Research on a Visual Electronic Nose System Based on Spatial Heterodyne Spectrometer
Abstract
:1. Introduction
2. Visual Gas Sensing Mechanism Based on SHS
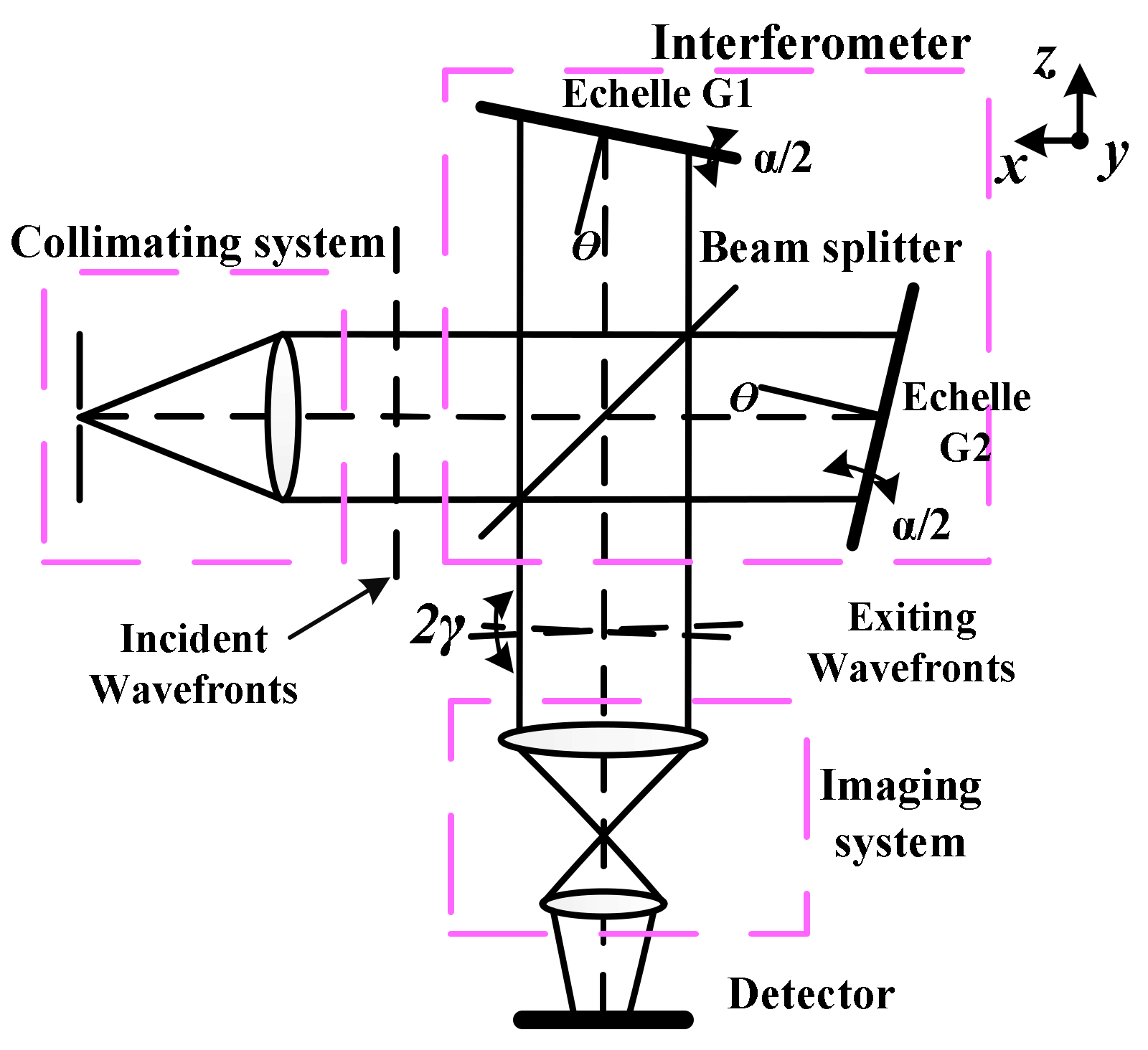
2.1. Wide Spectral Spatial Heterodyne Spectrometer
2.1.1. Wide Spectral Spatial Heterodyne Spectrometer
2.1.2. Basic Properties of the WS-SHS System
2.2. Sensing Mechanism of the Visual E-Nose
3. Visual E-Nose System Based on SHS
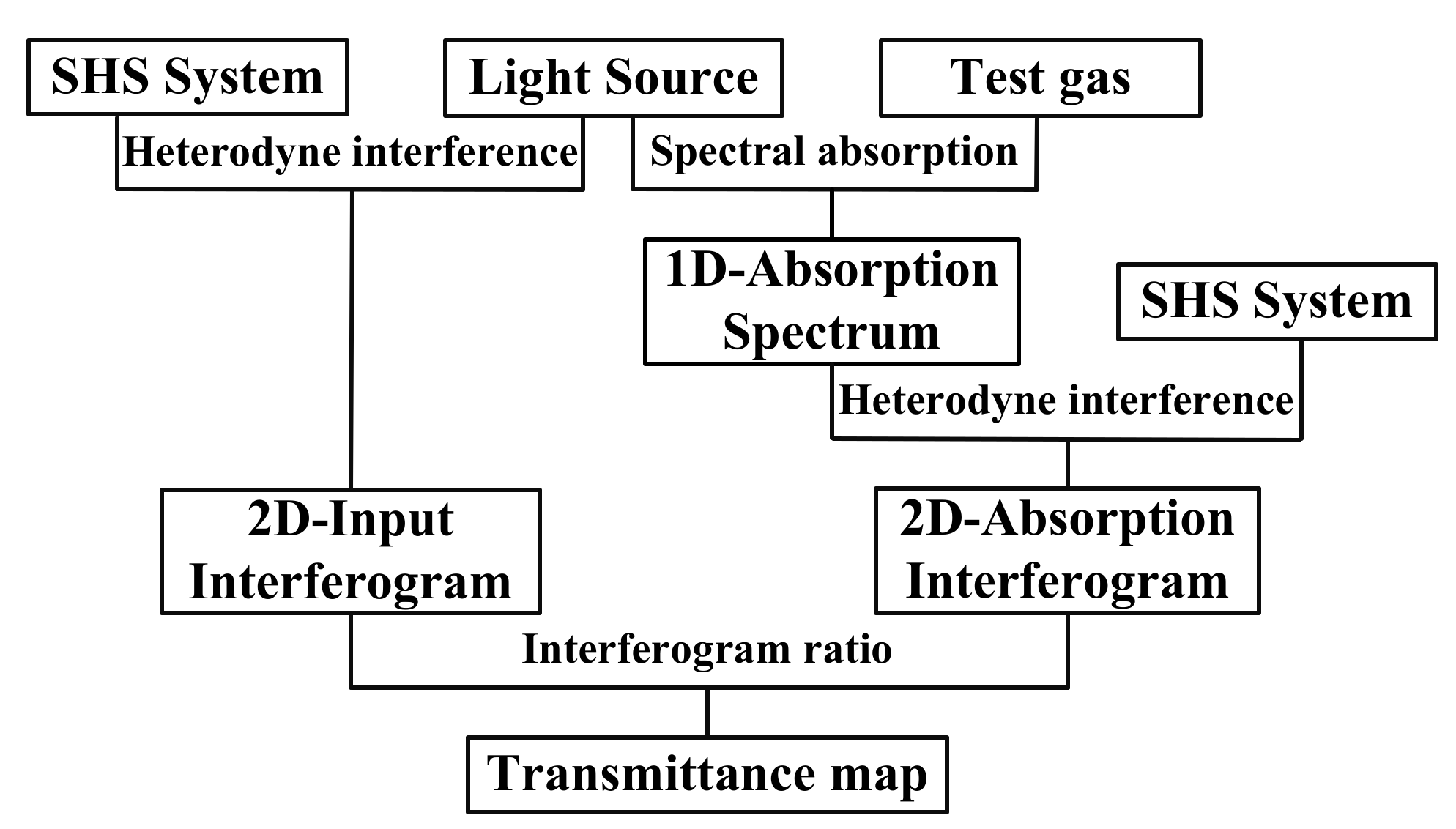
3.1. Construction of Visual E-Nose System
3.1.1. System Structure
3.1.2. Flowchart of the Visual E-Nose
3.2. Experiment
3.2.1. Experimental Steps
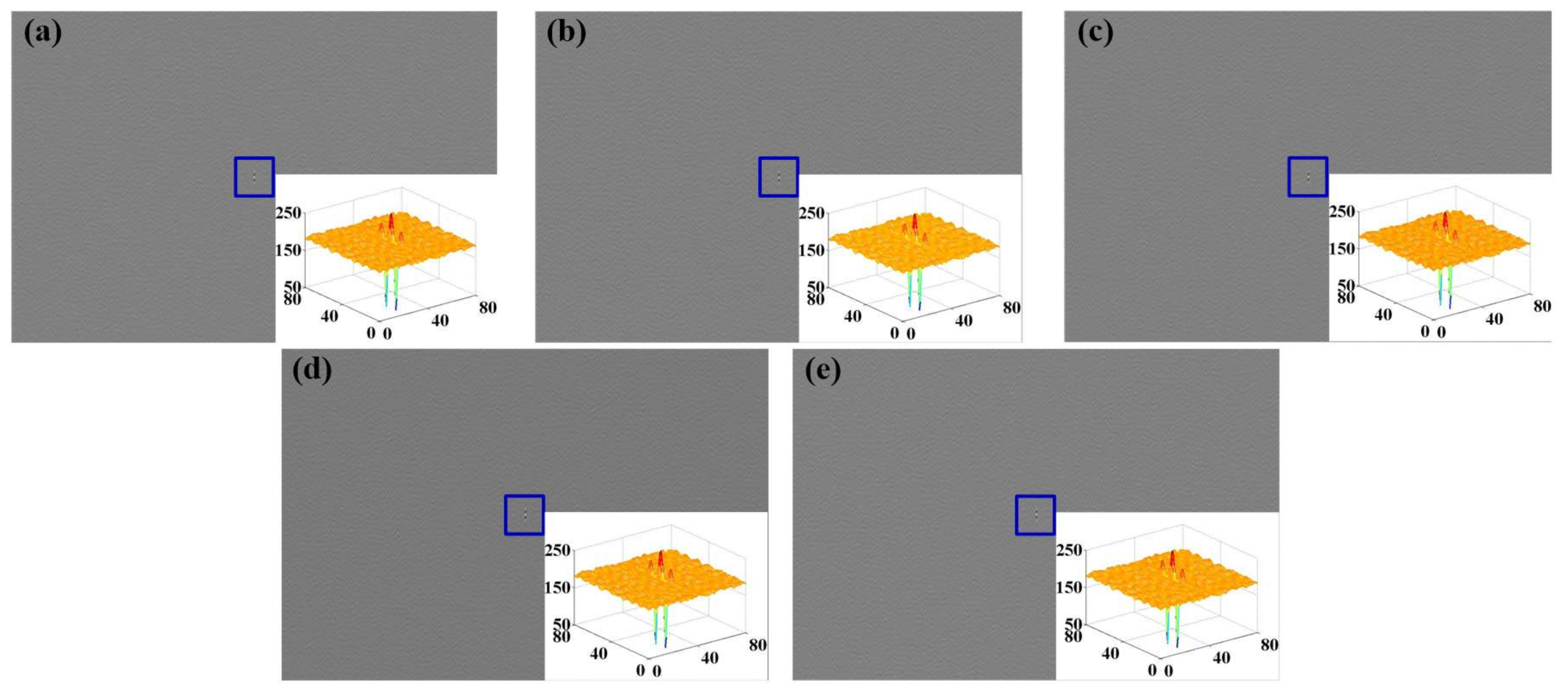
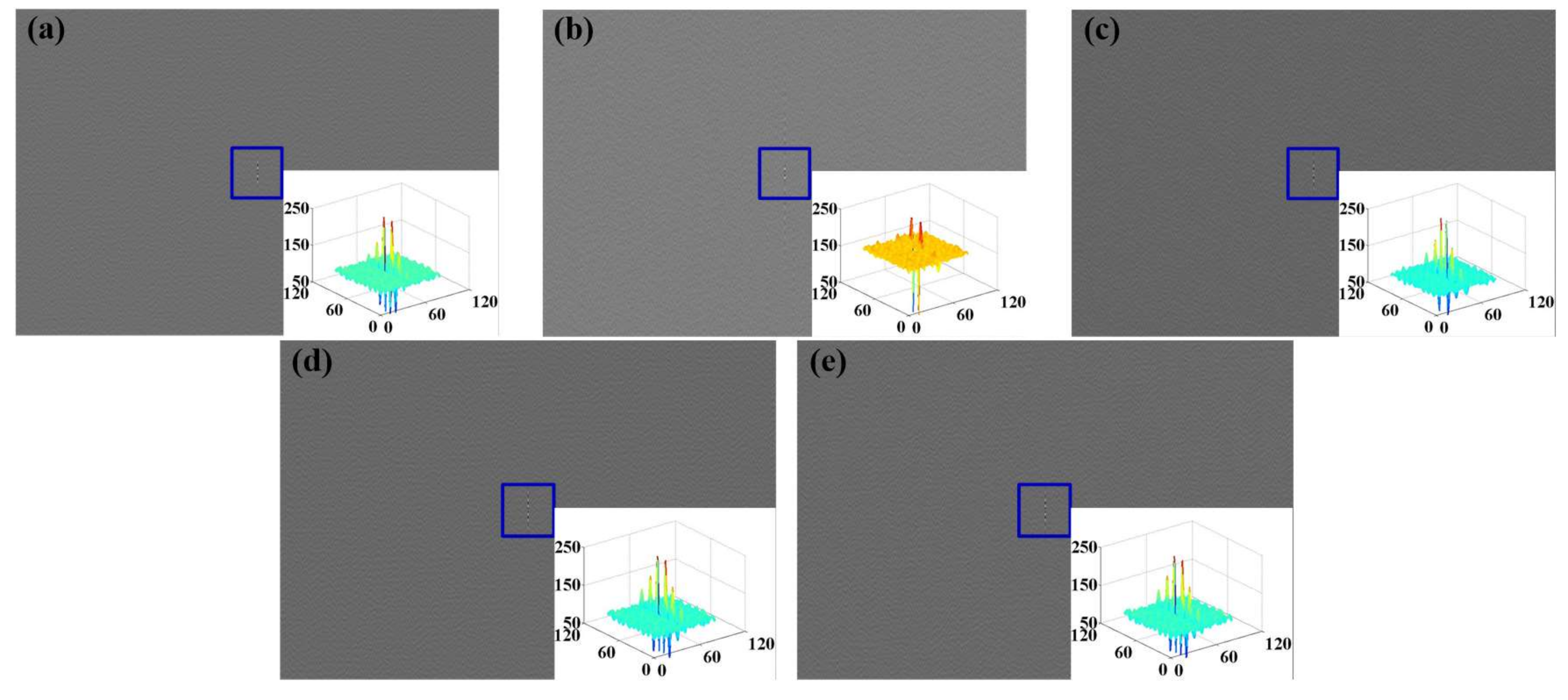
3.2.2. Acquisition and Analysis of Sensing Data
4. Analysis of the Visual E-Nose Sensing Data
4.1. Feature Extraction of Experiment Sensing Data
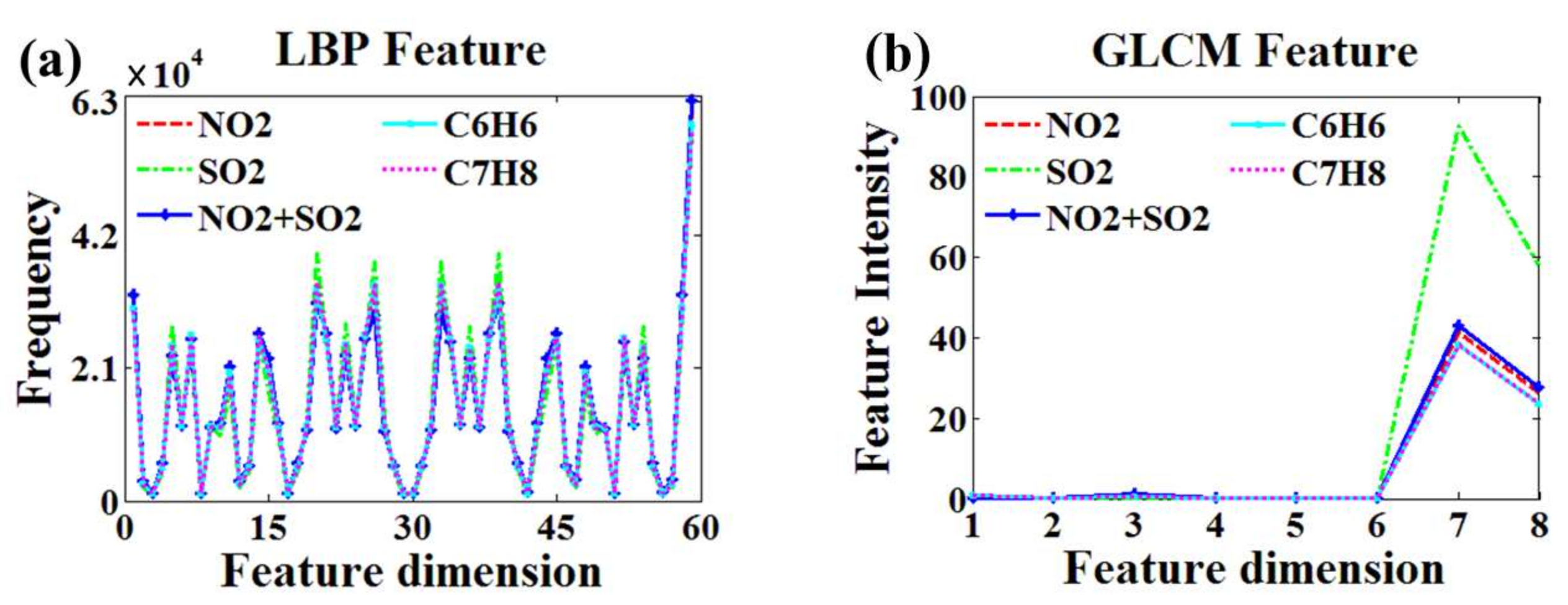
4.1.1. Feature Extraction
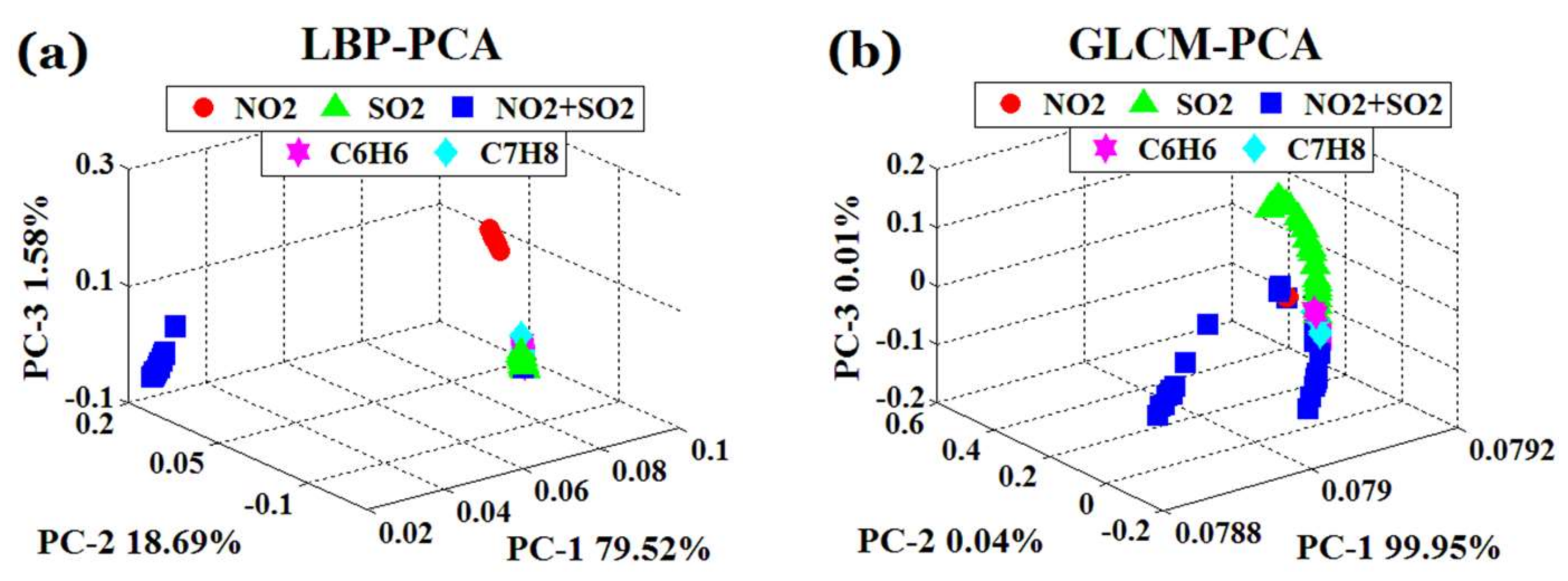
4.1.2. PCA Analysis
4.2. Type Recognition of the Experiment Sensing Data
4.2.1. Classifiers and Experimental Data
4.2.2. Recognition and Analysis of Gas Type
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef] [PubMed]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Wojnowskia, W.; Majchrzaka, T.; Dymerskia, T.; Gębickib, J.; Namieśnika, J. Electronic noses: Powerful tools in meat quality assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.M.; Agudo, J.E.; Manso, A.G.; Orellana, C.J.G.; Velasco, H.M.G.; Caballero, R.G. A compact and low cost electronic nose for aroma detection. Sensors 2013, 13, 5528–5541. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Macagnano, A.; Davide, F.; Amico, A.D.; Paolesse, R.; Boschi, T.; Faccio, M.; Ferri, G. An electronic nose for food analysis. Sens. Actuators B 1997, 44, 521–526. [Google Scholar] [CrossRef]
- Pavlou, A.K.; Magan, N.; McNulty, C.; Jones, J.M.; Sharp, D.; Brown, J.; Turner, A.P. Use of an electronic nose system for diagnoses of urinary tract infections. Biosens. Bioelectron. 2002, 17, 893–899. [Google Scholar] [CrossRef]
- Kodogiannis, V.S.; Lygouras, J.N.; Tarczynski, A.; Chowdrey, H.S. Artificial Odor Discrimination System Using Electronic Nose and Neural Networks for the Identification of Urinary Tract Infection. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; Westenbrink, E.W.; Ouaret, N.; Harbord, R.; Bailey, C.; O’Connell, N.; Cullis, J.; Williams, N.; Nwokolo, C.U.; Bardhan, K.D.; et al. Application of a Novel Tool for Diagnosing Bile Acid 865 Diarrhoea. Sensors 2013, 13, 11899–11912. [Google Scholar] [CrossRef] [PubMed]
- D’Amicoa, A.; Pennazza, G.; Santonicoa, M.; Martinelli, E.; Roscioni, C.; Galluccio, G.; Paolesse, R.; Natalea, C.D. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010, 68, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Guo, H.R.; Chang, Y.H.; Kao, M.T.; Wang, H.H.; Hong, R.I. Application of the electronic nose for uremia diagnosis. Sens. Actuators B 2001, 76, 177–180. [Google Scholar] [CrossRef]
