E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Collection
2.2. Fruit Ripening Evaluation
2.3. Volatile Compounds Analysis by E-Nose
2.4. GC-MS Analysis of Volatiles
2.5. Statistical Analysis
3. Results
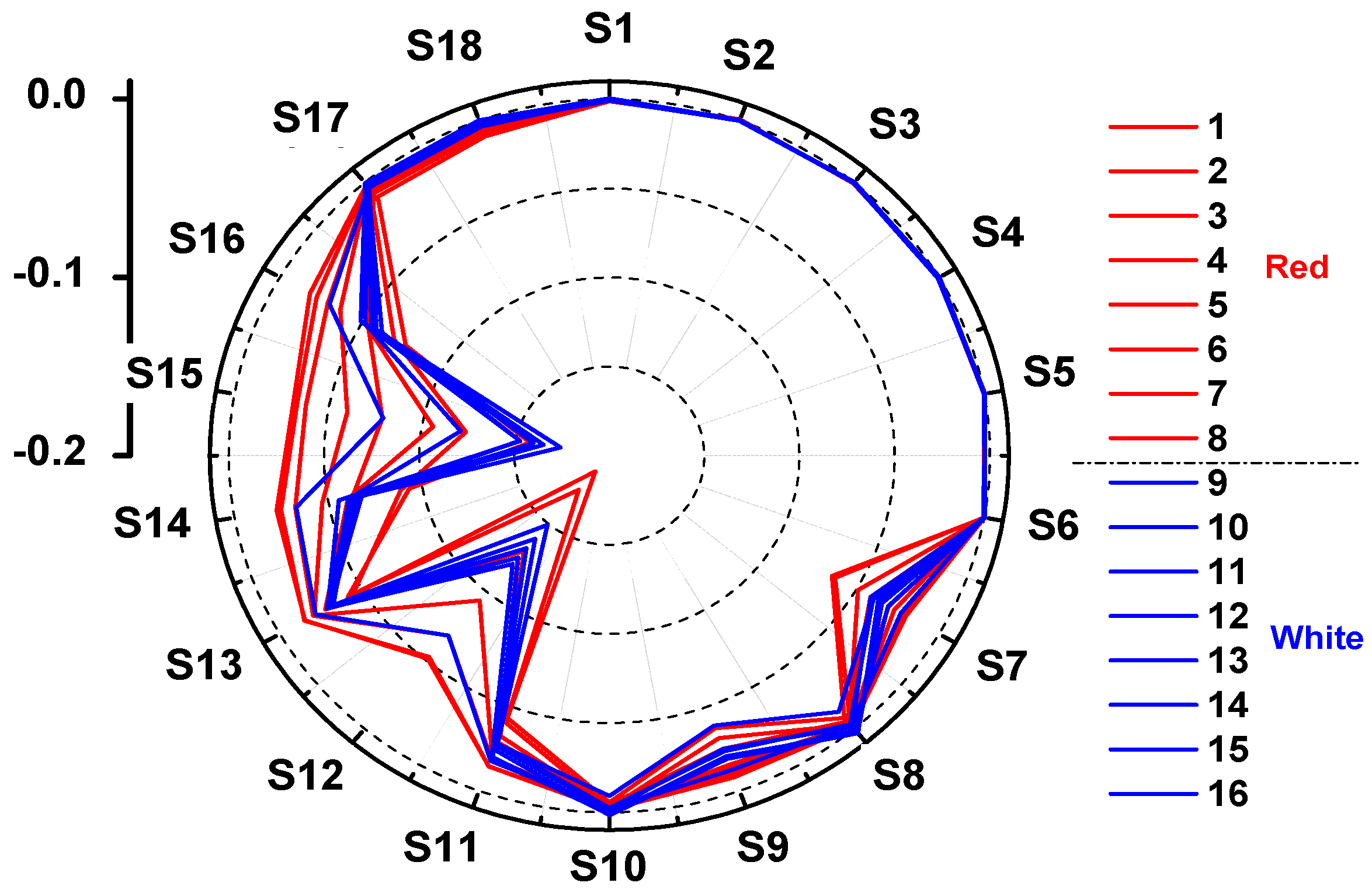
3.1. Response of E-Nose to Peach Fruit
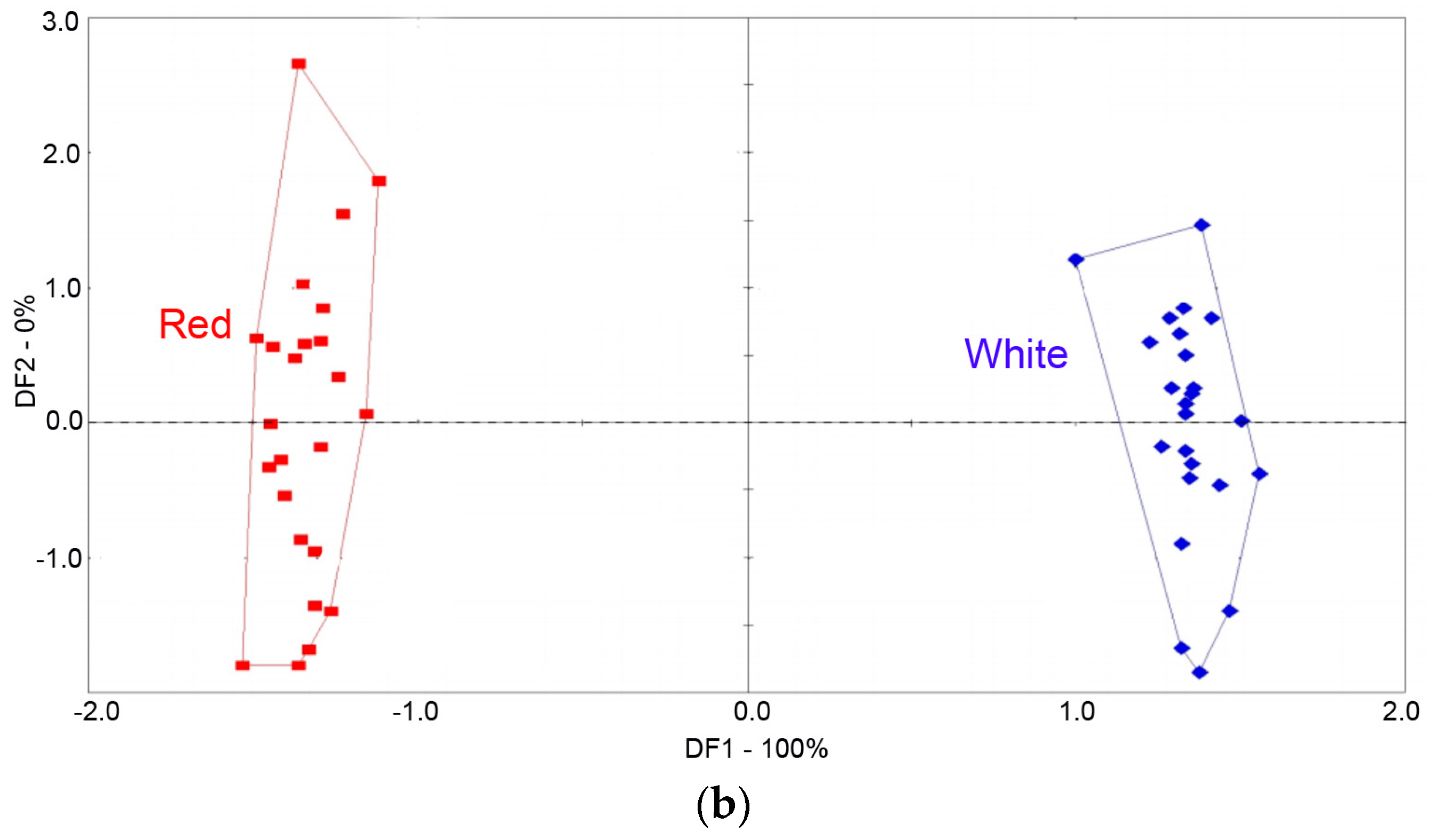
3.2. Classification of Peach Fruit Samples with Different Colors by E-Nose
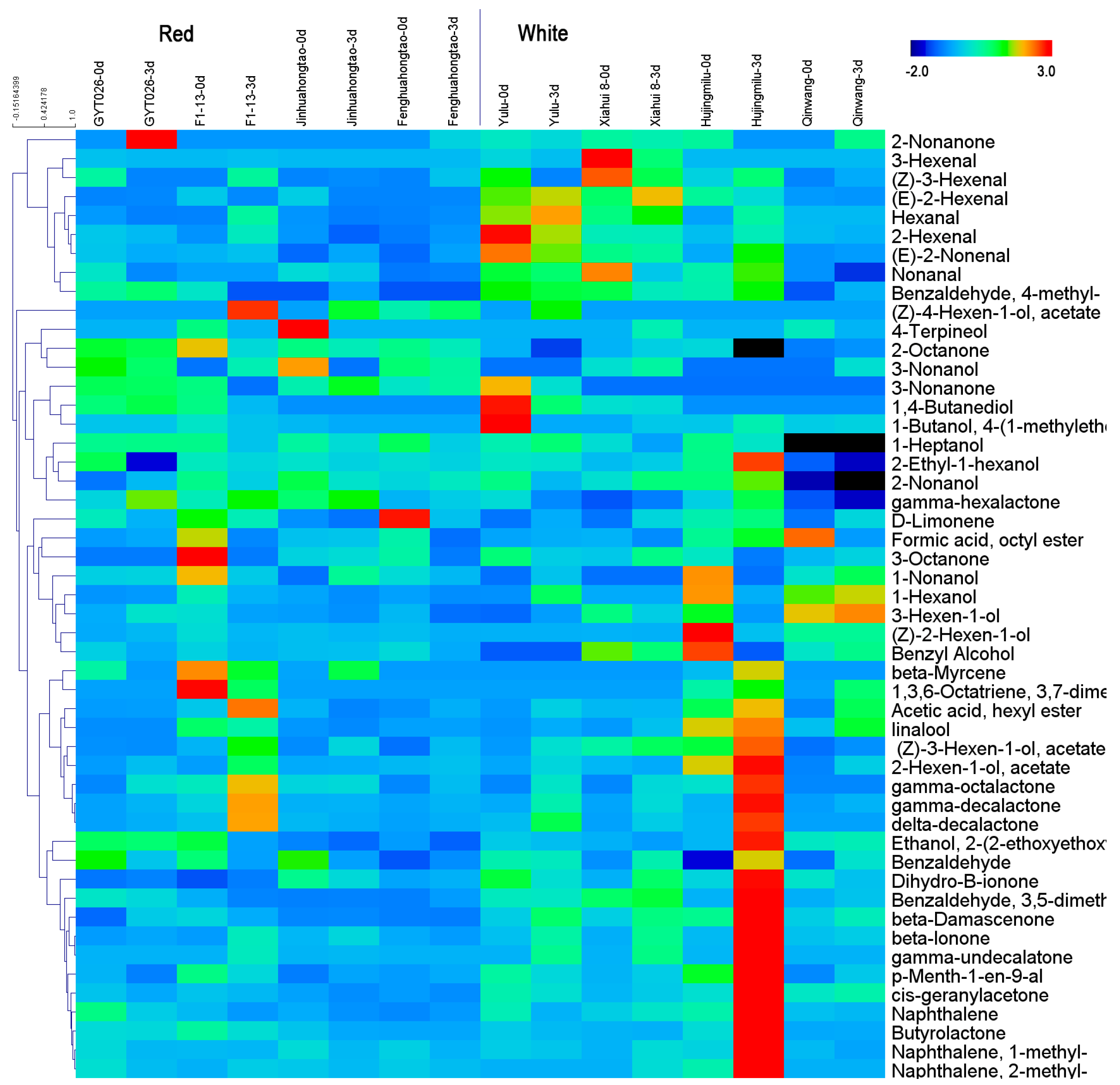
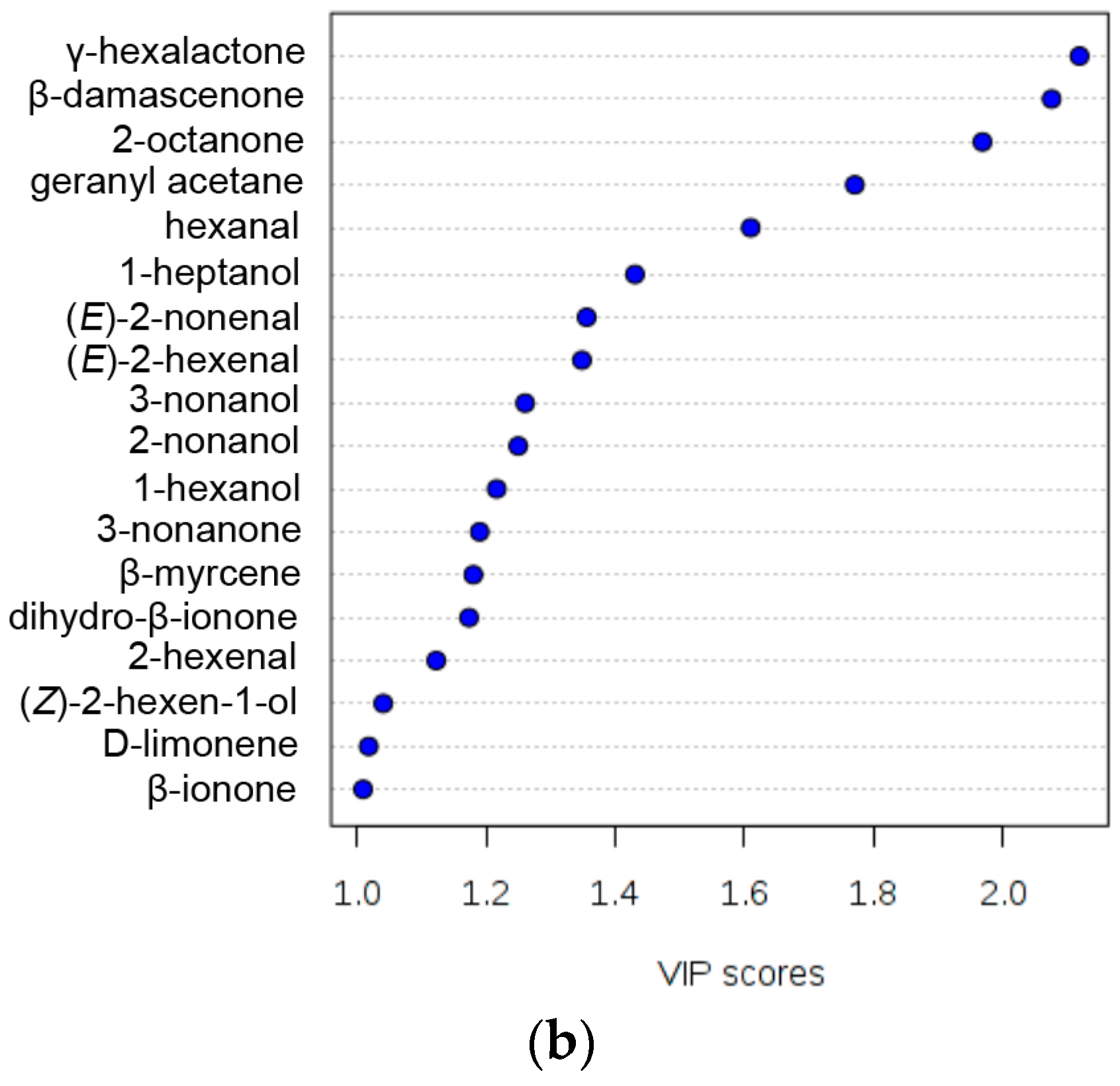
3.3. Volatiles Play a Role in Discriminating Peach Fruit Samples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, H.; Wang, K.L.; Wang, H.; Gu, C.; Dare, A.P.; Espley, E.V.; He, H.; Allan, A.C.; Han, Y. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J. 2015, 82, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horvath, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Rosati, C. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dixoygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.; Weesepoel, Y.; Koot, A.; Iglesias, I.; Eduardo, I.; Gratacos-Gubarsi, M.; Guerrero, L.; Hortos, M.; van Ruth, S. Investigation of the aroma of commercial peach (Prunus persica L. Batsch) types by Proton Transfer Reaction-Mass Spectrometry (PTR-MS) and sensory analysis. Food Res. Int. 2017, 99, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Montero-Prado, P.; Bentayeb, K.; Nerin, C. Pattern