Using a Second Order Sigma-Delta Control to Improve the Performance of Metal-Oxide Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
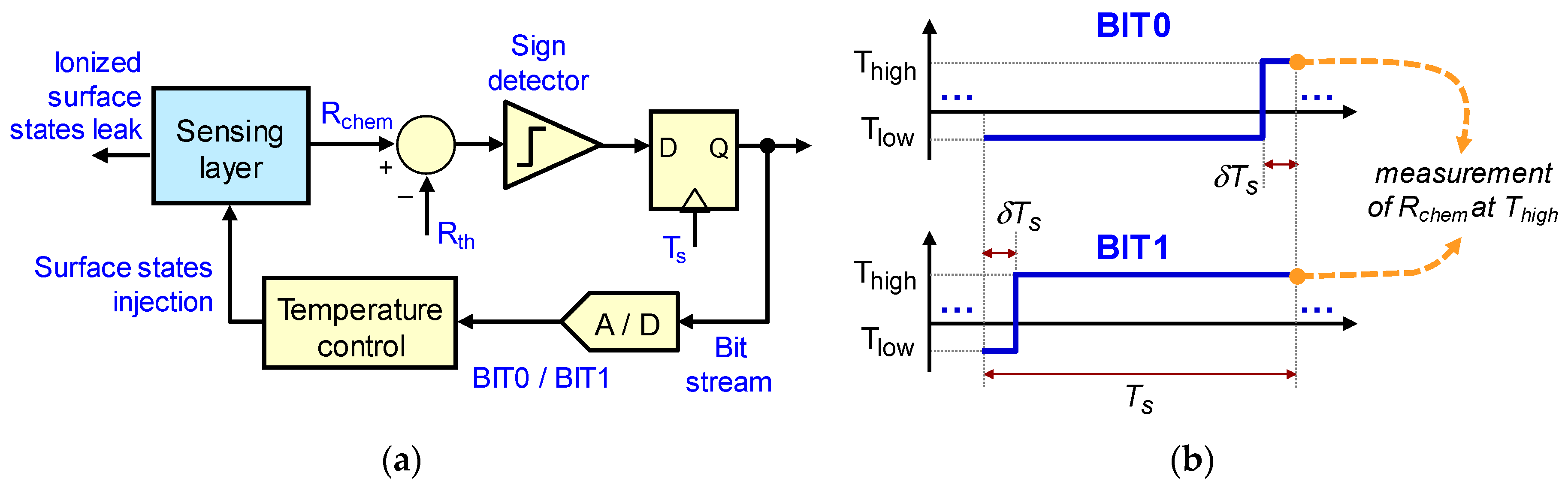
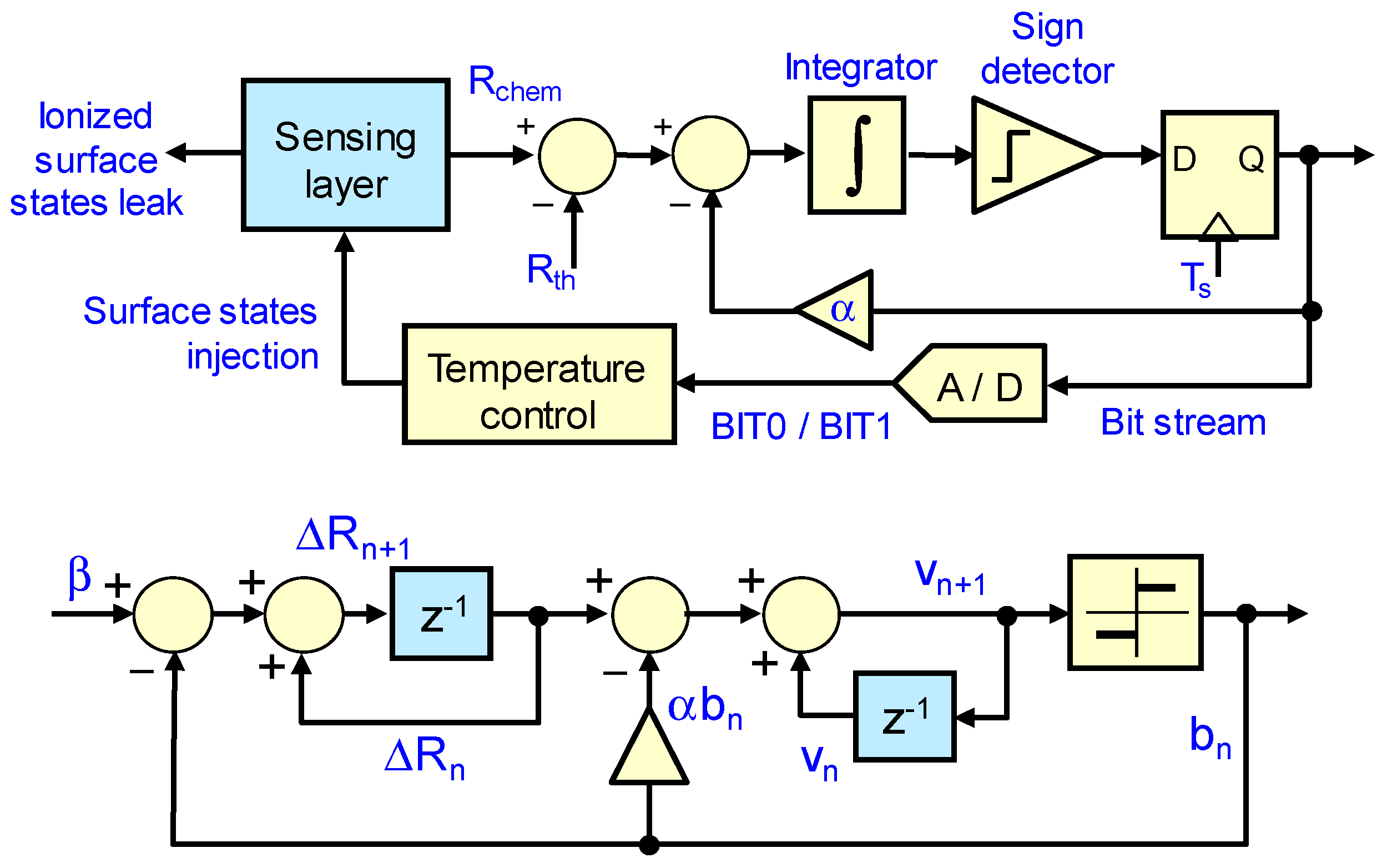
2.1. First and Second Order Sigma-Delta Loops for Gas Sensing
2.2. Description of the Gas Sensors
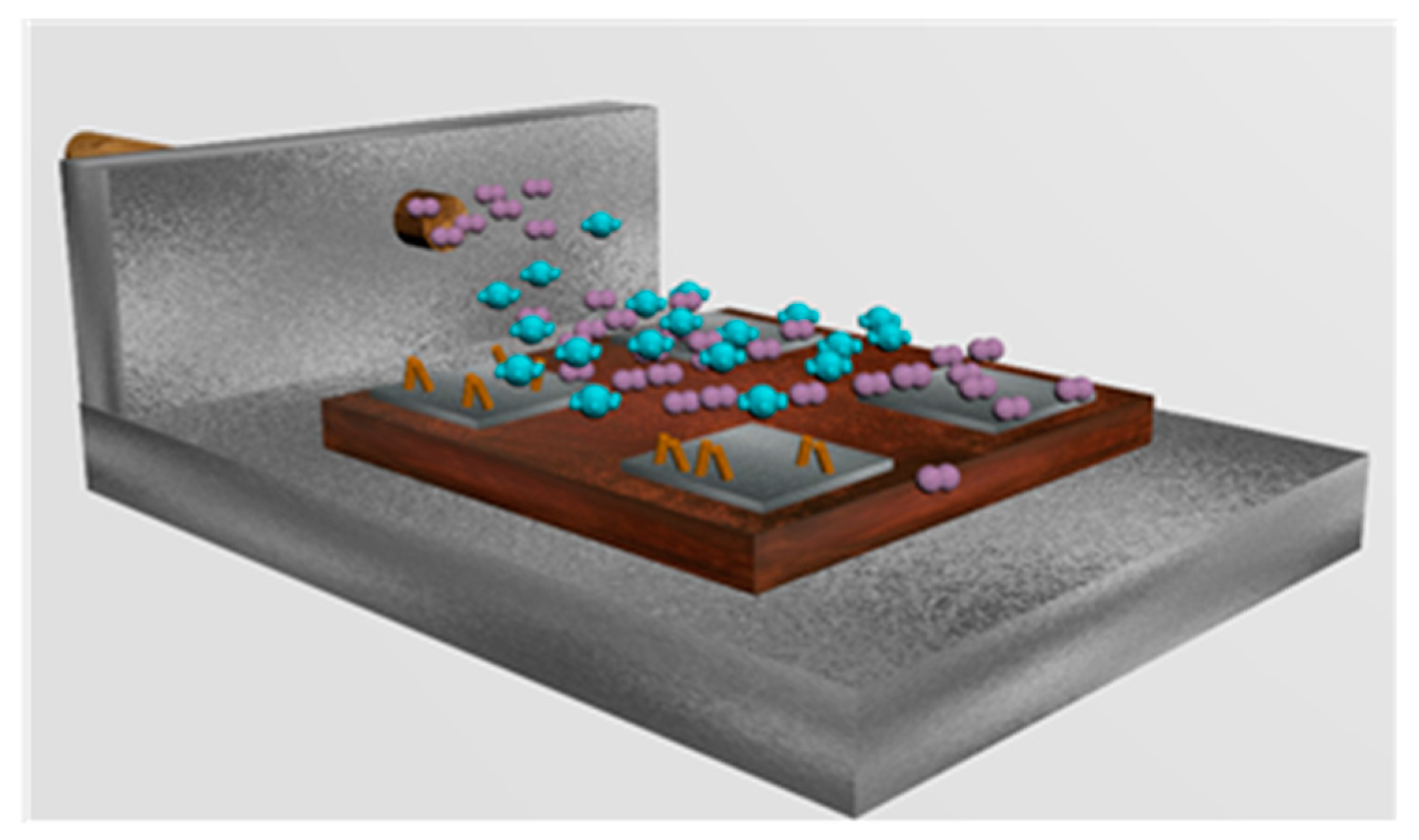
2.2.1. Sensing Layer Synthesis
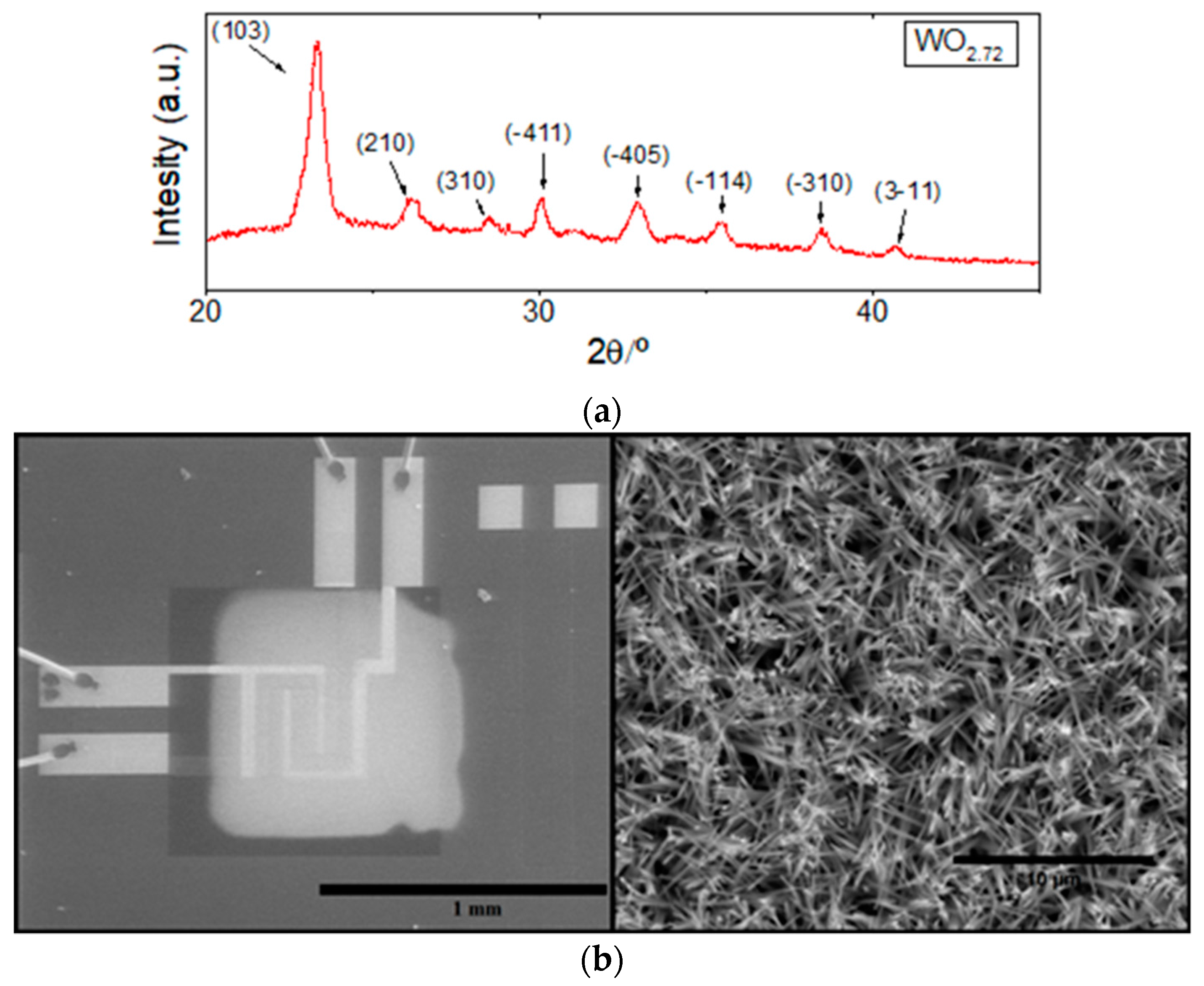
2.2.2. Material Characterization
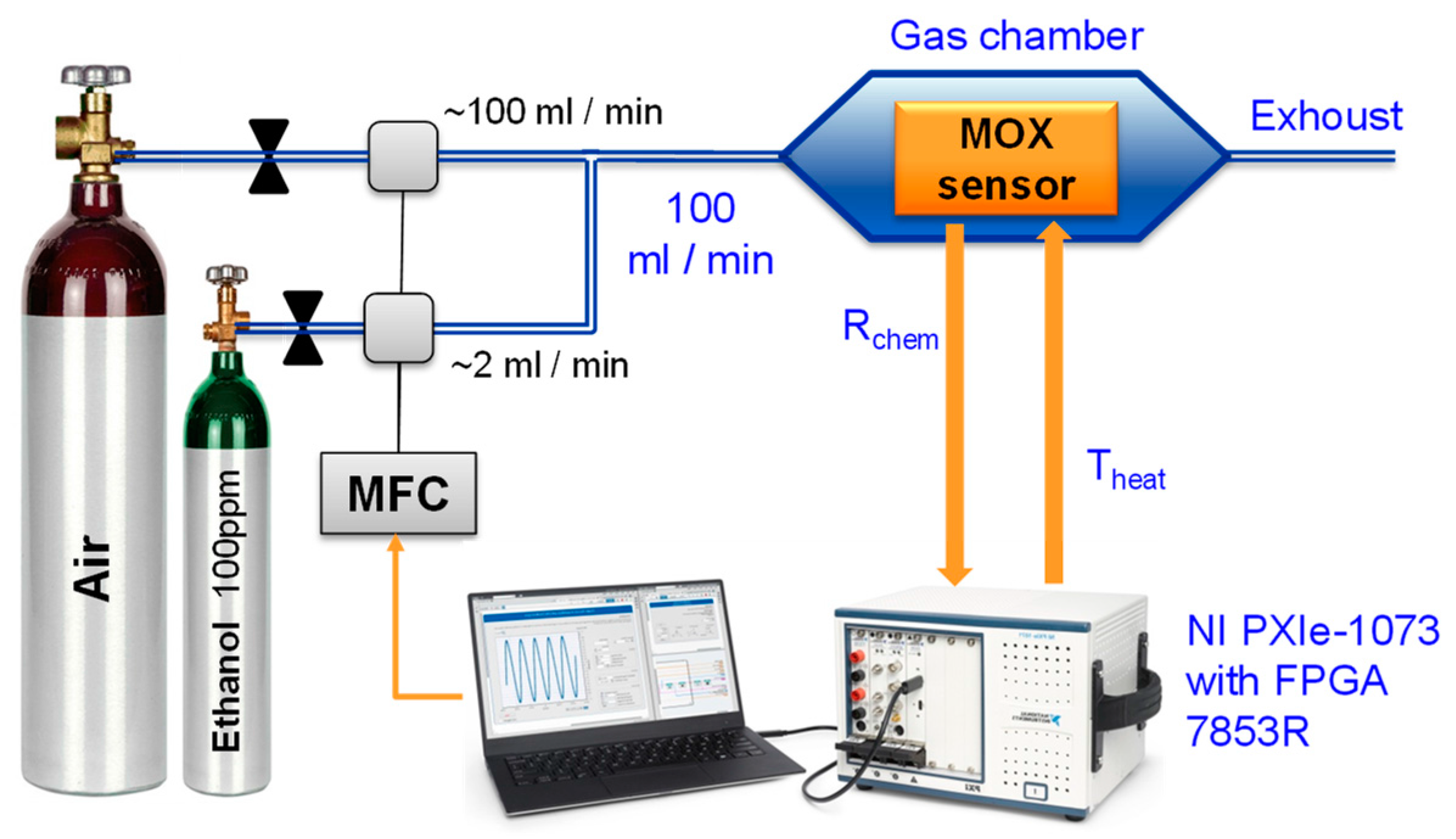
2.3. Experimental Setup
3. Results and Discussion
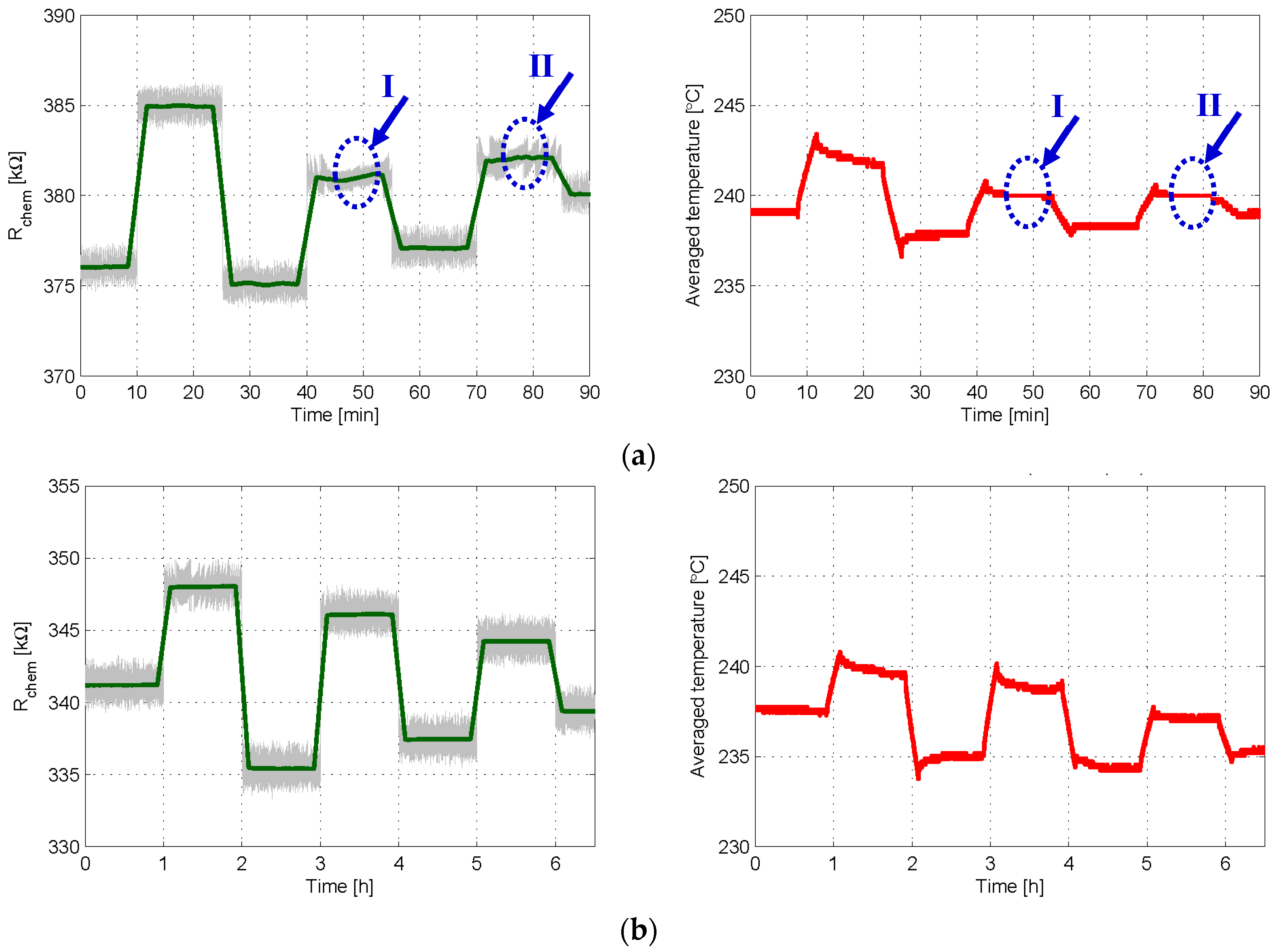
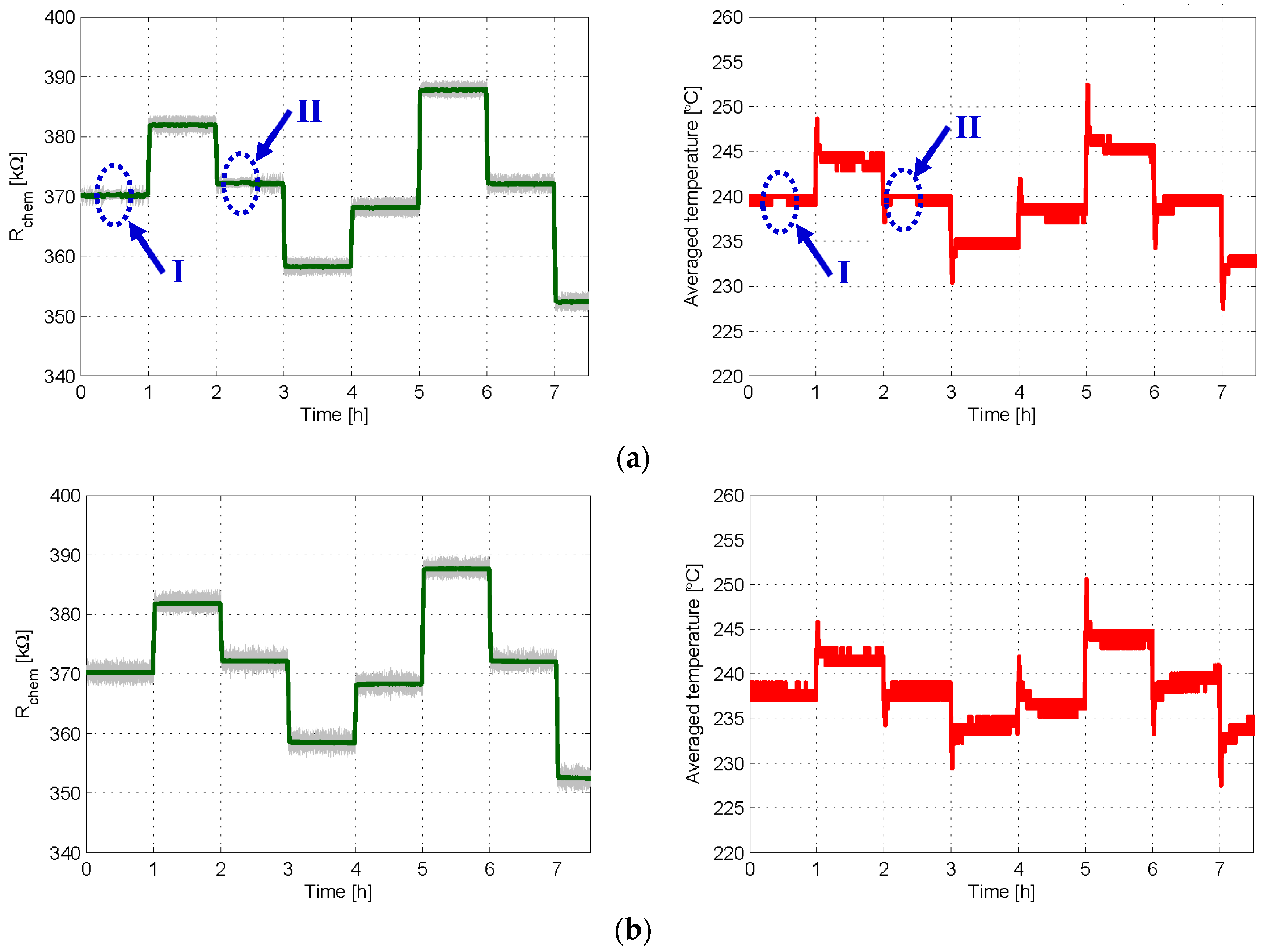
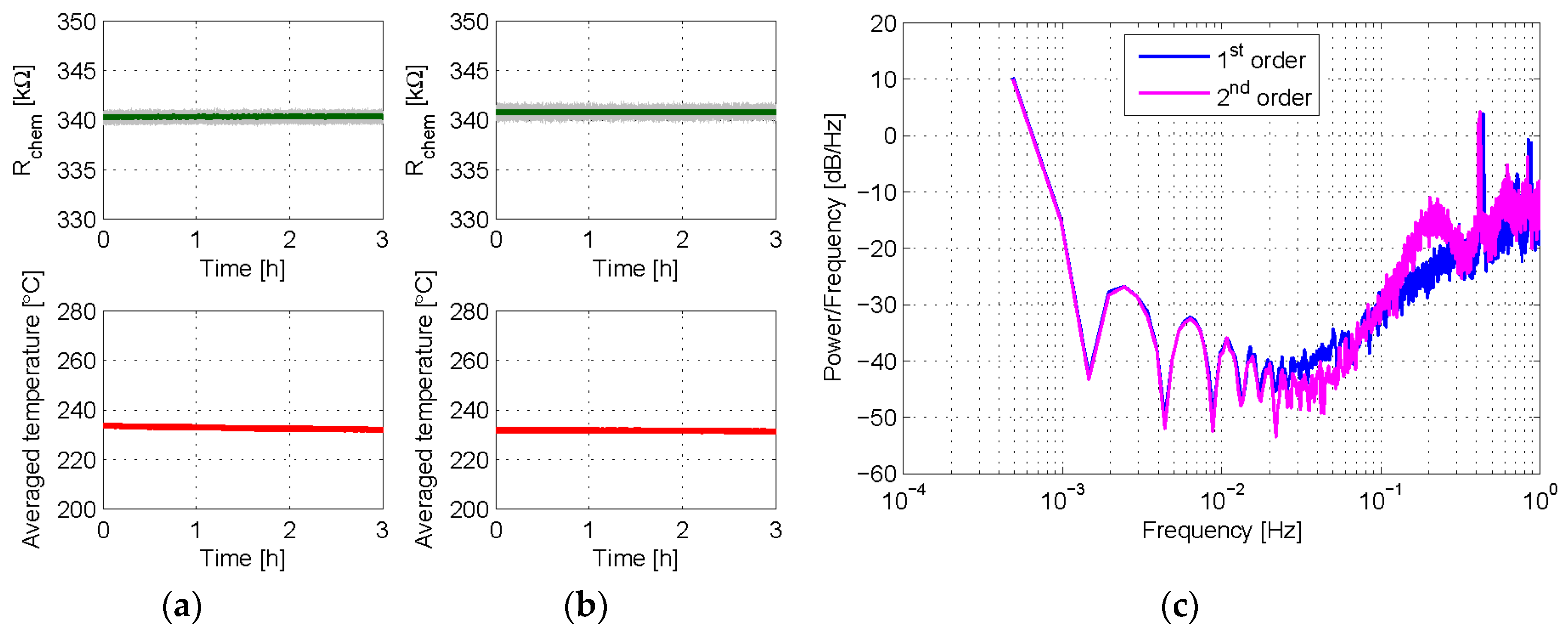
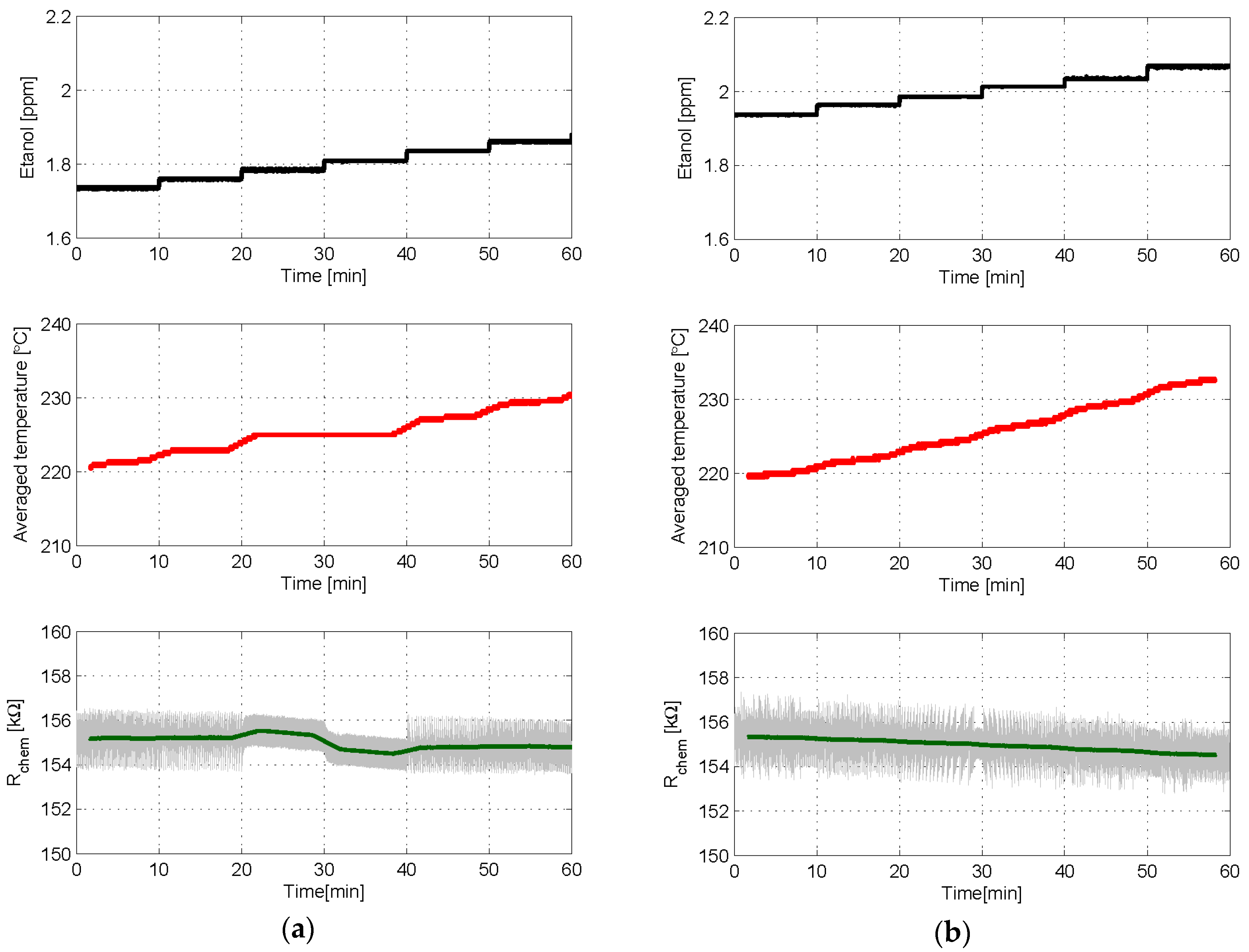
3.1. Experiment Set 1—Chemical Resistance Control with First and Second Order Sigma-Delta Loops
3.2. Experiment Set 2—Gas Sensing with First and Second Order Sigma-Delta Loops
