Estimation of Handgrip Force from SEMG Based on Wavelet Scale Selection
Abstract
:1. Introduction
2. Experimentation and Methodology
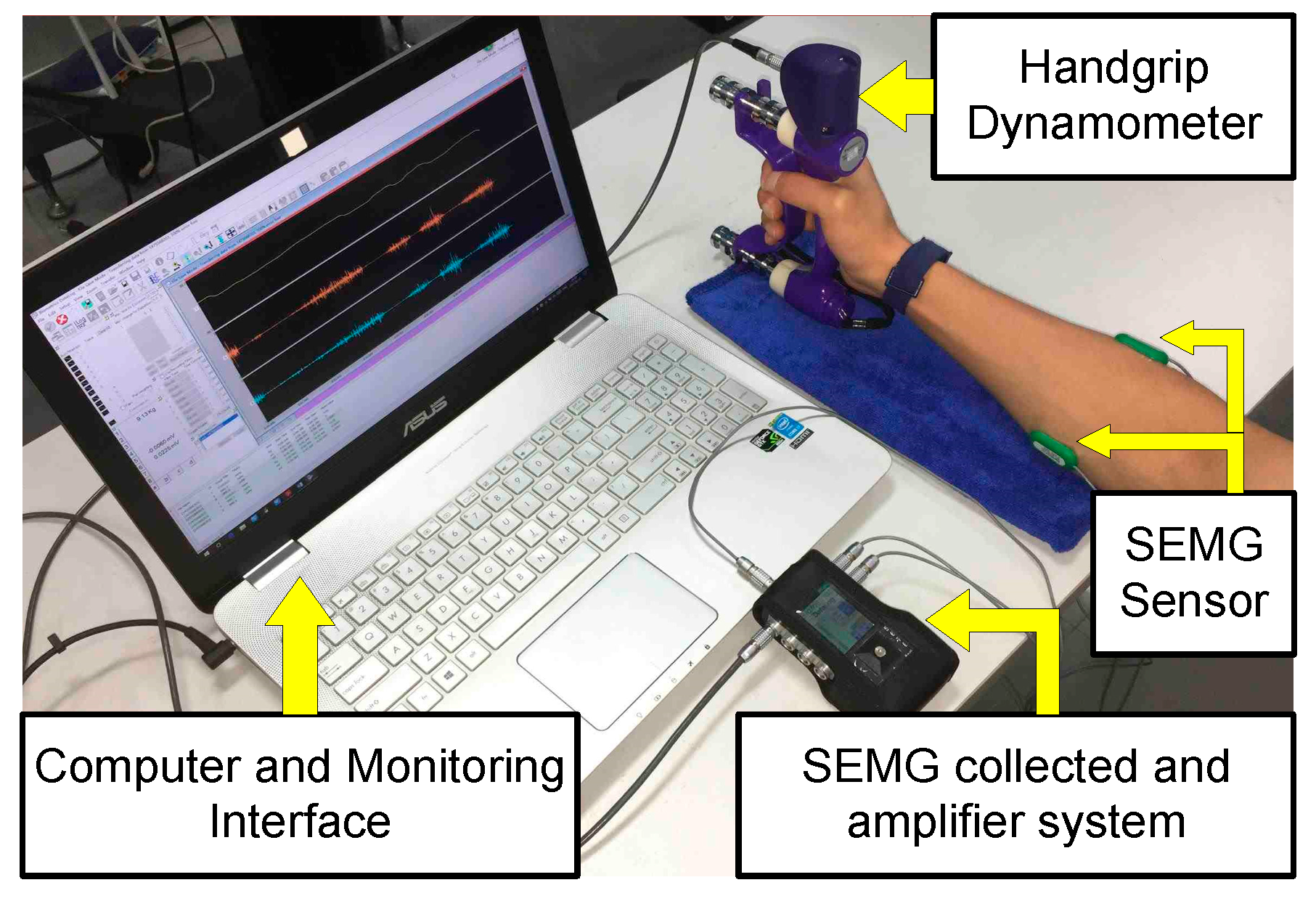
2.1. Experimental Setup
2.2. Experimental Procedure
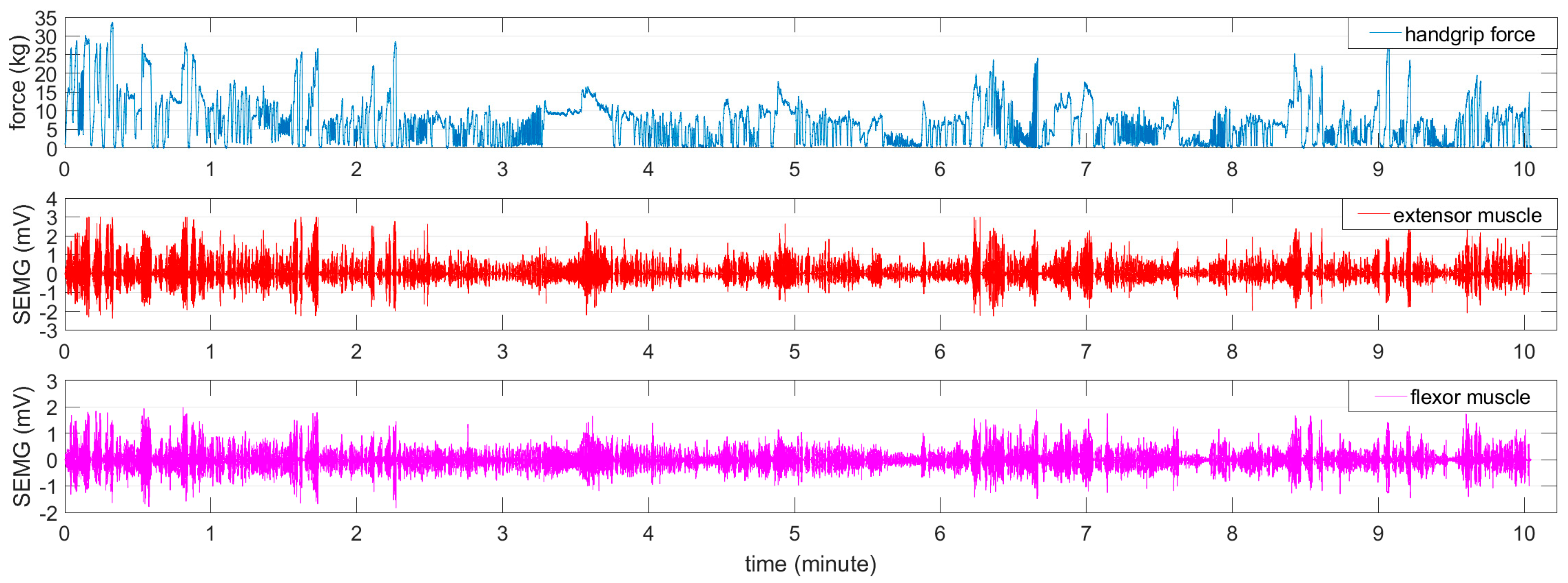
2.2.1. Force-Varying Analysis Task (Force-Varying Contraction)
2.2.2. Static Validation Task (Isometric Muscle Contraction)
2.2.3. Force-Varying Validation Task (Voluntary Contraction)