recognition of peach cultivars (Prunus persica L.) from their volatile components. Food Chem. 2013, 138, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, H.R.; Gao, J.; Huang, Y.J.; Zhang, B.; Chen, K.S. Two ω-3 FADs are associated with peach fruit volatile formation. Int. J. Mol. Sci. 2016, 17, 464. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, X.; Liu, X.; Xin, R.; Wang, J.J.; Gao, J.; Wu, B.P.; Gao, L.X.; Xu, C.J.; Zhang, B.; et al. UV-B irradiation differentially regulates terpene synthases and terpene content of peach. Plant Cell Environ. 2017, 40, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Vengas-Caleron, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genom. 2013, 14, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Xi, X.P.; Wei, W.W.; Shen, J.Y.; Ferguson, I.; Chen, K.S. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Xi, W.P.; Zhang, B.; Liang, L.; Shen, J.Y.; Wei, W.W.; Xu, C.J.; Allan, A.C.; Ferguson, I.B.; Chen, K.S. Postharvest temperature influences volatile lactone production via regulation of acyl-CoA oxidases in peach fruit. Plant Cell Environ. 2012, 35, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Besada, C.; Badenes, M.L.; Monforte, A.J.; Granell, A. A non-target approach unravels the volatile network in peach fruit. PLoS ONE 2012, 7, es38992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, M.A.