Evaluating the Influence of Chromatic and Luminance Stimuli on SSVEPs from Behind-the-Ears and Occipital Areas
Abstract
:1. Introduction
2. Materials and Methods
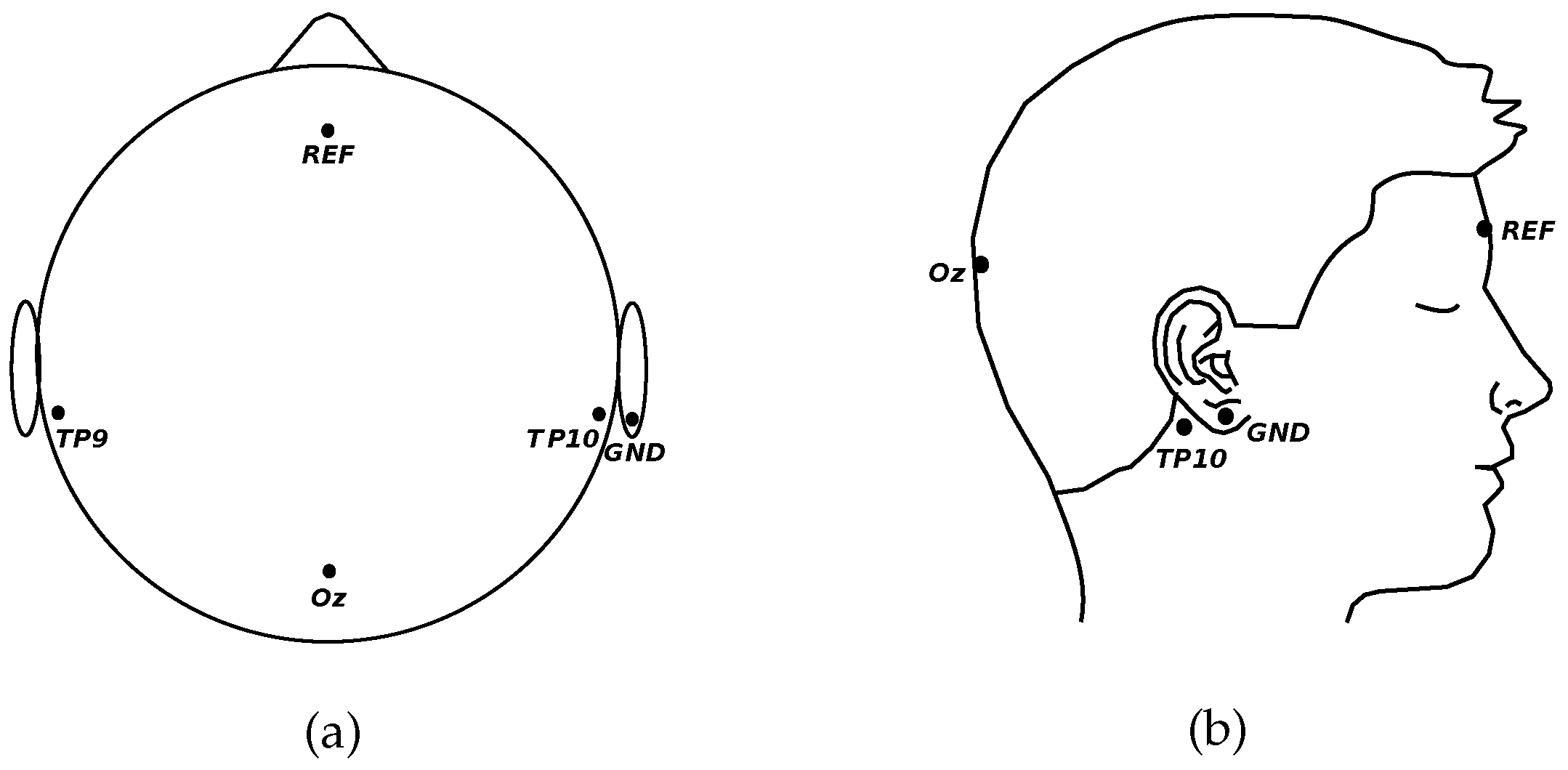
2.1. Data Acquisition