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sberveglieri, G. Recent developments in semiconducting thin-film gas sensors. Sens. Actuators B Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Li, Q.; Liu, H.; Li, Y. A review of recent developments in tin dioxide composites for gas sensing application. J. Ind. Eng. Chem. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Capone, S.; Zuppa, M.; Presicce, D.S.; Francioso, L.; Casino, F.; Siciliano, P. Metal oxide gas sensor array for the detection of diesel fuel in engine oil. Sens. Actuators B Chem. 2008, 131, 125–133. [Google Scholar] [CrossRef]
- Fleischer, M. Advances in application potential of adsorptive-type solid state gas sensors: High-temperature semiconducting oxides and ambient temperature GasFET devices. Meas. Sci. Technol. 2008, 19, 042001. [Google Scholar] [CrossRef]
- Ng, K.T.; Boussaid, F.; Bermak, A. A CMOS single-chip gas recognition circuit for metal oxide gas sensor arrays. IEEE Trans. Circ. Syst. I 2011, 58, 1569–1580. [Google Scholar] [CrossRef]
- Vuong, N.M.; Kim, D. Surface gas sensing kinetics of a WO3 nanowire sensor: Part 1—Oxidizing gases. Sens. Actuators B Chem. 2015, 220, 932–941. [Google Scholar] [CrossRef]
- Llobet, E.; Brezmes, J.; Vilanova, X.; Sueiras, J.E.; Correig, X. Qualitative and quantitative analysis of volatile organic compounds using transient and steady-state responses of a thick-film tin oxide gas sensor array. Sens. Actuators B Chem. 1997, 41, 13–21. [Google Scholar] [CrossRef]
- Di Natale, C.; Marco, S.; Davide, F.; D’Amico, A. Sensor-array calibration time reduction by dynamic modelling. Sens. Actuators B Chem. 1995, 25, 578–583. [Google Scholar] [CrossRef]
- Pardo, A.; Marco, S.; Samitier, J. Nonlinear inverse dynamic models of gas sensing systems based on chemical sensor arrays for quantitative measurements. IEEE Trans. Instrum. Meas. 1998, 47, 644–651. [Google Scholar] [CrossRef]