- Bourgeois, W.; Romain, A.C.; Nicolas, J.; Stuetz, R.M. The use of sensor arrays for environmental monitoring: Interests and limitations. J. Environ. Monit. 2003, 5, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. TRAC Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Sivret, E.C.; Parcsi, G.; Lebrero, R.; Wang, X.G.; Suffet, I.H.; Stuetz, R.M. Monitoring techniques for odour abatement assessment. Water Res. 2010, 44, 5129–5149. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Devadhasan, J.P.; Kim, D.; Lee, D.Y.; Kim, S. Smartphone coupled handheld array reader for real-time toxic gas detection. Anal. Chim. Acta 2017, 984, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic nose. Sens. Actuators B 1994, 18, 211–215. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Nie, H.; Dang, L. Classification of multiple indoor air contaminants by an electronic nose and a hybrid support vector machine. Sens. Actuators B 2012, 174, 114–125. [Google Scholar] [CrossRef]
- Liang, Z.; Tian, F.; Zhang, C.; Sun, H.; Liu, X. A correlated information removing based interference suppression technique in electronic nose for detection of bacteria. Anal. Chim. Acta 2017, 986, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Magna, G.; Vito, S.D.; Fuccio, R.D.; Francia, G.D. An adaptive classification model based on the Artificial Immune System for chemical sensor drift mitigation. Sens. Actuators B 2013, 177, 1017–1026. [Google Scholar] [CrossRef]
