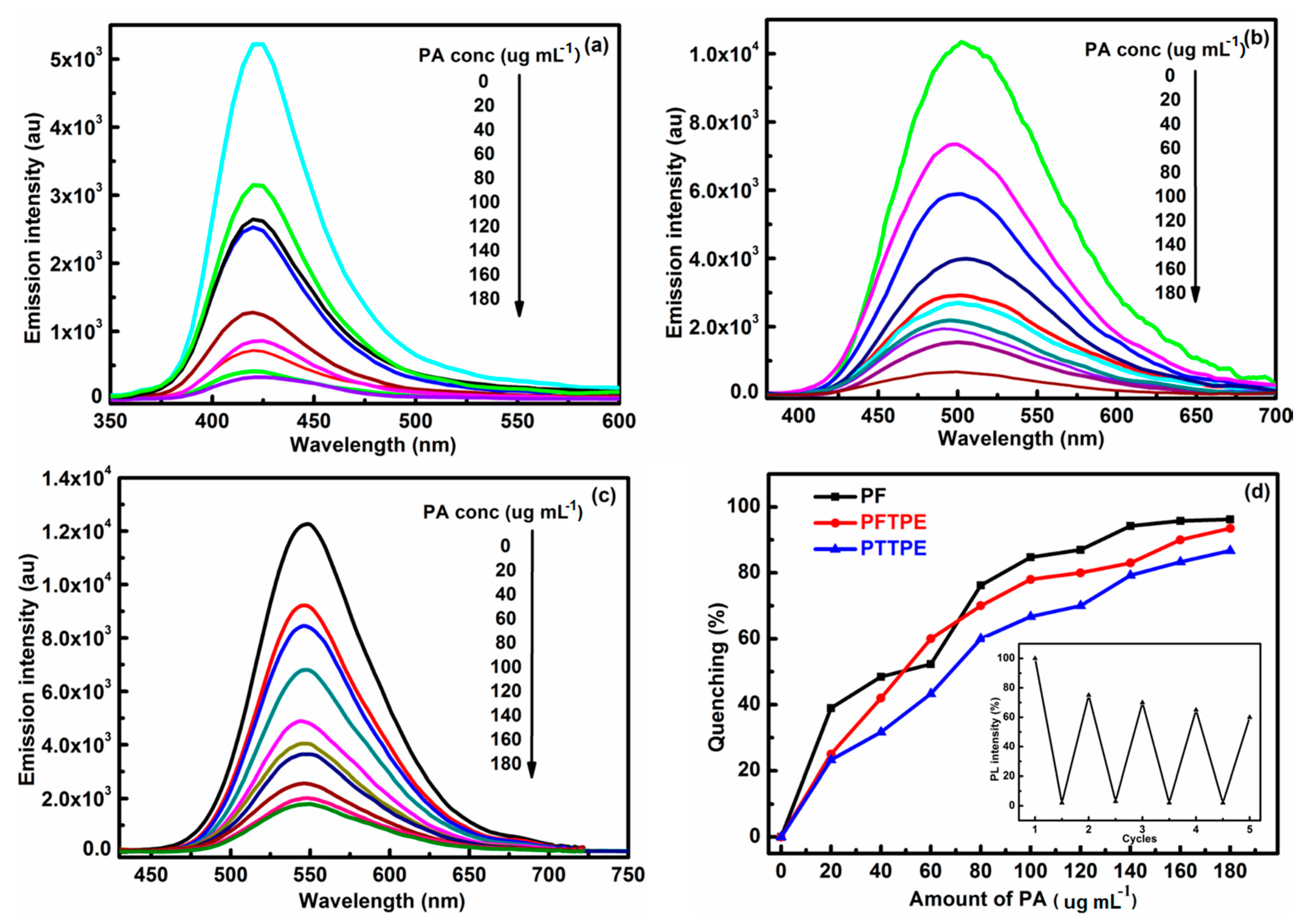
3.4. Comparison of Explosive Detection
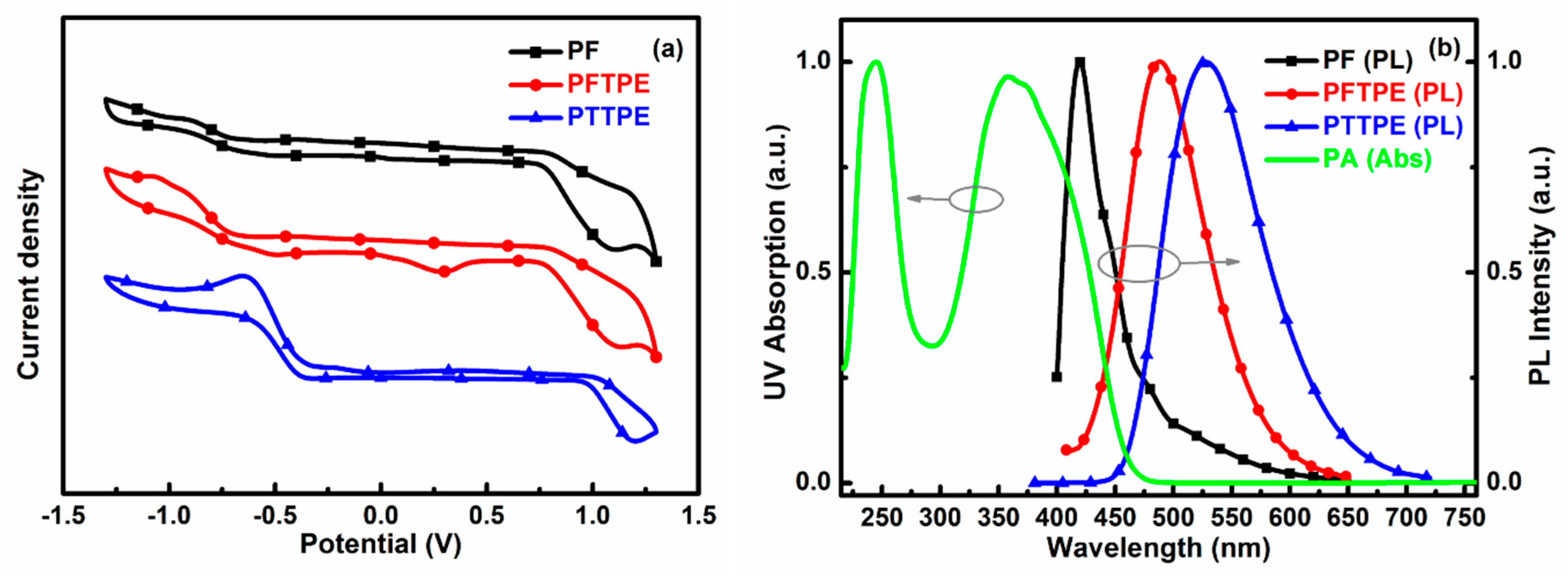
3.5. The Detection Principle of Sensors System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mäkinen, M.; Nousiainen, M.; Sillanpää, M. Ion spectrometric detection technologies for ultra-traces of explosives: A review. Mass Spectrom. Rev. 2011, 30, 940–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shang, J.; Lan, Y.; Tian, T.; Wang, H.; Chen, X.; Gu, J.Y.; Liu, J.Z.; Wan, L.J.; Zhu, W.; et al. Metal-organic polyhedra cages immobilized on a plasmonic substrate for sensitive detection of trace explosives. Adv. Funct. Mater. 2015, 25, 6009–6017. [Google Scholar] [CrossRef]
- Grate, J.W. Hydrogen-bond acidic polymers for chemical vapor sensing. Chem. Rev. 2008, 39, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical sensing of explosives. Electroanalysis 2007, 19, 415–423. [Google Scholar] [CrossRef]
- Popov, I.A.; Chen, H.; Kharybin, O.N.; Nikolaev, E.N.; Cooks, R.G. Detection of explosives on solid surfaces by thermal desorption and ambient ion/molecule reactions. Chem. Commun. 2005, 15, 1953–1955. [Google Scholar]
- Xu, B.; Xu, Y.; Wang, X.; Li, H.; Wu, X.; Tong, H.; Wang, L. Porous films based on a conjugated polymer gelator for fluorescent detection of explosive vapors. Polym. Chem. 2013, 4, 5056–5059. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Li, X.; Wang, N.; Wang, Z.Y.; Ma, J.; Bock, W.J.; Ma, D. Fiber-optic detection of explosives using readily available fluorescent polymers. Macromolecules 2009, 42, 921–926. [Google Scholar] [CrossRef]
- Nie, H.; Sun, G.; Zhang, M.; Baumgarten, M.; Müllen, K. Fluorescent conjugated polycarbazoles for explosives detection: Side chain effects on TNT sensor sensitivity. J. Mater. Chem. 2012, 22, 2129–2132. [Google Scholar] [CrossRef]
- Nie, H.; Zhao, Y.; Zhang, M.; Ma, Y.; Baumgarten, M.; Müllen, K. Detection of TNT explosives with a new fluorescent conjugated polycarbazole polymer. Chem. Commun. 2011, 47, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Swager, T.M. Porous shape persistent fluorescent polymer films: An approach to TNT sensory materials. J. Am. Chem. Soc. 1998, 120, 5321–5322. [Google Scholar] [CrossRef]
- Yang, J.S.; Swager, T.M. Fluorescent porous polymer films as TNT chemosensors: Electronic and structural effects. J. Am. Chem. Soc. 1998, 120, 11864–11873. [Google Scholar] [CrossRef]
- Li, Z.A.; Lou, X.; Yu, H.; Li, Z.; Qin, J. An imidazole-functionalized polyfluorene derivative as sensitive fluorescent probe for metal ions and cyanide. Macromolecules 2008, 41, 7433–7439. [Google Scholar] [CrossRef]