2.3. Proposed Method
2.3.1. Initial Nonlinear SEMG-Handgrip Force Relationship
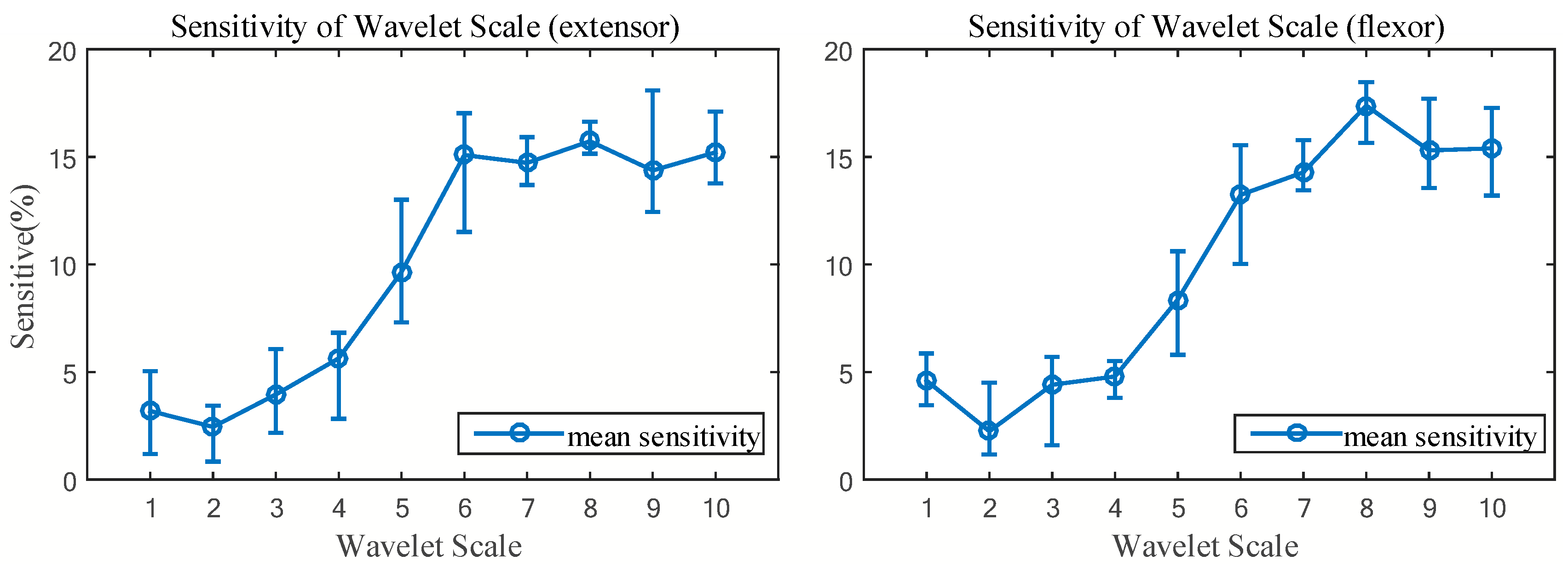
2.3.2. Sensitivity Analysis
- (1)
- CWT was used to convert the original SEMG signals to wavelet coefficients of the ten scales in the force-varying analysis task.
- (2)
- Wavelet coefficients of the ten scales normalized by MVC data were used to build the initial nonlinear SEMG-handgrip force relationship.
- (3)
- A specific number of random variables were used to conduct a simulation of the handgrip force in the initial nonlinear relationship.
- (4)
- Mean square deviation was used to calculate the simulated sensitivity value corresponding to each correlation between ten scales and handgrip force.
- (5)
- The sensitivity value of each correlation was normalized by the sum of the sensitivity values of the ten scales.
- (6)
- Steps 1–5 were repeated for every minute in the force-varying analysis task.