- Zhao, G.; Duan, Z.; Ming, L.; Li, Y.; Chen, R. Reflectance and fluorescence characterization of maize species using field laboratory measurements and lidar remote sensing. Appl. Opt. 2016, 55, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, L.; Niu, X.; Zheng, C.; Wang, Y.; Tittel, F.K. Mid-infrared absorption-spectroscopy-based carbon dioxide sensor network in greenhouse agriculture: Development and deployment. Appl. Opt. 2016, 55, 7029–7036. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Sui, Y.; Dong, M.; Ye, W.L.; Zheng, C.T.; Wang, Y.D. A carbon monoxide detection device based on mid-infrared absorption spectroscopy at 4.6 μm. Appl. Phys. B 2015, 119, 287–296. [Google Scholar] [CrossRef]
- Liudchik, A.M. Further advancement of differential optical absorption spectroscopy: Theory of orthogonal optical absorption spectroscopy. Appl. Opt. 2014, 53, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Aichele, C.; Froehlich, F.; Schuster, K.; Grimm, S. Fluorine incorporation into porous silica by gas phase doping with C2F6 in N2. Opt. Mater. Express 2013, 3, 1839–1854. [Google Scholar]
- Pogány, A.; Wagner, S.; Werhahn, O.; Ebert, V. Development and Metrological Characterization of a Tunable Diode Laser Absorption Spectroscopy (TDLAS) Spectrometer for Simultaneous Absolute Measurement of Carbon Dioxide and Water Vapor. Appl. Spectrosc. 