Lab-on-a-Disc Platform for Automated Chemical Cell Lysis
Abstract
:1. Introduction
2. Materials and Methods
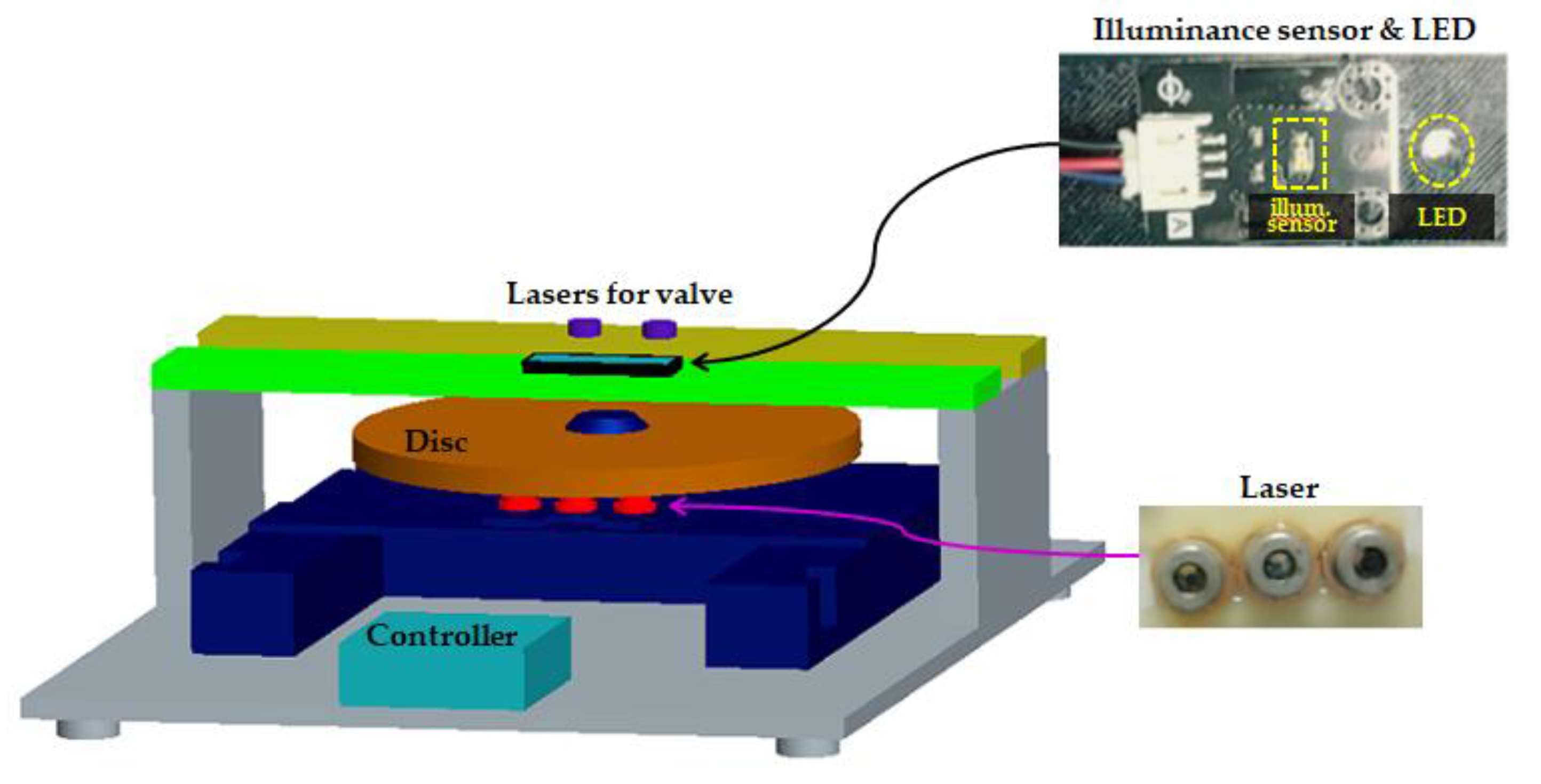
2.1. Processing System
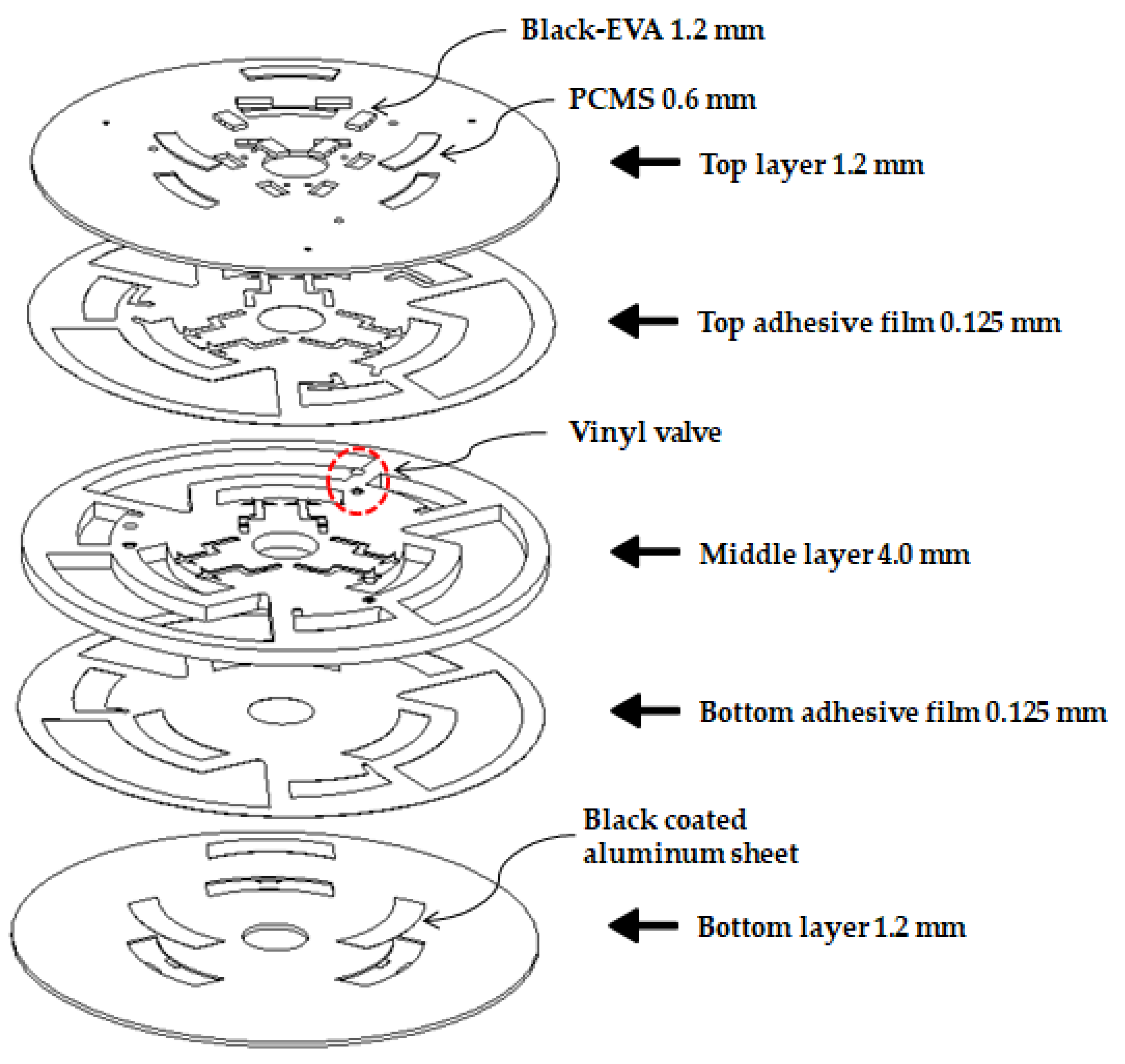
2.2. Disc Fabrication
2.3. Preparation of Bacterial Strain and Reagents
2.4. Experimental Procedure of the Disc for DNA Lysis
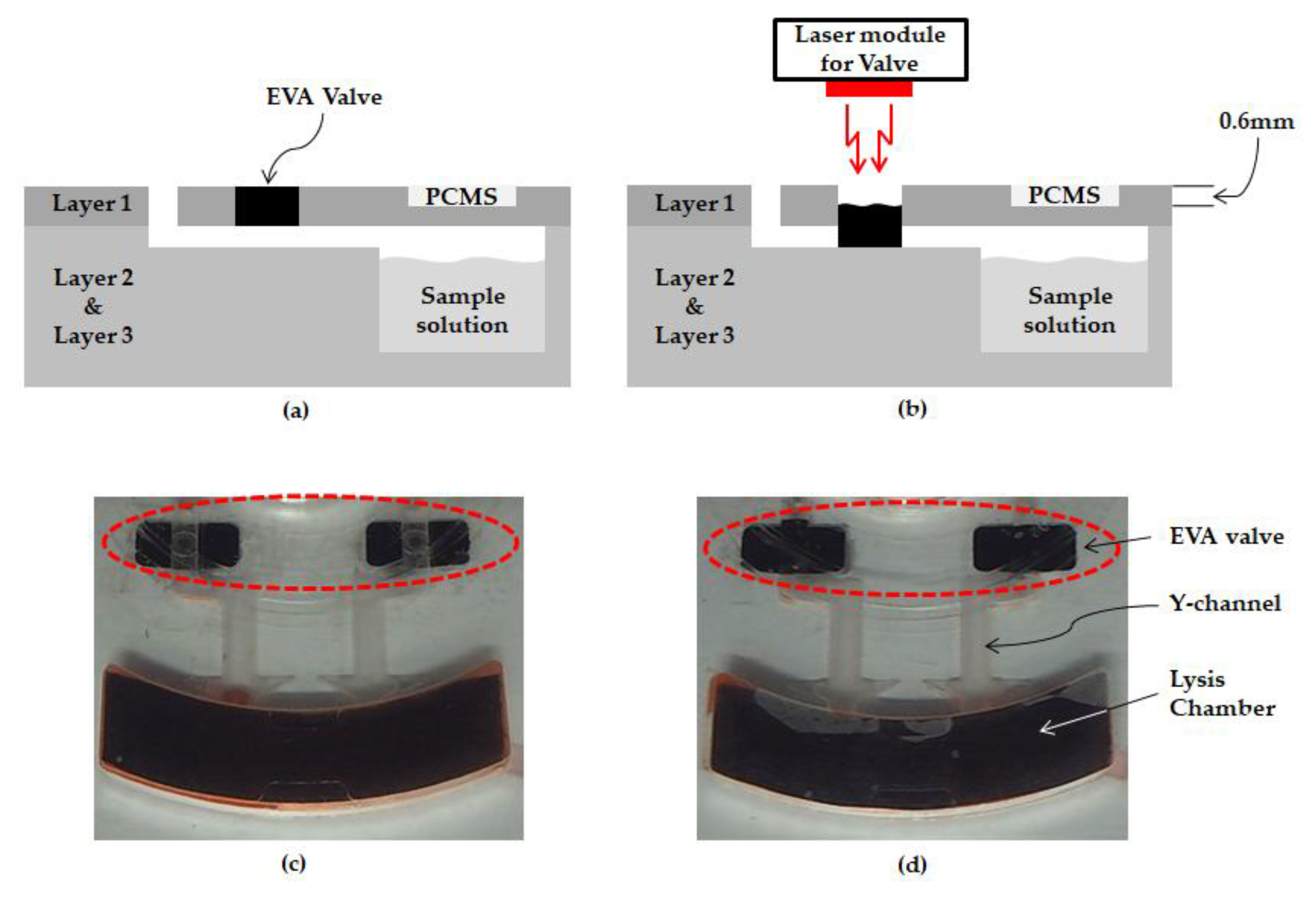
2.5. Ethylene Vinyl Acetate Used as a Valve
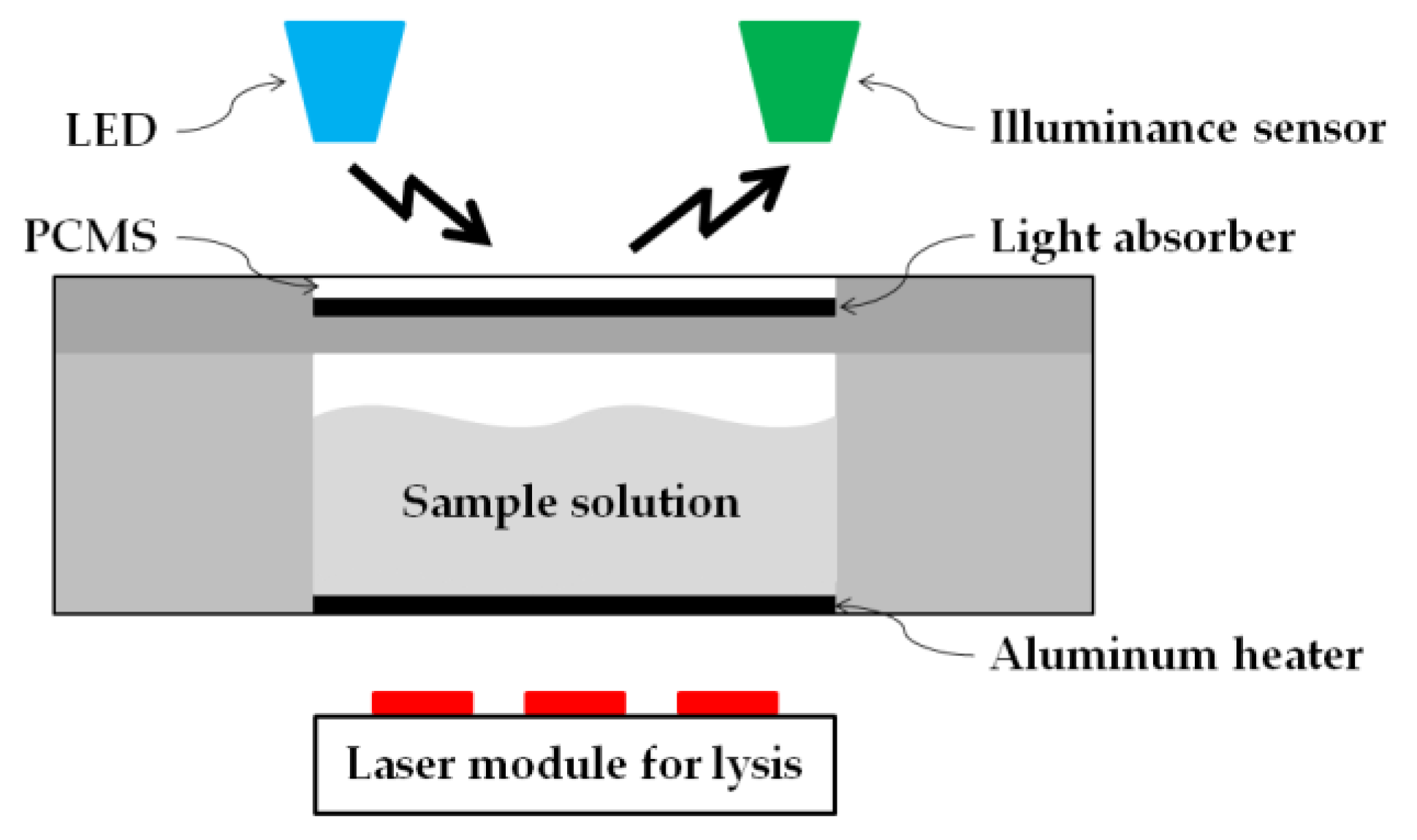
2.6. PCM Used as Illuminance Intensity Change
3. Results and Discussion
3.1. Disc Fabrication and Structure
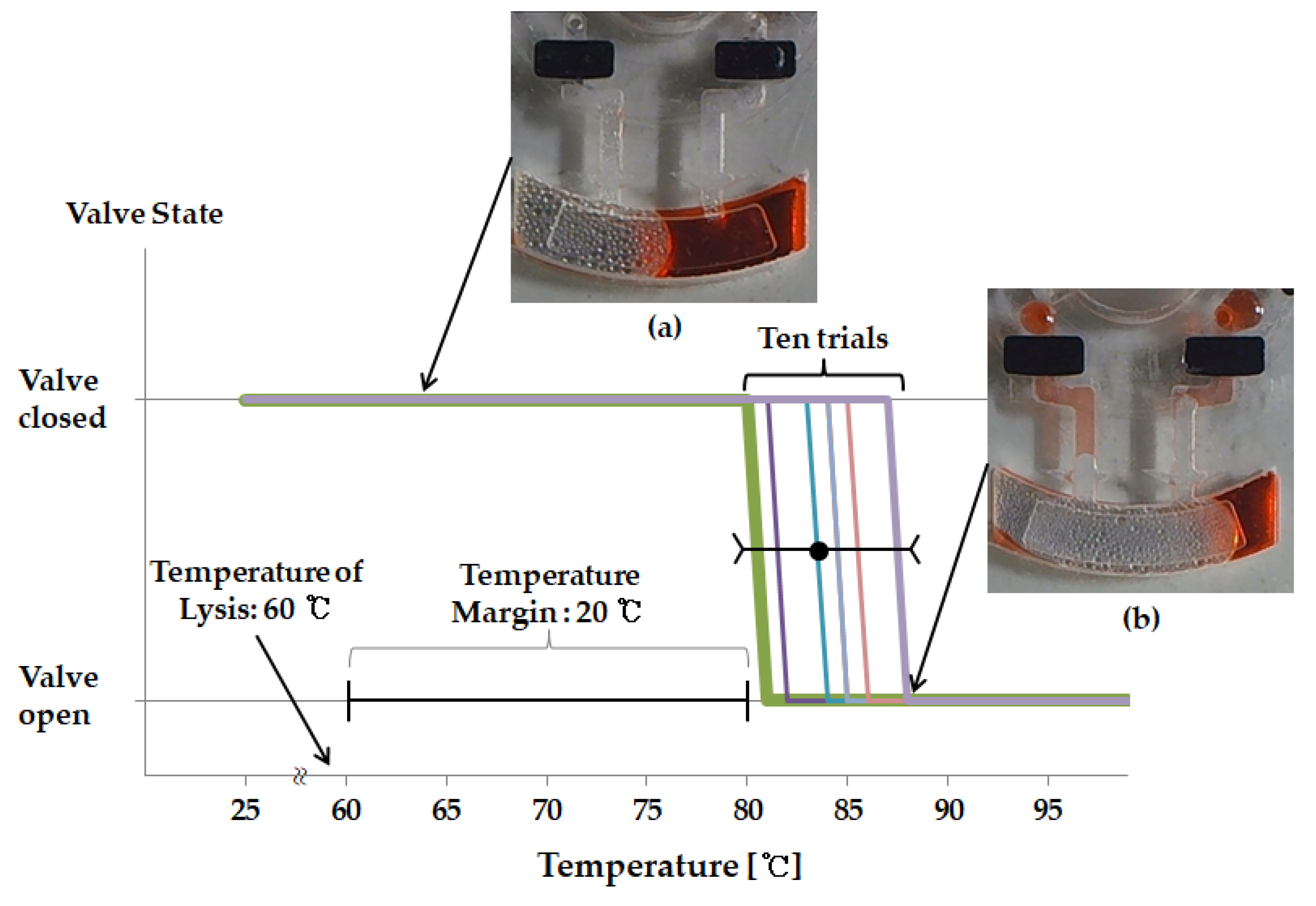
3.2. Durability of Ethylene Vinyl Acetate Used as a Valve
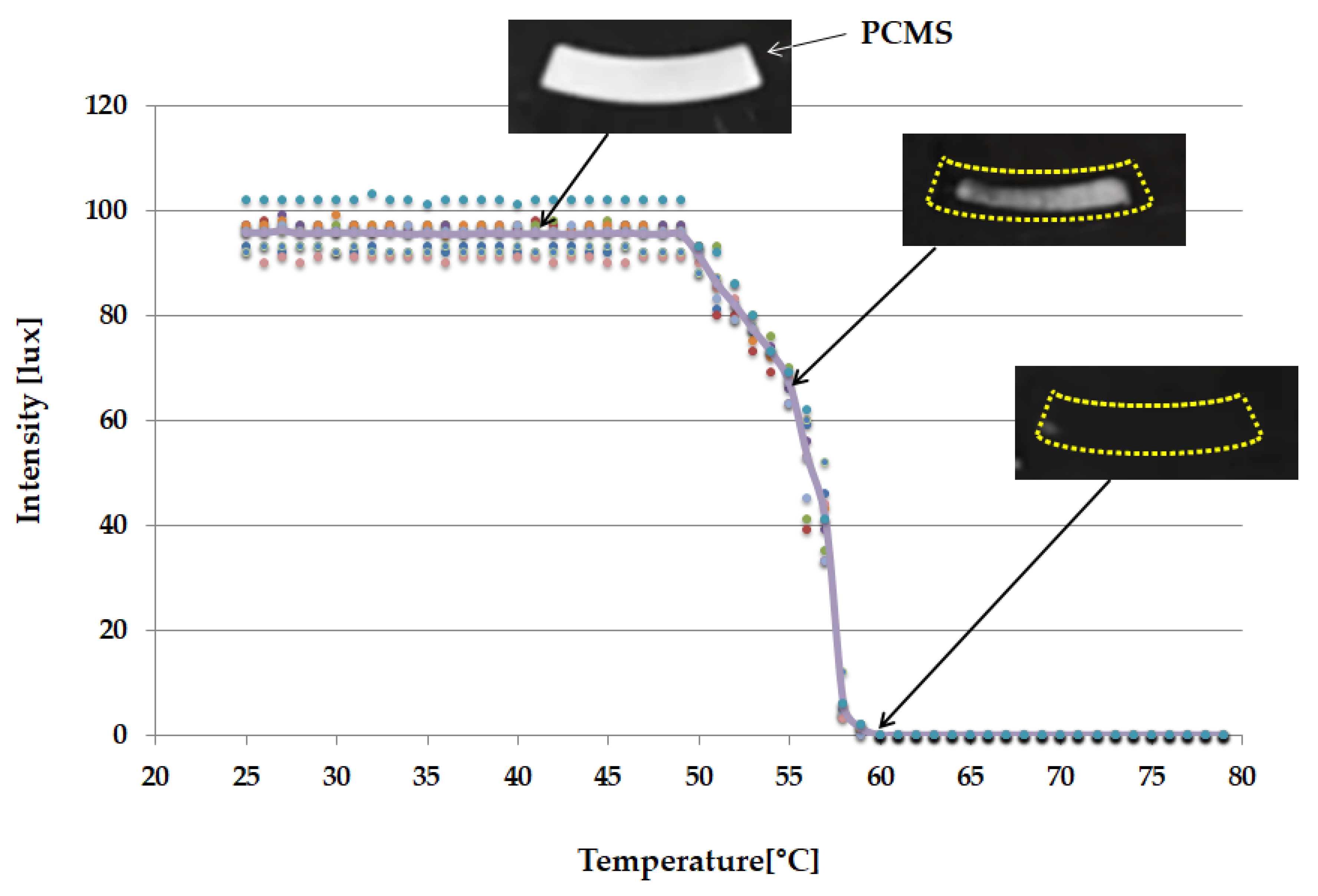
3.3. PCMS Performance
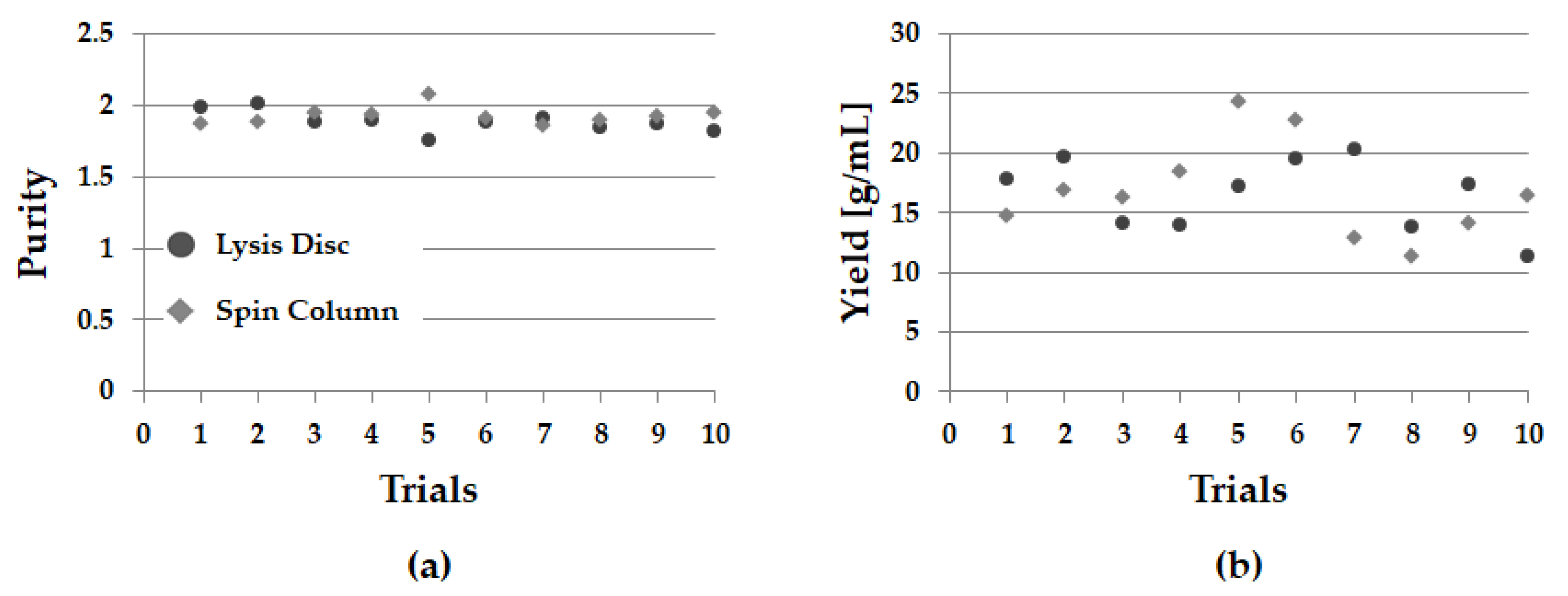
3.4. DNA Purification and Yield
3.5. PCR Electrophoresis Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics toward A Lab-on-a-Chip. Annu. Rev. Fluid Mech. 2004, 36, 381–412. [Google Scholar] [CrossRef]
- Boyd-Moss, M.; Baratchi, S.; DiVenere, M.; Khoshmanesh, K. Self-contained microfluidic systems: A review. Lab Chip 2016, 16, 3177–3192. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Jiang, Z.; Wei, X. Emerging microfluidic devices for cell lysis: A review. Lab Chip 2014, 14, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Lai, H.