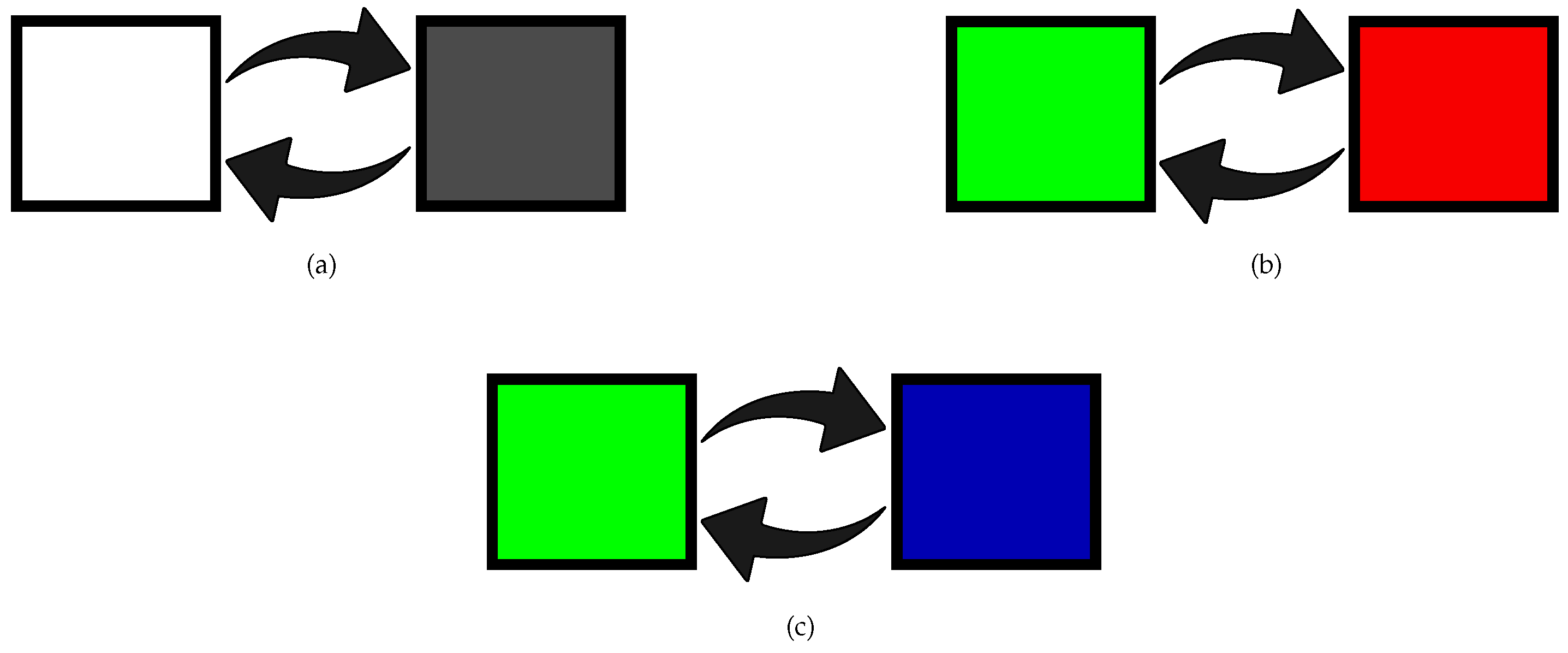
2.2. Visual Stimulation
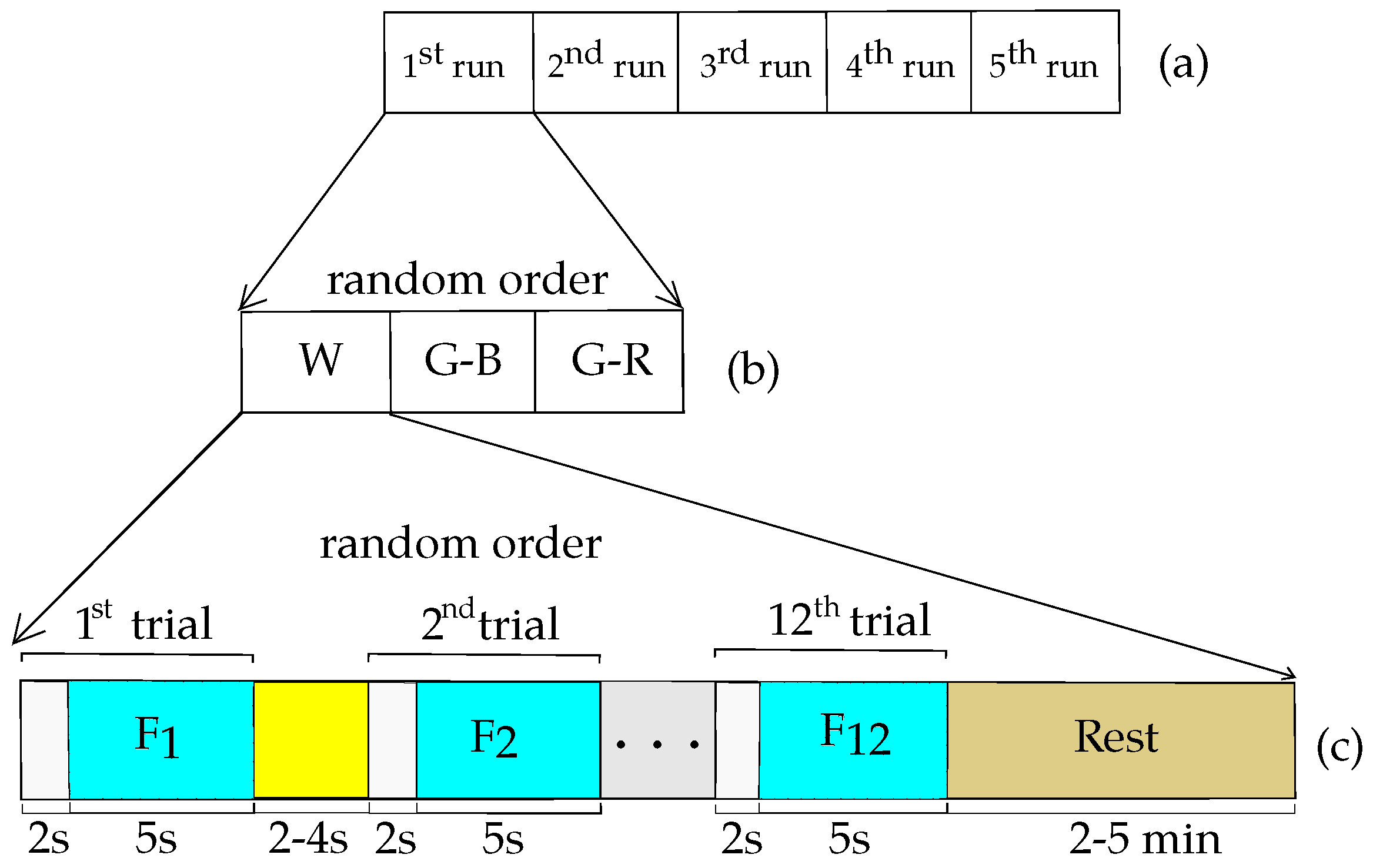