2015, 69, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Fang, Y.; Xiong, W.; Shi, H.; Qiao, Y. Abilitiy Analysis of Spatial Heterodyne Spectrometer in Atmospheric CO2 Detection. Acta Opt. Sin. 2011, 31. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, F.; Liao, H.; Yin, X.; Liu, Y. A novel spectrum analysis technique for odor sensing in optical electronic nose. Sens. Actuators B 2016, 222, 769–779. [Google Scholar] [CrossRef]
- Dong, L.; Tittel, F.K.; Li, C.; Sanchez, N.P.; Wu, H. Compact TDLAS based sensor design using interband cascade lasers for mid-IR trace gas sensing. Opt. Express 2016, 24, A528–A535. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Llobet, E.; Ramírez, J.L.; Ivanova, P.; Fonseca, L.; Zampolli, S. An RFID reader with onboard sensing capability for monitoring fruit quality. Sens. Actuators B 2007, 127, 143–149. [Google Scholar] [CrossRef]
- Harlander, J.; Reynolds, R.J.; Roesler, F.L. Spatial heterodyne spectroscopy for the exploration of diffuse interstellar emission lines at far-ultraviolet wavelengths. Astrophys. J. 2013, 396, 730–740. [Google Scholar] [CrossRef]
- Brault, J.W. New approach to high-precision Fourier transform spectrometer design. Appl. Opt. 2016, 35, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Englert, C.R.; Stevens, M.H.; Siskind, D.E.; Harlander, J.; Roesler, F.L. Spatial Heterodyne Imager for Mesospheric Radicals on STPSat-1. J. Geophys. Res. Atmos. 2012, 115, 898–907. [Google Scholar] [CrossRef]