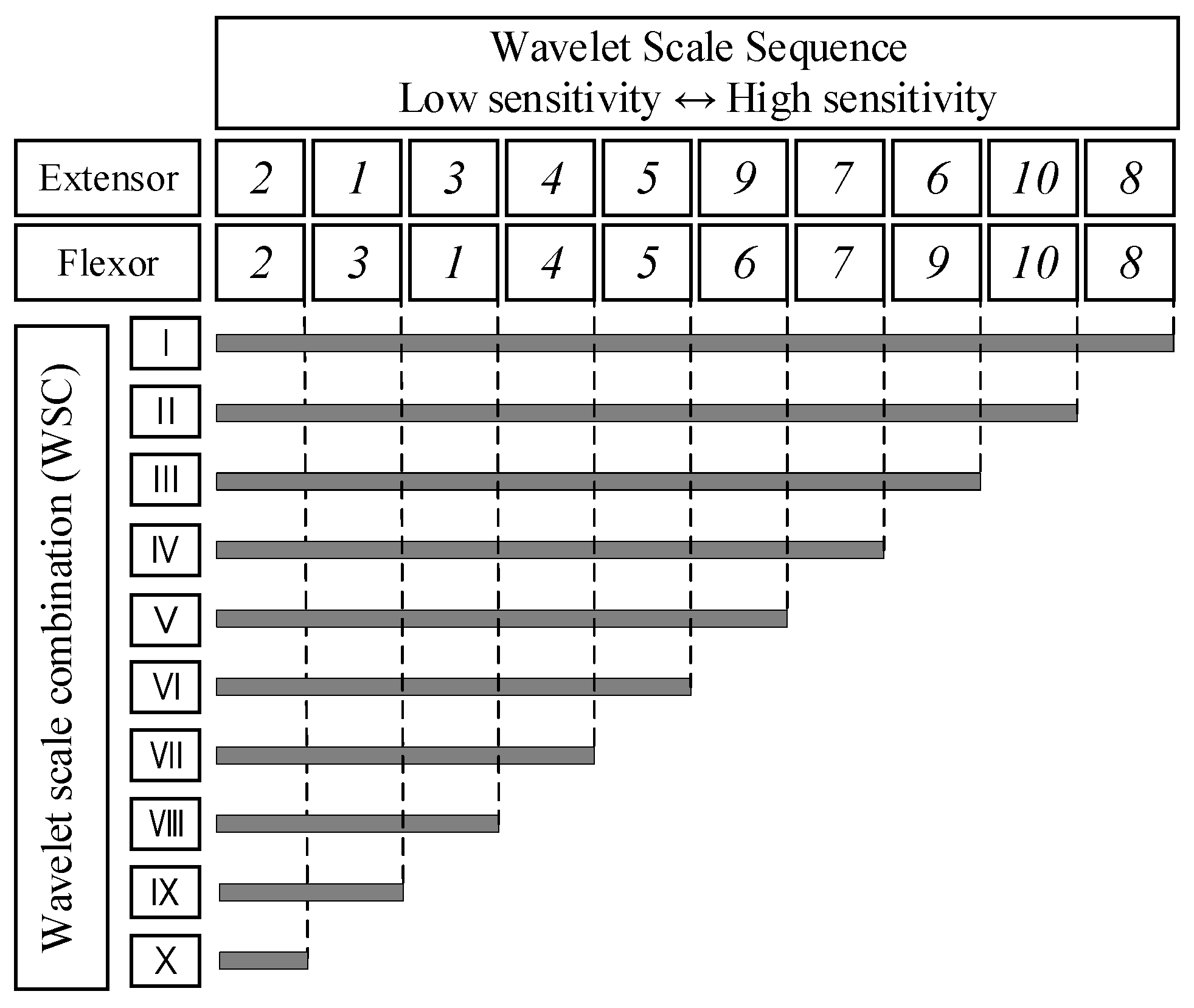
2.3.3. Sequence Combination Analysis (SCA)
2.4. Performance Comparison
2.4.1. Former Method
2.4.2. Performance Index
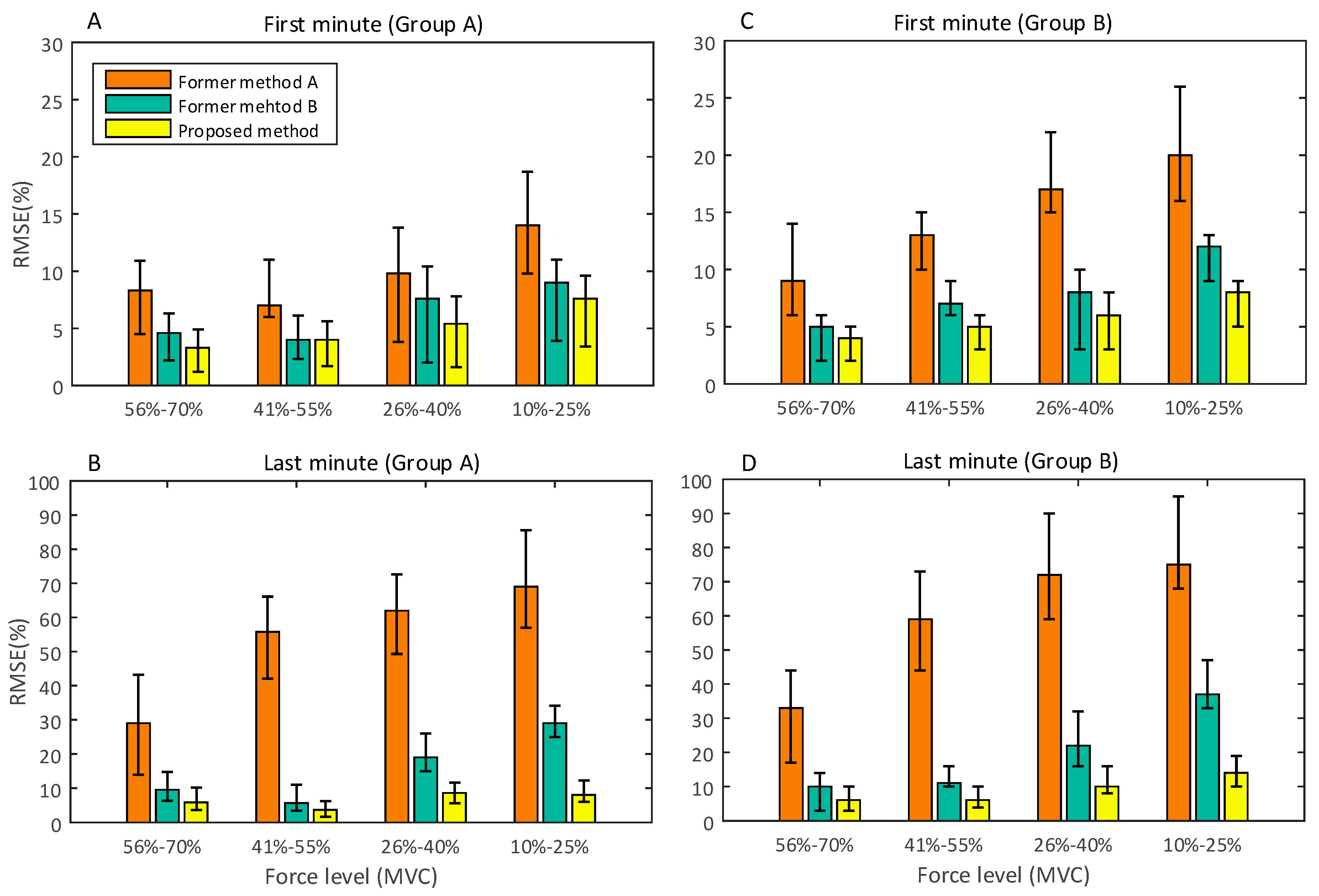
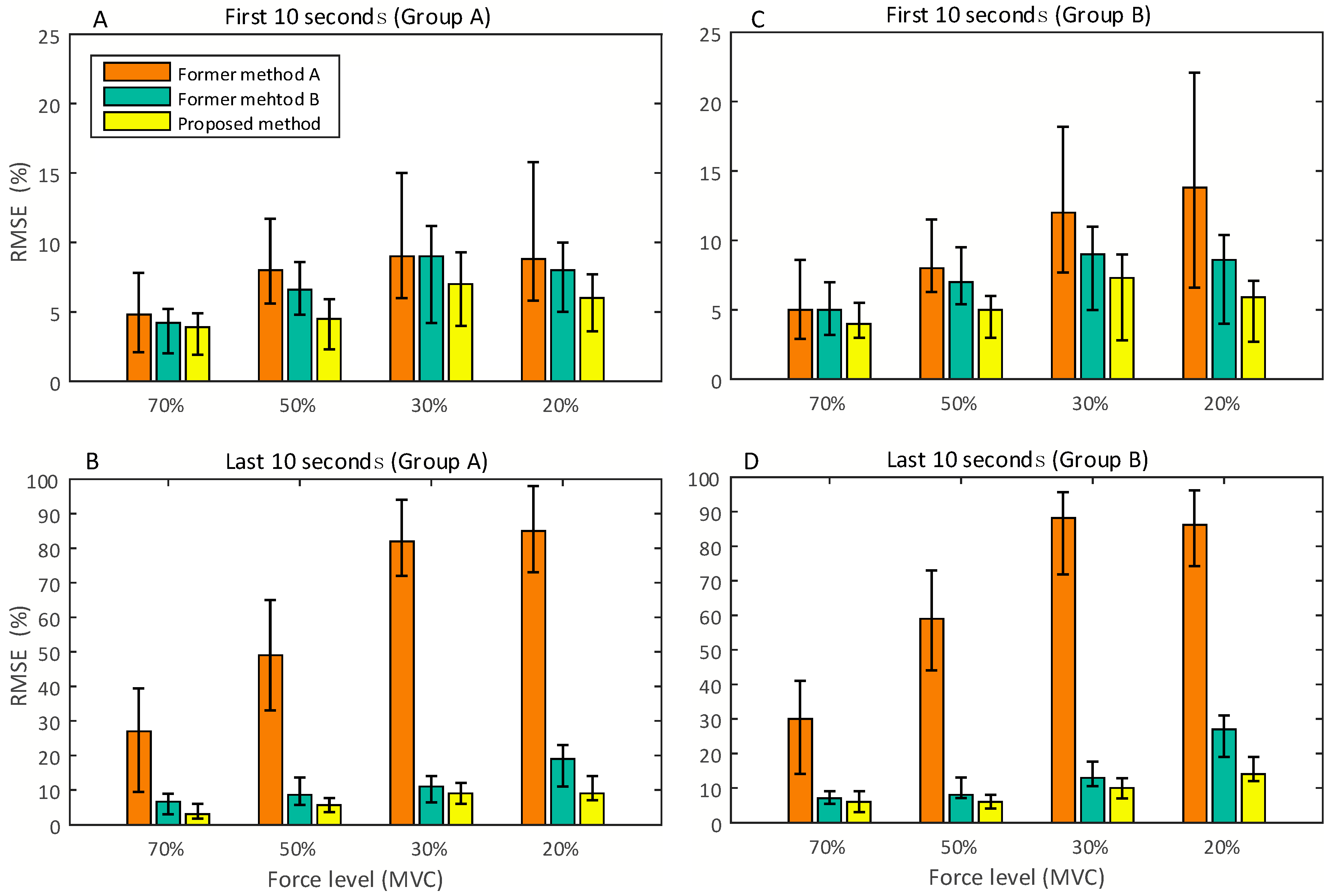
3. Results and Discussion
3.1. Nonlinear Correlation between Wavelet Scale and Handgrip Force
3.2. Selection of Wavelet Scale Combination
3.3. Performance Comparison
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieën, J.H. Methodological aspects of SEMG recordings for force estimation—A tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Scheme, E.; Englehart, K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Daley, H.; Englehart, K.; Hargrove, L.; Kuruganti, U. High density electromyography data of normally limbed and transradial amputee subjects for multifunction prosthetic control. J. Electromyogr. Kinesiol. 2012, 22, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hoozemans, M.J.M.; van Dieen, J.H. Prediction of handgrip forces using surface EMG of forearm muscles. J. Electromyogr. Kinesiol. 2005, 15, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Soo, Y.; Sugi, M.; Yokoi, H.; Arai, T.; Nishino, M.; Kato, R.; Nakamura, T.; Ota, J. Estimation of handgrip force using frequency-band technique during fatiguing muscle contraction. J. Electromyogr. Kinesiol. 2010, 20, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Brown, R.; Yue, G. A dynamical model of muscle activation, fatigue, and recovery. Biophys. J. 2002, 82, 2344–2359. [Google Scholar] [CrossRef]
