Rapid Iodine Sensing on Mechanically Treated Carbon Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Mechanically-Treated Carbon Nanofiber (MCNF) Electrodes
2.3. Electrochemical Characterization
3. Results/Discussion
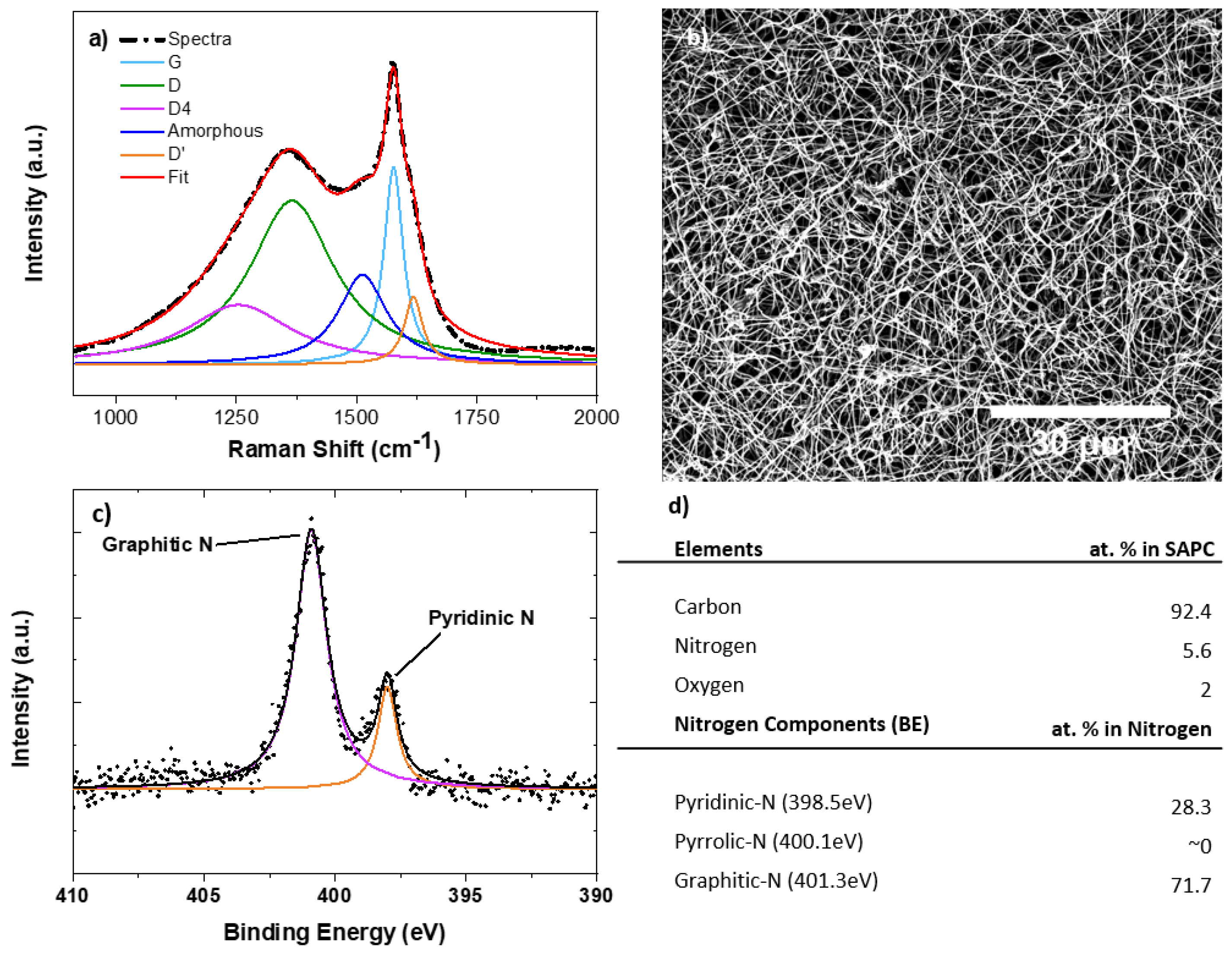
3.1. Material Characterization
3.2. Electrochemical Characterization
3.2.1. Cyclic Voltammetry
3.2.2. Differential Pulse Voltammetry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delange, F. The Disorders Induced by Iodine Deficiency. Thyroid 1994, 4, 107–128. [Google Scholar] [CrossRef] [PubMed]
- De Benoist, B.; McLean, E.; Andersson, M.; Rogers, L. Iodine deficiency in 2007: Global progress since 2003. Food Nutr. Bull. 2008, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Urinary Iodine Concentrations for Determining Iodine Status in Populations. 2007. Available online: http://whqlibdoc.who.int/publications/2007/978924 1595827eng.pdf (accessed on 20 August 2013).