- Lawler, J.E.; Labby, Z.E.; Harlander, J.; Roesler, F.L. Broadband, high-resolution spatial heterodyne spectrometer. Appl. Opt. 2008, 47, 6371–6384. [Google Scholar] [CrossRef] [PubMed]
- Englert, C.R.; Harlander, J. Flatfielding in spatial heterodyne spectroscopy. Appl. Opt. 2006, 45, 4583–4590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, F.; Zhao, Z.; Zhang, L.; Yang, S.X.; Song, A. Interferogram correction of spatial heterodyne spectrometer. Opto-Electron. Eng. 2017, 44, 488–497. [Google Scholar]
- Brock, J.R. A note on the Beer-Lambert law. Anal. Chim. Acta 1962, 27, 95–97. [Google Scholar] [CrossRef]
- Rothman, L.S.; Rinsland, C.P.; Goldman, A.; Massie, S.T.; Edwards, D.P.; Flaud, J.M.; Perrin, A.; Peyret, C.C.; Dana, V.; Mandin, J.Y.; et al. The HITRAN molecular spectroscopic database and hawks (hitran atmospheric workstation): 1996 Edition. J. Quant. Spectrosc. Radiat. Ransf. 1998, 60, 665–710. [Google Scholar] [CrossRef]
- Lin, L. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M.; Shanmugam, K. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Ojala, T.; Pietikäinen, M.; Mäenpää, T. Gray Scale and Rotation Invariant Texture Classification with Local Binary Patterns. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 971–987. [Google Scholar] [CrossRef]
- Zulpe, N.S.; Pawar, V.P. GLCM Textural Features for Brain Tumor Classification. Int. J. Comput. Sci. Issues 2012, 9, 354–359. [Google Scholar]