- Edwards, R.H. Human muscle function and fatigue. Ciba Found. Symp. 1981, 82, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Marco, G.; Alberto, B.; Taian, V. Surface EMG and muscle fatigue: Multi-Channel approaches to the study of myoelectric manifestations of muscle fatigue. Physiol. Meas. 2017, 38, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; Bisi, M.C.; Stagni, R.; Botter, A. Changes in tibialis anterior architecture affect the amplitude of surface elexctromyograms. J. Neuroeng. Rehabil. 2017, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Klaver-krol, E.G.; Rasker, J.J.; Henriquez, N.R.; Verheijen, W.G.; Zwarts, M.J. Muscle fiber velocity and electromyographic signs of fatigue in fibromyalgia. Muscle Nerve 2012, 46, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Krou-Lund, C.; Jørgensen, K. Myo-electric fatigue manifestations revieited: Power spectrum, conduction velocity, and amplitude of human elbow flexor muscles during isolated and repetitive endurance contractions at 30% maximal voluntary contraction. Eur. J. Appl. Physiol. 1993, 66, 161–173. [Google Scholar] [CrossRef]
- Basmajian, J.V.; De Luca, C.J. Muscles Alive: Their Functions Revealed by Electromyography; Williams and Wilkins: Baltimore, MD, USA, 1985; pp. 201–222. [Google Scholar]
- Bonato, P.; Roy, S.H.; Knaflitz, M.; De Luca, C.J. Time–frequency parameters of the surface myoelectric signal for assessing muscle fatigue during cyclic dynamic contractions. IEEE Trans. Biomed. Eng. 2001, 48, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Knaflitz, M.; Bonato, P.; Actis, M.V. Electrical manifestations of muscle fatigue during concentric and eccentric isokinetic knee flexion–extension movements. IEEE Trans. Biomed. Eng. 2006, 53, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Farina, D. Interpretation of the surface electromyogram in dynamic contractions. Exerc. Sport Sci. Rev. 2006, 34, 121–127. [Google Scholar] [CrossRef] [PubMed]