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Brendolise, C.; Chagne, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P.; et al. Multiple Repeats of a Promoter Segment Causes Transcription Factor Autoregulation in Red Apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Farcuh, M.; Menenes, C. Monitoring the sensorial quality and aroma through an electronic nose in peaches during storage. J. Sci. Food Agric. 2008, 88, 2073–2078. [Google Scholar] [CrossRef]
- Su, M.S.; Zhang, B.; Ye, Z.W.; Chen, K.S.; Guo, J.; Gu, X.J.; Shen, J.Y. Pulp volatiles measured by an electronic nose are related to harvest season, TSS concentration and TSS/TA ratio among 39 peaches and nectarines. Sci. Hortic. 2013, 150, 146–153. [Google Scholar] [CrossRef]
- Tian, H.; Wang, P.; Zhan, P.; Yan, H.; Zhou, W.; Zhang, F. Effects of β-glucosidase on the aroma characteristics of flat peach juice as assessed by descriptive sensory analysis and gas chromatography and compared by partial least squares regression. LWT-Food Sci. Technol. 2017, 82, 113–120. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef] [PubMed]



| Code | Peach Cultivar | Flesh Color | Sample Description | TSS 1 (Brix) | Firmness (N) |
|---|---|---|---|---|---|
| 1 | GYT026 | red | Harvest day | 12.51 ± 0.65 | 13.23 ± 1.77 |
| 2 | 3 d shelf-life | 12.84 ± 0.51 | 3.54 ± 0.2 | ||