- Skrobot, V.L.; Castro, E.V.R. RCC Pereira VMD Pasa, ICP Fortes. Use of Principal Component Analysis (PCA) and Linear Discriminant Analysis (LDA) in Gas Chromatographic (GC) Data in the Investigation of Gasoline Adulteration. Energy Fuels 2016, 21, 5–19. [Google Scholar]
- Wang, J.; Zheng, N. A Novel Fractal Image Compression Scheme with Block Classification and Sorting Based on Pearson’s Correlation Coefficient. IEEE Trans. Image Process. 2013, 22, 3690–3702. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Chen, P.A.; Huang, C.C.; Chiang, H.K.; Chiu, A.W. Enhanced Raman sensitivity and magnetic separation for urolithiasis detection using phosphonic acid-terminated Fe3O4 nanoclusters. J. Mater. Chem. B 2015, 3, 4282–4290. [Google Scholar] [CrossRef]
- Sales, F.; Callao, M.P.; Rius, F.X. Multivariate standardization for correcting the ionic strength variation on potentiometric sensor arrays. Analyst 2000, 125, 883–888. [Google Scholar] [CrossRef]




| Category | Parameters |
|---|---|
| Technical principle | Light absorption sensing technology |
| Response range | 200–1100 nm |
| Size of sensing unit | 0.54 nm |
| Lower limit of detection | NO2: 0.2‰; SO2: 0.1‰; C6H6: 0.2‰ |
| Sensitivity | NO2: 0.2‰; SO2: 0.1‰; C6H6: 0.1‰ |
| Test gases | Inorganic gas: NO, NH3, O3, SO2, CS2, NO2, O2, etc. |
| Organic gas: C6H6, C7H8, C8H10, CH2O etc. |
| Class | Correlation Coefficient | ||||
|---|---|---|---|---|---|