- González-Izal, M.; Rodríguez-Carreño, I.; Malanda, A.; Mallor-Giménez, F.; Navarro-Amézqueta, I.; Gorostiaga, E.M.; Izquierdo, M. sEMG wavelet-based indices predicts muscle power loss during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Sadoyama, T.; Miyano, H. Frequency analysis of surface EMG to evaluation of muscle fatigue. Eur. J. Appl. Physiol. 1981, 47, 239–246. [Google Scholar] [CrossRef]
- Merletti, R.; Lo, C.L.R.; Orizio, C. Indices of muscle fatigue. J. Electromyogr. Kinesiol. 1991, 1, 20–33. [Google Scholar] [CrossRef]
- Gallina, A.; Merletti, R.; Vieira, T.M.M. Are the myoelectric manifestations of fatigue distributed regionally in the human medial gastrocnemius muscle? J. Electromyogr. Kinesiol. 2011, 21, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Leclerc, F.; Arendt-Nielsen, L.; Buttelli, O.; Madeleine, P. The change in spatial distribution of upper trapezius muscle activity is correlated to contraction duration. J. Electromyogr. Kinesiol. 2008, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.; Fujiwara, T. The relationship between EMG median frequency and low frequency band amplitude changes at different levels of muscle capacity. Clin. Biomech. 2002, 17, 464–469. [Google Scholar] [CrossRef]