-C.; Liou, T.-M.; Hsu, K.-F.; Chou, C.-Y.; Lee, G.-B. A DNA methylation assay for detection of ovarian cancer cells using a HpaII/MspI digestion-based PCR assay in an integrated microfluidic system. Microfluid. Nanofluid. 2013, 15, 575–585. [Google Scholar] [CrossRef]
- Chen, L.; Patrone, N.; Liang, J.F. Peptide Self-Assembly on Cell Membranes to Induce Cell Lysis. Biomacromolecules 2012, 13, 3327–3333. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.A.; Wu, L.L.; Babikian, S.; Bachman, M.; Santiago, J.G. Integrated Printed Circuit Board Device for Cell Lysis and Nucleic Acid Extraction. Anal. Chem. 2012, 84, 9640–9645. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.N.; Kim, S. Microfluidic Platforms for Single-Cell Analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Irimia, D.; Tompkins, R.G.; Toner, M. Single-Cell Chemical Lysis in Picoliter-Scale Closed Volumes Using a Microfabricated Device. Anal. Chem. 2004, 76, 6137–6143. [Google Scholar] [CrossRef] [PubMed]
- Sasuga, Y.; Iwasawa, T.; Terada, K.; Oe, Y.; Sorimachi, H.; Ohara, O.; Harada, Y. Single-Cell Chemical Lysis Method for Analyses of Intracellular Molecules Using an Array of Picoliter-Scale Microwells. Anal. Chem. 2008, 80, 9141–9149. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.-P.; Hsiao, J.-H.; Maslov, N.A. Single-Cell Chemical Lysis on Microfluidic Chips with Arrays of Microwells. Sensors 2012, 12, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, F.; Schwemmer, F.; Hutzenlaub, T.; Baumann, D.; Strohmeier, O.; Dingemanns, G.; Simons, G.; Sager, C.; Plobner, L.; von Stetten, F.; et al. LabDisk with complete reagent prestorage for sample-to-answer nucleic acid based detection of respiratory pathogens verified with influenza A H3N2 virus. Lab Chip 2016, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Sewart, R.; Becker, H.; Roux, P.; Land, K. Blister pouches for effective reagent storage on microfluidic chips for blood cell counting. Microfluid. Nanofluid. 2016, 20, 163. [Google Scholar] [CrossRef]
- Clingenpeel, S.; Clum, A.; Schwientek, P.; Rinke, C.; Woyke, T. Reconstructing each cell’s genome within complex microbial communities—Dream or reality? Front Microbiol. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.D.; Wise, F.; Baeumner, A.J. Development of a laser-induced cell lysis system. Anal. Bioanal. Chem. 2002, 374, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.X.; Parate, K.; Abi-Samra, K.; Madou, M. Multifunctional wax valves for liquid handling and incubation on a microfluidic CD. Microfluid. Nanofluid. 2015, 18, 1031–1037. [Google Scholar] [CrossRef]
- Al-Faqheri, W.; Thio, T.H.G.; Qasaimeh, M.A.; Dietzel, A.; Madou, M.; Al-Halhouli, A. Particle/cell separation on microfluidic platforms based on centrifugation effect: A review. Microfluid. Nanofluid. 2017, 21, 102. [Google Scholar] [CrossRef]
- La, M.; Park, S.J.; Kim, H.W.; Park, J.J.; Ahn, K.T.; Ryew, S.M.; Kim, D.S. A centrifugal force-based serpentine micromixer (CSM) on a plastic lab-on-a-disk for biochemical assays. Microfluid. Nanofluid. 2013, 15, 87–98. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F. A Colorimetric Enzyme-Linked Immunosorbent Assay (ELISA) Detection Platform for a Point-of-Care Dengue Detection System on a Lab-on-Compact-Disc. Sensors 2015, 15, 11431–11441. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sunkara, V.; Kim, T.H.; Hwang, H.; Cho, Y.K. Lab-on-a-Disc for Fully Integrated Multiplex Immunoassays. Anal. Chem. 2012, 84, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Ibrahim, F.; Sayad, A.; Thiha, A.; Pei, K.; Mohktar, M.; Thong, K. A Portable Automatic Endpoint Detection System for Amplicons of Loop Mediated Isothermal Amplification on Microfluidic Compact Disk Platform. Sensors 2015, 15, 5376–5389. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Huang, L.; Wang, J. Analysis and experiment of transient filling flow into a rectangular microchannel on a rotating disk. Microfluid. Nanofluid. 2016, 20, 52. [Google Scholar] [CrossRef]
- Soroori, S.; Rodriguez-Delgado, J.M.; Kido, H.; Dieck-Assad, G.; Madou, M.; Kulinsky, L. The use of polybutene for controlling the flow of liquids in centrifugal microfluidic systems. Microfluid. Nanofluid. 2016, 20, 26. [Google Scholar] [CrossRef]
- Godino, N.; Vereshchagina, E.; Gorkin, R.; Ducrée, J. Centrifugal automation of a triglyceride bioassay on a low-cost hybrid paper-polymer device. Microfluid. Nanofluid. 2014, 16, 895–905. [Google Scholar] [CrossRef]
- Lee, B.; Lee, J.N.; Park, J.M.; Lee, J.G.; Kim, S.; Cho, Y.K.; Ko, C. A fully automated immunoassay from whole blood on a disc. Lab Chip 2009, 9, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Huang, P.C.; Lin, M.G. Analysis and experiment of capillary valves for microfluidics on a rotating disk. Microfluid. Nanofluid. 2008, 4, 427–437. [Google Scholar] [CrossRef]
- Lim, D.; Yoo, J.C. Chemical Cell Lysis System Applicable to Lab-on-a-Disc. Appl. Biochem. Biotechnol. 2017, 183, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Yoo, J.C. Automated Centrifugal-Microfluidic Platform for DNA Purification Using Laser Burst Valve and Coriolis Effect. Appl. Biochem. Biotechnol. 2015, 175, 3778–3787. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Belgrader, P.; Young, S.; Yuan, B.; Primeau, M.; Christel, L.A.; Pourahmadi, F.; Northrup, M.A. A Battery-Powered Notebook Thermal Cycler for Rapid Multiplex Real-Time PCR Analysis. Anal. Chem. 2001, 73, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Peng, N.; Li, L.; Li, Z.; Hu, F.; Zhang, Z.; Wang, C. Centrifugal Microfluidic System for Nucleic Acid Amplification and Detection. Sensors 2015, 15, 27954–27968. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.; Stewart, G.; Mounier, M.; Malic, L.; Peytavi, R.; Clime, L.; Veres, T. From cellular lysis to microarray detection, an integrated thermoplastic elastomer (TPE) point of care Lab on a Disc. Lab Chip 2015, 15, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, Y.; Kim, H.S.; Kim, T.H.; Park, J.; Lee, J.G.; Cho, Y.K. Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood. Lab Chip 2011, 11, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Nakajima, H.; Itoh, K.; Arakawa, E.; Inoue, M. Degradation of a Polymerase Chain Reaction (PCR) Product by Heat-Stable Deoxyribonuclease (DNase) Produced from Yersinia enterocolitica. Microbiol. Immunol. 1994, 38, 153–156. [Google Scholar] [CrossRef] [PubMed]


| Step | Procedure | Laser ON/OFF (s) | Time (s) |
|---|---|---|---|
| 1 | Load the sample into the main chamber | - | - |
| 2 | Spin the LOD-1500 rpm (To move the liquid remaining in the channel to the main chamber) | - | 30 |
| 3 | Ethylene Vinyl Acetate Valve closing | ON | 60 |
| 4 | Automated lysis system-reach to 60 °C | ON | 90~100 |
| 5 | Automated lysis system-retain 60 °C | 2 s/4.5 s | 600 |
| 6 | Laser burst valve opening | ON | 10 |
| 7 | Spin the LOD-5000 rpm (the lysis solution moves to out chamber) | - | 30 |
| Sample Number | Proposed LOD | Spin Column | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A260 | A280 | A320 | Purity | Yield | A260 | A280 | A320 | Purity | Yield | |
| 1 | 0.385 | 0.209 | 0.029 | 1.982 | 17.800 | 0.301 | 0.164 | 0.006 | 1.864 | 14.755 |
| 2 | 0.419 | 0.222 | 0.027 | 2.011 | 19.600 | 0.359 | 0.201 | 0.022 | 1.881 | 16.830 |