- Li, Z.A.; Lou, X.; Li, Z.; Qin, J. A new approach to fluorescence “Turn-On” sensing of α-amino acids. ACS Appl. Mater. Interfaces 2009, 1, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, W.; Fan, Z.; Li, J.; Chen, X.; Luo, G.; Zhang, X. Synthesis and characterization of high triplet energy polyfluorene bearing m-tetraphenylsilane segment as a polymer host for green phosphorescent polymer light emitting diodes. Synth. Met. 2015, 204, 70–75. [Google Scholar] [CrossRef]
- Lim, S.F.; Friend, R.H.; Rees, I.D.; Li, J.; Ma, Y.; Robinson, K.; Holmes, A.B.; Hennebicq, E.; Beljonne, D.; Cacialli, F. Suppression of Green Emission in a New Class of Blue-Emitting Polyfluorene Copolymers with Twisted Biphenyl Moieties. Adv. Funct. Mater. 2005, 15, 981–988. [Google Scholar] [CrossRef]
- Lee, Y.T.; Chiang, C.L.; Chen, C.T. Solid-state highly fluorescent diphenylaminospirobifluorenyl- fumaronitrile red emitters for non-doped organic light-emitting diodes. Chem. Commun. 2007, 2, 217–219. [Google Scholar]
- Hu, Y.; Liu, J.; You, X.; Wang, C.; Li, Z.; Xie, W. A light-up probe for detection of adenosine in urine samples by a combination of an AIE molecule and an aptamer. Sensors 2017, 17, 2246. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, A.; Tang, B.Z. Polymerizations based on triple-bond building blocks. Prog. Polym. Sci. 2017, 78, 92–138. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Wu, W.; Ye, S.; Tang, R.; Huang, L.; Li, Q.; Yu, G.; Liu, Y.; Qin, J.; Li, Z. New tetraphenylethylene-containing conjugated polymers: Facile synthesis, aggregation-induced emission enhanced characteristics and application as explosive chemosensors and PLEDs. Polymer 2012, 53, 3163–3171. [Google Scholar] [CrossRef]
- Jana, D.; Ghorai, B.K. Synthesis and aggregation-induced emission properties of tetraphenyleth-ylene-based oligomers containing triphenylethylene moiety. Tetrahedron Lett. 2012, 53, 6838–6842. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Lin, T.T.; Wu, J.; Xu, J.W. Aggregation-induced emission active 3,6-bis(1,2,2-triphen-ylvinyl)carbazole and bis(4-(1,2,2-triphenylvinyl)phenyl)amine-based poly(acrylates) for explosive detection. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 672–681. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Yao, B.; Wang, J.; Mei, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. A polytriazole synthesized by 1,3-dipolar polycycloaddition showing aggregation-enhanced emission and utility in explosive detection. Macromol. Rapid Commun. 2013, 34, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, R.; Tang, X.; Shi, Y.; Li, C.; Wang, Y. One-pot click access to a cyclodextrin dimer-based novel aggregation induced emission sensor and monomer-based chiral stationary phase. Sensors 2016, 16, 1985. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Bai, Q.; Shan, T.; Lu, P.; Ma, Y. Electropolymerized AIE-active polymer film with high quantum efficiency and its application in OLED. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 707–715. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Neo, W.T.; Song, J.; Yan, H.; Zong, Y.; Tang, B.Z.; Hor, T.A.; Xu, J. Electrospun aggregation-induced emission active POSS-based porous copolymer films for detection of explosives. Chem. Commun. 2014, 50, 13785–13788. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.; Tang, B.Z. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiang, T.; Guo, Y.; Ding, L.; He, B.; Chang, Z.; Lam, J.W.; Liu, J.; Chan, C.Y.; Lu, P.; et al. Silole-containing poly(silylenevinylene)s: Synthesis, characte-rization, aggregation enhanced emission, and explosive detection. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2265–2274. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q. Mussel-inspired direct immobilization of nanoparticles and application for oil-water separation. ACS NANO 2014, 8, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 77908–77912. [Google Scholar] [CrossRef]
- Fan, Z.X.; Zhao, Q.H.; Wang, S.; Bai, Y.; Wang, P.P.; Li, J.J.; Chu, Z.W.; Chen, G.H. Polyurethane foam functionalized with an AIE-active polymer using an ultrasonication-assisted method: Preparation and application for the detection of explosives. RSC Adv. 2016, 6, 26950–26953. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Jim, C.K.; Tang, Y.; Lam, J.W.; Liu, J.; Mahtab, F.; Gao, P.; Tang, B.Z. Aggregation-enhanced emissions of intramolecular excimers in disubstituted polyacetylenes. J. Phys. Chem. B 2008, 112, 9281–9288. [Google Scholar] [CrossRef] [PubMed]
- Doktycz, S.J.; Suslick, K.S. Interparticle collisions driven by ultrasound. Science 1990, 247, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, B.; Gu, Y.; Xiong, Z.; Sun, J.; Zhao, X.S. Graphene-based electrodes for electrochemical energy storage. Energy Environ. Sci. 2013, 6, 1388–1414. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.; Wong, J.S.; Hu, M.; Li, R.K. Controllable morphology and wettability of polymer microspheres prepared by nonsolvent assisted electrospraying. Polymer 2014, 55, 2913–2920. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.; Shi, H.; Hu, M.; Li, R.K. Preparation, morphology, and mechanical properties of carbon nanotube anchored polymer nanofiber composite. Compos. Sci. Technol. 2014, 92, 95–102. [Google Scholar] [CrossRef]