- Kumar, D.K.; Pah, N.D.; Bradley, A. Wavelet analysis of surface electromyography to determine muscle fatigue. IEEE Trans. Neural Syst. Rehabilit. 2003, 11, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Yu, J.; Akay, M. Time–frequency analysis of myoelectric signals during dynamic contractions: A comparative study. IEEE Trans. Biomed. Eng. 2000, 47, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Sparto, P.J.; Parnianpour, M.; Jagadeesh, J.M. Wavelet analysis of electromyography for back muscle fatigue detection during dynamic constant-torque exertions. Spine 1999, 24, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.G.; Valero-Cuevas, F.J. Experimentally Valid Predictions of Muscle Force and EMG in Models of Motro-Unit Function Are Most Sensitive to Neural Properties. J. Neurophysiol. 2007, 98, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, X.; Li, H.; Wei, J.; Fatikow, S. Nonlinear analysis and optimal design of a novel piezoelectric-driven compliant microgripper. Mech. Mach. Theory 2017, 118, 32–78. [Google Scholar] [CrossRef]
- Mogk, J.; Keir, P. The effects of posture on forearm muscle loading during gripping. Ergonomics 2003, 46, 956–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, Y.; Zhang, X. Estimation of Handgrip Force from Nonlinear SEMG-Force Relationship during Dynamic Contraction Tasks. In Proceedings of the IEEE International Conference on Robotics and Biomimetics, Macau, China, 5–8 December 2017; pp. 412–417. [Google Scholar]
- Lawrence, J.H.; De Luca, C.J. Myoelectric signal versus force relationship in different human muscle. J. Appl. Physiol. 1983, 54, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Christopher, T.; Gilbert, P.A. Practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Asefi, M.; Moghimi, S.; Kalani, H.; Moghimi, A. Dynamic modeling of SEMG-force relation in the presence of muscle fatigue during isometric contractions. Biomed. Signal Process. 