| 3 | F1-13 | red | Harvest day | 10.42 ± 0.41 | 7.68 ± 1.49 |
| 4 | 3 d shelf-life | 11.73 ± 0.28 | 3.15 ± 0.09 | ||
| 5 | Fenghuahong | red | Harvest day | 11.88 ± 0.83 | 15.64 ± 2.26 |
| 6 | 3 d shelf-life | 11.31 ± 0.75 | 4.62 ± 0.43 | ||
| 7 | Jinghuahong | red | Harvest day | 7.92 ± 0.51 | 30.58 ± 2.65 |
| 8 | 3 d shelf-life | 8.74 ± 0.46 | 26.17 ± 1.89 | ||
| 9 | Yulu | white | Harvest day | 14.22 ± 0.32 | 29.38 ± 2.76 |
| 10 | 3 d shelf-life | 15.54 ± 0.47 | 5.61 ± 0.38 | ||
| 11 | Xiahui 8 | white | Harvest day | 16.44 ± 0.41 | 32.45 ± 3.22 |
| 12 | 3 d shelf-life | 15.99 ± 0.3 | 15.82 ± 2.22 | ||
| 13 | Hujingmilu | white | Harvest day | 13.61 ± 0.54 | 42.36 ± 1.58 |
| 14 | 3 d shelf-life | 14.72 ± 0.62 | 4.76 ± 0.75 | ||
| 15 | Qinwang | white | Harvest day | 11.88 ± 0.36 | 21.28 ± 3.17 |
| 16 | 3 d shelf-life | 13.58 ± 0.57 | 28.33 ± 2.15 |
| Red | White | |||||||
|---|---|---|---|---|---|---|---|---|
| High | Low | Fold Difference | Average | High | Low | Fold Difference | Average | |
| Hexanal | 378.82 | 9.12 | 41.52 | 70.56 | 941.94 | 0.90 | 1045.44 | 355.74 |
| 3-Hexenal | 6.11 | 2.71 | 2.25 | 3.96 | 314.32 | 0.50 | 623.40 | 78.87 |
| (Z)-3-Hexenal | 5.28 | 0.14 | 36.65 | 3.15 | 18.84 | 1.13 | 16.71 | 6.27 |
| 2-Hexenal | 175.37 | 11.18 | 15.68 | 73.14 | 705.87 | 11.94 | 59.12 | 187.58 |
| (E)-2-Hexenal | 72.90 | 0.98 | 74.11 | 32.32 | 349.49 | 5.97 | 58.58 | 121.68 |