| NO2 | SO2 | NO2 + SO2 | C6H6 | C7H8 | |
| NO2 | 1 | 0.55 | 0.67 | 0.95 | 0.92 |
| SO2 | 0.55 | 1 | 0.44 | 0.53 | 0.50 |
| NO2 + SO2 | 0.67 | 0.44 | 1 | 0.61 | 0.58 |
| C6H6 | 0.95 | 0.53 | 0.61 | 1 | 0.94 |
| C7H8 | 0.92 | 0.50 | 0.58 | 0.94 | 1 |
| Gas | NO2 | SO2 | NO2 + SO2 | C6H6 | C7H8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (‰) | 0.2 | 0.4 | 0.6 | 0.3 | 0.6 | 0.9 | 3.2 + 0.1 | 3.5 + 0.3 | 3.0 + 0.6 | 3 | 3 |
| Number | 10 | 10 | 12 | 10 | 10 | 12 | 10 | 10 | 12 | 32 | 32 |
| Total | 32 | 32 | 32 | 32 | 32 | ||||||
| Class | Classification Accuracy (%) | |||
|---|---|---|---|---|
| CC | EDC | |||
| LBP | GLCM | LBP | GLCM | |
| NO2 | 100 | 100 | 100 | 100 |
| SO2 | 50 | 70 | 80 | 80 |
| NO2 + SO2 | 90 | 30 | 100 | 60 |
| C6H6 | 60 | 70 | 90 | 90 |
| C7H8 | 60 | 50 | 80 | 80 |
| Mean | 72 | 64 | 90 | 82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Tian, F.; Song, A.; Hu, Y. Research on a Visual Electronic Nose System Based on Spatial Heterodyne Spectrometer. Sensors 2018, 18, 1188. https://doi.org/10.3390/s18041188
Zhang W, Tian F, Song A, Hu Y. Research on a Visual Electronic Nose System Based on Spatial Heterodyne Spectrometer. Sensors. 2018; 18(4):1188. https://doi.org/10.3390/s18041188
Chicago/Turabian StyleZhang, Wenli, Fengchun Tian, An Song, and Youwen Hu. 2018. "Research on a Visual Electronic Nose System Based on Spatial Heterodyne Spectrometer" Sensors 18, no. 4: 1188. https://doi.org/10.3390/s18041188