- Shelor, C.P.; Dasgupta, P.K. Review of analytical methods for the quantification of iodine in complex matrices. Anal. Chim. Acta 2011, 702, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Haap, M.; Roth, H.J.; Huber, T.; Dittmann, H.; Wahl, R. Urinary iodine: Comparison of a simple method for its determination in microplates with measurement by inductively-coupled plasma mass spectrometry. Sci. Rep. 2017, 7, 39835. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y. A Novel Sensor for Sensitive and Selective Detection of Iodide Using Turn-on Fluorescence Graphene Quantum Dots/Ag Nanocomposite. Anal. Sci. 2015, 31, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, Z.H.; Khun, K.; Willander, M. A Selective Iodide Ion Sensor Electrode Based on Functionalized ZnO Nanotubes. Sensors 2013, 13, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-H.; Wang, K.-M.; Xiao, D.; Yang, X.-H. Development of an iodine sensor based on fluorescence energy transfer. Analyst 2000, 125, 1441–1445. [Google Scholar] [CrossRef]
- Chen, T.-W.; Tsai, T.-H.; Chen, S.-M.; Lin, K.-C. Using PEDOT Film Modified Electrode to Monitor Iodide and Its Enhancement of Arsenite Sensing. Int. J. Electrochem. Sci. 2011, 6, 15. [Google Scholar]
- Toh, H.S.; Tschulik, K.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical quantification of iodide ions in synthetic urine using silver nanoparticles: A proof-of-concept. Analyst 2014, 139, 3986–3990. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.R.I.; Rubino, A.; Laviola, M.C.; Ciriello, R. Comparison of silver, gold and modified platinum electrodes for the electrochemical detection of iodide in urine samples following ion chromatography. J. Chromatogr. B 2005, 827, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Dielacher, B.; Tiefenauer, R.F.; Junesch, J.; Vörös, J. Iodide sensing via electrochemical etching of ultrathin gold films. Nanotechnology 2015, 26, 025202. [Google Scholar] [CrossRef] [PubMed]
- Lowe Eleanor, R.; Banks Craig, E.; Compton Richard, G. Edge Plane Pyrolytic Graphite Electrodes for Halide Detection in Aqueous Solutions. Electroanalysis 2005, 17, 1627–1634. [Google Scholar] [CrossRef]
- Ghazinejad, M.; Holmberg, S.; Pilloni, O.; Oropeza-Ramos, L.; Madou, M. Graphitizing Non-graphitizable Carbons by Stress-induced Routes. Sci. Rep. 2017, 7, 16551. [Google Scholar] [CrossRef] [PubMed]
- Pollack, B.; Holmberg, S.; George, D.; Tran, I.; Madou, M.; Ghazinejad, M. Nitrogen-Rich Polyacrylonitrile-Based Graphitic Carbons for Hydrogen Peroxide Sensing. Sensors 2017, 17, 2407. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Nagaiah, T.C.; Xia, W.; Wang, Y.; Dommele, S.V.; Bitter, J.H.; Santa, M.; Grundmeier, G.; Bron, M.; Schuhmann, W.; et al. Electrocatalytic Activity and Stability of Nitrogen-Containing Carbon Nanotubes in the Oxygen Reduction Reaction. J. Phys. Chem. C 2009, 113, 14302–14310. [Google Scholar] [CrossRef]
- Li, S.-M.; Yang, S.-Y.; Wang, Y.-S.; Lien, C.-H.; Tien, H.-W.; Hsiao, S.-T.; Liao, W.-H.; Tsai, H.-P.; Chang, C.-L.; Ma, C.-C.; et al. Controllable synthesis of nitrogen-doped graphene and its effect on the simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Carbon 2013, 59, 418–429. [Google Scholar] [CrossRef]
- Lopez-Giacoman, S.; Madero, M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol. 2015, 4, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative analysis of urea in human urine and serum by 1H nuclear magnetic resonance. Analyst 2012, 137, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.; Wilder, L.; Caudill, S.; Gonzalez, A.; Needham, L.; Pirkle, J. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]



| Component | Concentration (g/L) |
|---|---|
| Urea | 25 |
| Sodium Chloride | 9 |
| Disodium Hydrogen Orthophosphate, anhydrous | 2.5 |
| Potassium Dihydrogen Orthophosphate | 2.5 |
| Ammonium Chloride | 3 |
| Creatinine | 2 |
| Sodium Sulphite, hydrated | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Perebikovsky, A.; Benice, O.; Holmberg, S.; Madou, M.; Ghazinejad, M. Rapid Iodine Sensing on Mechanically Treated Carbon Nanofibers. Sensors 2018, 18, 1486. https://doi.org/10.3390/s18051486
Cho E, Perebikovsky A, Benice O, Holmberg S, Madou M, Ghazinejad M. Rapid Iodine Sensing on Mechanically Treated Carbon Nanofibers. Sensors. 2018; 18(5):1486. https://doi.org/10.3390/s18051486
Chicago/Turabian StyleCho, Eunbyul, Alexandra Perebikovsky, Olivia Benice, Sunshine Holmberg, Marc Madou, and Maziar Ghazinejad. 2018. "Rapid Iodine Sensing on Mechanically Treated Carbon Nanofibers" Sensors 18, no. 5: 1486. https://doi.org/10.3390/s18051486