- Gosangi, R.; Gutiérrez-Osuna, R. Active temperature modulation of metal-oxide sensors for quantitative analysis of gas mixtures. Sens. Actuators B Chem. 2013, 185, 201–210. [Google Scholar] [CrossRef]
- Fonollosa, J.; Sheik, S.; Huerta, R.; Marco, S. Reservoir computing compensates slow response of chemo sensor arrays exposed to fast varying gas concentrations in continuous monitoring. Sens. Actuators B Chem. 2015, 215, 618–629. [Google Scholar] [CrossRef]
- De Vito, S.; Castaldo, A.; Loffredo, F.; Massera, E.; Polichetti, T.; Nasti, I.; Vacca, P.; Quercia, L.; Di Francia, G. Gas concentration estimation in ternary mixtures with room temperature operating sensor array using tapped delay architectures. Sens. Actuators B Chem. 2007, 124, 309–316. [Google Scholar] [CrossRef]
- Vergara, A.; Martinelli, E.; Llobet, E.; D’Amico, A.; Di Natale, C. Optimized feature extraction for temperature-modulated gas sensors. J. Sens. 2009, 2009, 716316. [Google Scholar] [CrossRef]
- Vergara, A.; Llobet, E.; Brezmes, J.; Vilanova, X.; Ivanov, P.; Gràcia, I.; Cané, C.; Correig, X. Optimized temperature modulation of micro-hotplate gas sensors through pseudo-random binary sequences. IEEE Sens. J. 2005, 5, 1369–1378. [Google Scholar] [CrossRef]
- Sudarmaji, A.; Kitagawa, A. Application of temperature modulation-SDP on MOS gas sensors: Capturing soil gaseous profile for discrimination of soil under different nutrient addition. J. Sens. 2016, 2016, 1035902. [Google Scholar] [CrossRef]
- Fonollosa, J.; Fernández, L.; Huerta, R.; Gutiérrez-Gálvez, A.; Marco, S. Temperature optimization of metal oxide sensor arrays using mutual information. Sens. Actuators B Chem. 2013, 187, 331–339. [Google Scholar] [CrossRef]