| 1-Hexanol | 117.48 | 5.10 | 23.05 | 31.82 | 451.44 | 2.61 | 172.65 | 135.07 |
| (Z)-2-Hexen-1-ol | 32.77 | 2.47 | 13.25 | 11.77 | 263.28 | 2.15 | 122.49 | 45.29 |
| 3-Hexen-1-ol | 9.46 | 0.66 | 14.39 | 5.48 | 36.76 | 1.98 | 18.54 | 9.87 |
| Nonanal | 19.66 | 5.51 | 3.57 | 11.41 | 52.97 | 1.49 | 35.44 | 21.12 |
| (E)-2-Nonenal | 6.51 | 1.62 | 4.02 | 3.52 | 20.01 | 1.36 | 14.72 | 9.51 |
| Acetic acid, hexyl ester | 100.40 | 2.37 | 42.43 | 17.75 | 104.05 | 3.92 | 26.54 | 25.79 |
| (Z)-3-Hexen-1-ol, acetate | 151.03 | 13.24 | 11.40 | 53.82 | 246.43 | 10.74 | 22.95 | 88.78 |
| (Z)-4-Hexen-1-ol, acetate | 24.71 | 6.77 | 3.65 | 13.28 | 32.97 | 2.99 | 11.02 | 13.37 |
| 2-Hexen-1-ol, acetate | 27.84 | 1.23 | 22.69 | 7.71 | 64.10 | 1.59 | 40.20 | 14.76 |
| Formic acid, octyl ester | 18.75 | 3.70 | 5.06 | 6.67 | 23.36 | 3.76 | 6.22 | 7.53 |
| β-Myrcene | 14.92 | 0.28 | 53.43 | 5.93 | 7.83 | 1.01 | 7.78 | 2.74 |
| D-Limonene | 32.84 | 1.28 | 25.65 | 9.26 | 12.73 | 1.15 | 11.10 | 6.40 |
| 1,3,6-Octatriene, 3,7-dimethyl- | 9.47 | 2.77 | 3.42 | 5.08 | 4.34 | 2.16 | 2.01 | 2.98 |
| Linalool | 146.30 | 1.75 | 83.75 | 40.96 | 407.75 | 2.33 | 175.07 | 93.59 |
| 4-Terpineol | 9.31 | 1.63 | 5.72 | 4.15 | 2.01 | 1.54 | 1.30 | 1.77 |
| p-Menth-1-en-9-al | 6.69 | 0.88 | 7.57 | 2.90 | 15.00 | 0.76 | 19.65 | 5.44 |
| β-Damascenone | 3.61 | 0.52 | 6.99 | 1.58 | 15.72 | 2.13 | 7.38 | 5.64 |