- Zha, J.W.; Gao, Y.; Zhang, D.L.; Wen, Y.; Li, R.K.; Shi, C.Y.; Dang, Z.M. Flexible electrospun polyvinylidene fluoride nanofibrous composites with high electrical conductivity and good mechanical properties by employing ultrasonication induced dispersion of multiwalled carbon nanotubes. Compos. Sci. Technol. 2016, 128, 201–206. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, L.; Bogale, R.F.; Gao, Y.; Wang, X.; Qian, X.; Guo, S.; Zhao, J.; Ning, G. Highly selective detection of 2,4,6-trinitrophenol and Cu2+ ions based on a fluorescent cadmium-pamoate metal-organic framework. Chemistry 2015, 21, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Wollin, K.; Dieter, H.H. Toxicological guidelines for monocyclic nitro-, amino- and aminonitroa-romatics, nitramines, and nitrate esters in drinking water. Arch. Environ. Contam. Toxicol. 2005, 49, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, M.; Liu, X.Q.; Sun, L.B.; Jiang, H.L. Rational synthesis of an exceptionally stable Zn(II) metal-organic framework for the highly selective and sensitive detection of picric acid. Chem. Commun. 2016, 52, 5734–5737. [Google Scholar] [CrossRef] [PubMed]
- Rd, T.S.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar]
- Lin, R.B.; Li, F.; Liu, S.Y.; Qi, X.L.; Zhang, J.P.; Chen, X.M. A noble-metal-free porous coordination framework with exceptional sensing efficiency for oxygen. Angew. Chem. Int. Ed. 2013, 52, 13429–13433. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.J.; Chen, N.J.; Li, Q.Y.; Wu, X.J.; Huang, X.L.; Lin, Z.H.; Zhao, Y.G. Highly fluorescent metal-organic Frameworks Based on benzene -cored tetraphenylethene derivative with the ability to detection of 2,4,6-trinitrophenol in water. Cryst. Growth Des. 2017, 17, 3170–3177. [Google Scholar] [CrossRef]
- Mostakim, S.K.; Biswas, S. A thiadiazole-functionalized Zr(IV)-based metal-organic framework as a highly fluorescent probe for the selective detection of picric acid. CrystEngComm 2016, 18, 3104–3113. [Google Scholar]

| Substance | HOMO (eV) 2 | LUMO (eV) 3 | Band Gap (eV) 4 |
|---|---|---|---|
| PA 1 | −8.29 | −3.87 | 4.42 |
| PF | −5.49 | −2.54 | 2.95 |
| PFTPE | −5.52 | −2.59 | 2.93 |
| PTTPE | −5.71 | −3.14 | 2.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Fan, Z.; Zhang, X.; Tan, X.; Li, D.; Chen, G.; Zhao, Q. A Comparison of ACQ, AIE and AEE-Based Polymers Loaded on Polyurethane Foams as Sensors for Explosives Detection. Sensors 2018, 18, 1565. https://doi.org/10.3390/s18051565
Chu Z, Fan Z, Zhang X, Tan X, Li D, Chen G, Zhao Q. A Comparison of ACQ, AIE and AEE-Based Polymers Loaded on Polyurethane Foams as Sensors for Explosives Detection. Sensors. 2018; 18(5):1565. https://doi.org/10.3390/s18051565
Chicago/Turabian StyleChu, Zhiwei, Zhuxin Fan, Xiang Zhang, Xiaofeng Tan, Dongxu Li, Guohua Chen, and Qinghua Zhao. 2018. "A Comparison of ACQ, AIE and AEE-Based Polymers Loaded on Polyurethane Foams as Sensors for Explosives Detection" Sensors 18, no. 5: 1565. https://doi.org/10.3390/s18051565