- Burgués, J.; Marco, S. Low power operation of temperature-modulated Metal Oxide Semiconductor gas sensors. Sensors 2018, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Ghafarinia, V.; Maleki, M. Self-regulated bias circuits for efficient adjustment of the operating temperature of chemoresistive gas sensors. IEEE Sens. J. 2017, 17, 2984–2991. [Google Scholar] [CrossRef]
- Magno, M.; Jelicic, V.; Chikkadi, K.; Roman, C.; Hierold, C.; Bilas, V.; Benini, L. Low-power gas sensing using single walled Carbon nano tubes in wearable devices. IEEE Sens. J. 2016, 16, 8329–8337. [Google Scholar] [CrossRef]
- Domínguez-Pumar, M.; Kowalski, L.; Calavia, R.; Llobet, E. Smart control of chemical gas sensors for the reduction of their time response. Sens. Actuators B Chem. 2016, 229, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sira-Ramirez, H. Sliding Mode Control: The Delta-Sigma Modulation Approach; Birkhäuser: Basel, Switzerland, 2015; ISBN 978-3-319-17256-9. [Google Scholar]
- Gorreta, S.; Pons-Nin, J.; Blokhina, E.; Feely, O.; Domínguez-Pumar, M. Delta-sigma control of dielectric charge for contactless capacitive MEMS. J. Microelectromech. Syst. 2014, 23, 829–841. [Google Scholar] [CrossRef]
- Utkin, V.I. Sliding Modes in Control and Optimization; Springer: Heidelberg, Germany, 1992; ISBN 978-3-642-84381-5. [Google Scholar]
- Norsworthy, S.R.; Schreier, R.; Temes, G.C. Delta-Sigma Data Converters: Theory, Design, and Simulation; Wiley-IEEE Press: Piscataway, NJ, USA, 1996; ISBN 978-0-7803-1045-2. [Google Scholar]
- Makinwa, K.; Huijsing, J. A 2nd order thermal sigma-delta modulator for flow sensing. In Proceedings of the IEEE Sensors, Irvine, CA, USA, 30 October–3 November 2005. [Google Scholar] [CrossRef]
- Gorreta, S.; Pons-Nin, J.; Blokhina, E.; Domínguez-Pumar, M. A Second Order Delta-Sigma Control of Dielectric Charge for Contactless Capacitive MEMS. J. Microelectromech. Syst. 2015, 24, 259–261. [Google Scholar] [CrossRef]
- Bheesayagari, C.; Gorreta, S.; Pons-Nin, J.; Domínguez-Pumar, M. Second order sigma-delta control of charge trapping for MOS capacitors. J. Microelecron. Reliab. 2017, 76–77, 635–639. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, L.; Pons-Nin, J.; Navarrete, E.; Llobet, E.; Domínguez-Pumar, M. Using a Second Order Sigma-Delta Control to Improve the Performance of Metal-Oxide Gas Sensors. Sensors 2018, 18, 654. https://doi.org/10.3390/s18020654
Kowalski L, Pons-Nin J, Navarrete E, Llobet E, Domínguez-Pumar M. Using a Second Order Sigma-Delta Control to Improve the Performance of Metal-Oxide Gas Sensors. Sensors. 2018; 18(2):654. https://doi.org/10.3390/s18020654
Chicago/Turabian StyleKowalski, Lukasz, Joan Pons-Nin, Eric Navarrete, Eduard Llobet, and Manuel Domínguez-Pumar. 2018. "Using a Second Order Sigma-Delta Control to Improve the Performance of Metal-Oxide Gas Sensors" Sensors 18, no. 2: 654. https://doi.org/10.3390/s18020654