| Dihydro-β-ionone | 13.51 | 1.50 | 9.04 | 4.99 | 31.72 | 2.50 | 12.67 | 10.63 |
| cis-geranylacetone | 4.72 | 0.93 | 5.09 | 2.36 | 24.58 | 2.32 | 10.58 | 7.28 |
| β-Ionone | 2.53 | 0.14 | 17.64 | 0.94 | 16.54 | 0.34 | 48.13 | 2.62 |
| 3-Octanone | 6.18 | 1.03 | 6.00 | 2.75 | 3.63 | 0.45 | 7.99 | 1.39 |
| 2-Octanone | 460.72 | 251.74 | 1.83 | 328.41 | 280.70 | 41.90 | 6.70 | 208.58 |
| 3-Nonanone | 6.97 | 3.17 | 2.20 | 5.38 | 11.33 | 3.50 | 3.23 | 8.02 |
| 2-Nonanone | 11.61 | 1.29 | 8.97 | 6.03 | 3.17 | 1.17 | 2.71 | 2.52 |
| 1-Heptanol | 38.13 | 28.79 | 1.32 | 31.95 | 42.19 | 20.00 | 2.11 | 28.95 |
| 2-Ethyl-1-hexanol | 15.11 | 0.81 | 18.54 | 10.53 | 25.32 | 3.96 | 6.40 | 11.48 |
| 3-Nonanol | 37.86 | 11.04 | 3.43 | 21.34 | 16.19 | 11.53 | 1.40 | 13.79 |
| 2-Nonanol | 12.96 | 4.89 | 2.65 | 7.79 | 13.39 | 0.57 | 23.48 | 7.25 |
| Ethanol, 2-(2-ethoxyethoxy) | 30.94 | 4.53 | 6.82 | 13.92 | 45.90 | 9.94 | 4.62 | 16.98 |
| 1-Nonanol | 6.80 | 0.91 | 7.44 | 2.61 | 6.85 | 1.52 | 4.50 | 4.06 |
| Benzyl Alcohol | 3.03 | 0.93 | 3.25 | 1.46 | 7.05 | 1.38 | 5.13 | 3.73 |
| 1,4-Butanediol | 3.00 | 0.17 | 17.70 | 1.54 | 7.47 | 0.77 | 9.69 | 2.00 |
| 1-Butanol, 4-(1-methylethoxy) | 6.93 | 0.40 | 17.37 | 2.24 | 68.89 | 1.82 | 37.86 | 7.58 |
| Benzaldehyde | 20.64 | 4.59 | 4.50 | 10.77 | 23.96 | 3.88 | 6.17 | 10.69 |
| Benzaldehyde, 4-methyl | 5.07 | 1.13 | 4.50 | 2.79 | 5.02 | 1.39 | 3.62 | 3.67 |