| 3 | 0.295 | 0.163 | 0.014 | 1.888 | 14.045 | 0.350 | 0.192 | 0.025 | 1.946 | 16.270 |
| 4 | 0.285 | 0.154 | 0.008 | 1.895 | 13.870 | 0.374 | 0.196 | 0.007 | 1.938 | 18.375 |
| 5 | 0.351 | 0.205 | 0.008 | 1.747 | 17.130 | 0.495 | 0.244 | 0.010 | 2.072 | 24.265 |
| 6 | 0.401 | 0.219 | 0.010 | 1.876 | 19.555 | 0.471 | 0.255 | 0.016 | 1.903 | 22.710 |
| 7 | 0.411 | 0.218 | 0.006 | 1.912 | 20.260 | 0.284 | 0.166 | 0.028 | 1.850 | 12.805 |
| 8 | 0.291 | 0.165 | 0.016 | 1.839 | 13.730 | 0.242 | 0.134 | 0.014 | 1.894 | 11.375 |
| 9 | 0.377 | 0.216 | 0.030 | 1.865 | 17.340 | 0.306 | 0.171 | 0.024 | 1.920 | 14.105 |
| 10 | 0.249 | 0.148 | 0.024 | 1.815 | 11.245 | 0.347 | 0.187 | 0.017 | 1.944 | 16.455 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-J.; Yoo, J.-C. Lab-on-a-Disc Platform for Automated Chemical Cell Lysis. Sensors 2018, 18, 687. https://doi.org/10.3390/s18030687
Seo M-J, Yoo J-C. Lab-on-a-Disc Platform for Automated Chemical Cell Lysis. Sensors. 2018; 18(3):687. https://doi.org/10.3390/s18030687
Chicago/Turabian StyleSeo, Moo-Jung, and Jae-Chern Yoo. 2018. "Lab-on-a-Disc Platform for Automated Chemical Cell Lysis" Sensors 18, no. 3: 687. https://doi.org/10.3390/s18030687