2016, 28, 41–49. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Kaya, M.; Temple, G.K.; Johnston, I.A.; Herzog, W. Determining patterns of motor recruitment during locomotion. J. Exp. Biol. 2002, 205, 359–369. [Google Scholar] [PubMed]
- Begg, R.; Lai, D.T.H.; Palaniswami, M. Computational Intelligence in Biomedical Engineering; CRC: New York, NY, USA, 2007. [Google Scholar]
- Henneman, E.; Clamann, H.P.; Gillies, J.D.; Skinner, R.D. Rank order of motoneurons within a pool: Law of combination. J. Neurophysiol. 1974, 37, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Solnik, S.; Gruder, A.; Rider, P.; Steinweg, K.; Helseth, J.; DeVita, P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture 2009, 29, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Nardo, F.D.; Maranesi, E.; Mengarelli, A.; Ghetti, G.; Burattini, L.; Fioretti, S. Assessment of the variability of vastii myoelectric activity in young healthy females during walking: A statistical gait analysis. J. Electromyogr. Kinesiol. 2015, 25, 800–807. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Zhang, X.; Ota, J.; Huang, Y. Estimation of Handgrip Force from SEMG Based on Wavelet Scale Selection. Sensors 2018, 18, 663. https://doi.org/10.3390/s18020663
Wang K, Zhang X, Ota J, Huang Y. Estimation of Handgrip Force from SEMG Based on Wavelet Scale Selection. Sensors. 2018; 18(2):663. https://doi.org/10.3390/s18020663
Chicago/Turabian StyleWang, Kai, Xianmin Zhang, Jun Ota, and Yanjiang Huang. 2018. "Estimation of Handgrip Force from SEMG Based on Wavelet Scale Selection" Sensors 18, no. 2: 663. https://doi.org/10.3390/s18020663