| Benzaldehyde, 3,5-dimethyl | 5.49 | 0.33 | 16.50 | 2.00 | 16.75 | 1.43 | 11.75 | 6.30 |
| Butyrolactone | 14.01 | 2.29 | 6.11 | 6.01 | 46.82 | 2.01 | 23.24 | 7.10 |
| γ-hexalactone | 10.26 | 2.39 | 4.28 | 5.54 | 6.76 | 0.14 | 46.73 | 2.92 |
| γ-octalactone | 4.13 | 0.31 | 13.18 | 1.47 | 4.12 | 0.53 | 7.79 | 1.81 |
| γ-decalactone | 142.67 | 1.09 | 130.91 | 25.28 | 164.70 | 0.38 | 431.31 | 37.67 |
| δ-decalactone | 28.90 | 0.60 | 48.44 | 5.85 | 35.32 | 1.22 | 29.07 | 10.34 |
| γ-undecalatone | 7.00 | 0.05 | 135.219 | 2.39 | 49.24 | 0.40 | 122.78 | 12.70 |
| Naphthalene | 6.91 | 1.57 | 4.39 | 3.12 | 19.15 | 2.48 | 7.73 | 5.11 |
| Naphthalene, 1-methyl | 5.71 | 0.66 | 8.67 | 2.06 | 42.03 | 0.59 | 71.57 | 5.88 |
| Naphthalene, 2-methyl | 2.74 | 0.32 | 8.53 | 1.16 | 29.61 | 1.57 | 18.90 | 8.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit. Sensors 2018, 18, 765. https://doi.org/10.3390/s18030765
Xin R, Liu X, Wei C, Yang C, Liu H, Cao X, Wu D, Zhang B, Chen K. E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit. Sensors. 2018; 18(3):765. https://doi.org/10.3390/s18030765
Chicago/Turabian StyleXin, Rui, Xiaohong Liu, Chunyan Wei, Chong Yang, Hongru Liu, Xiangmei Cao, Di Wu, Bo Zhang, and Kunsong Chen. 2018. "E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit" Sensors 18, no. 3: 765. https://doi.org/10.3390/s18030765