2.3. Experimental Protocol
2.4. EEG Signal Processing
2.5. Statistical Evaluation
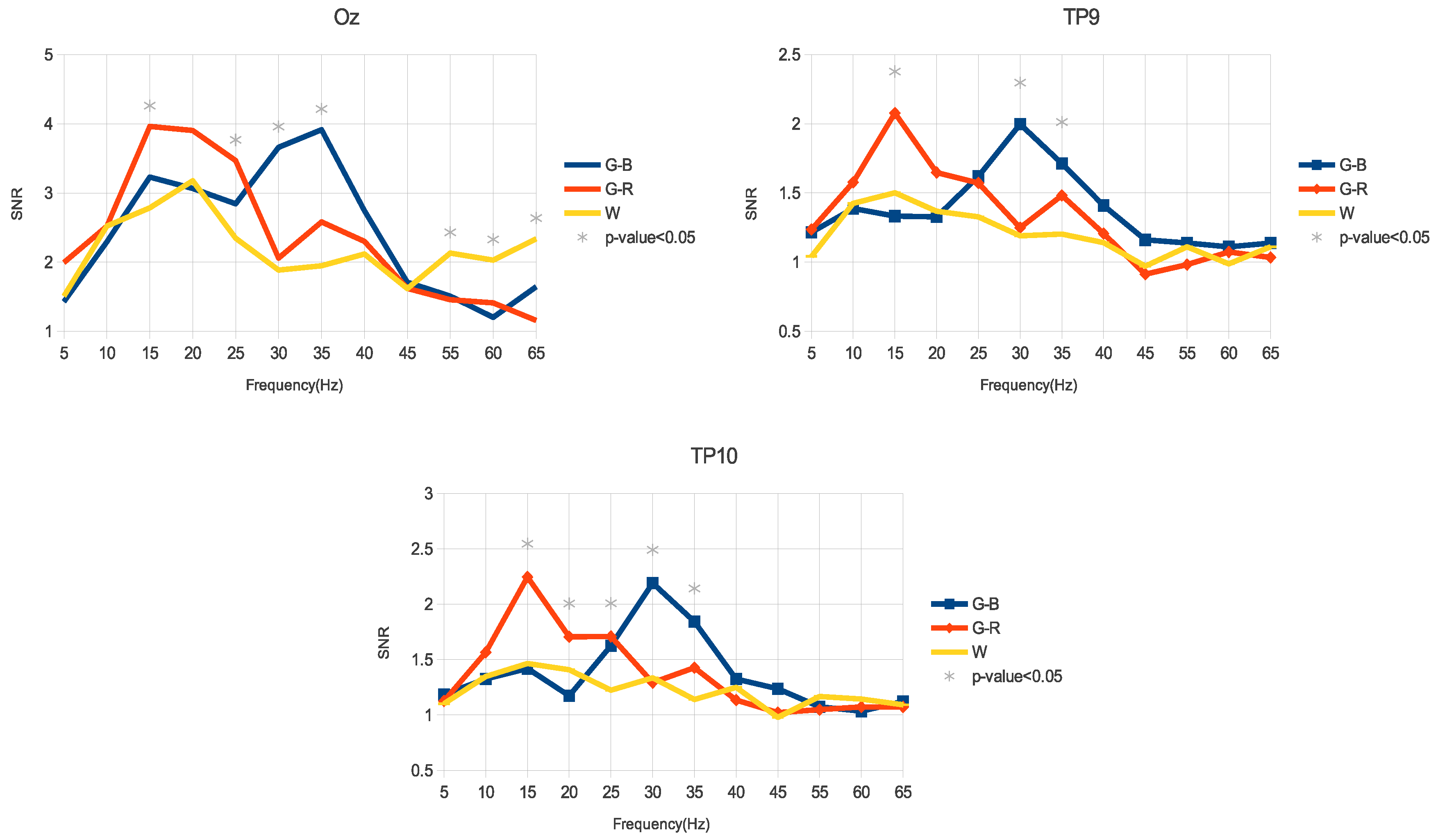
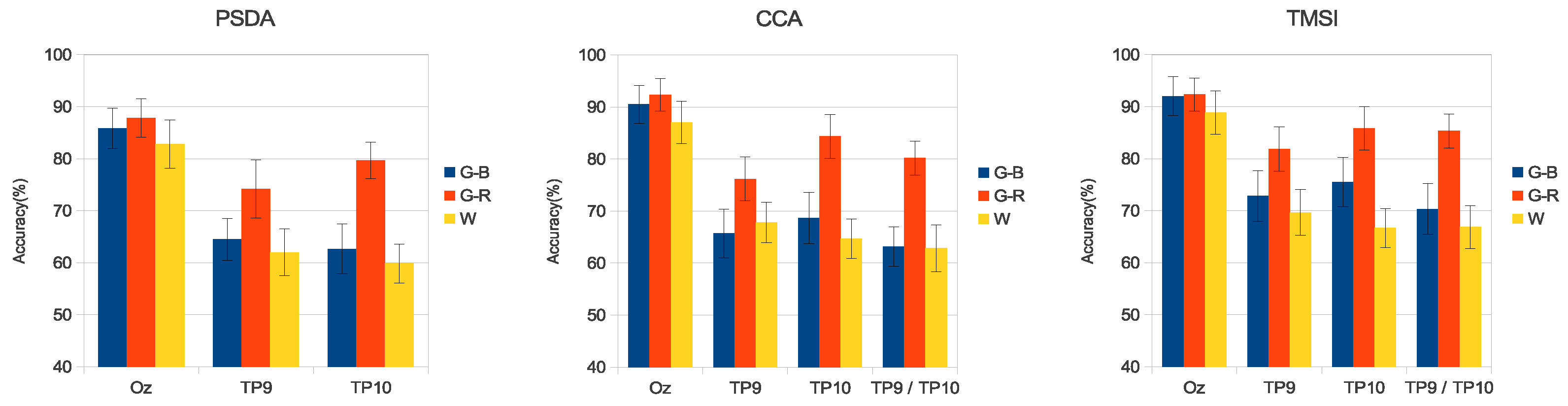
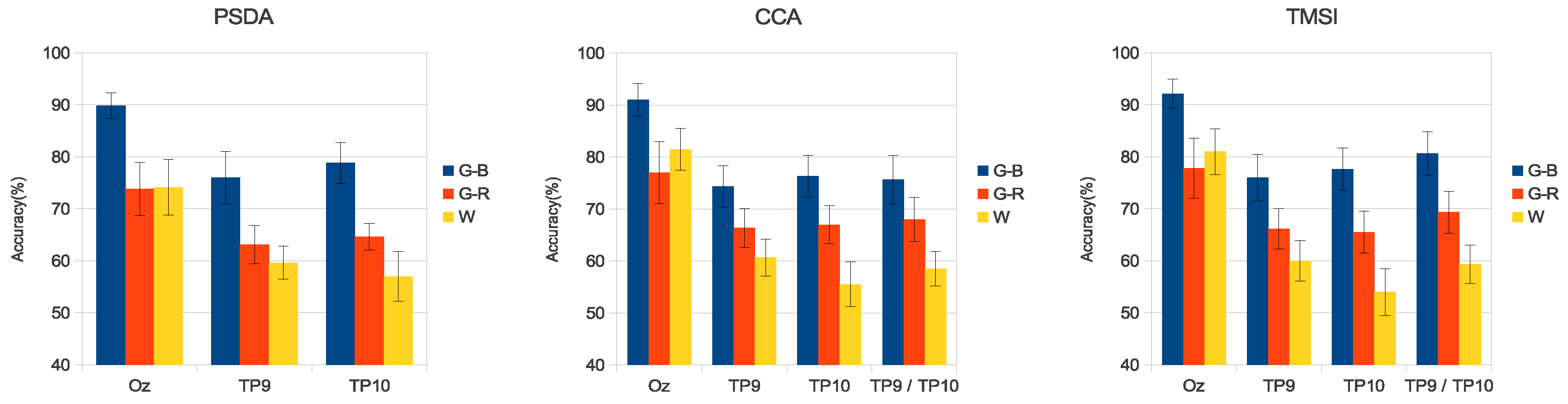
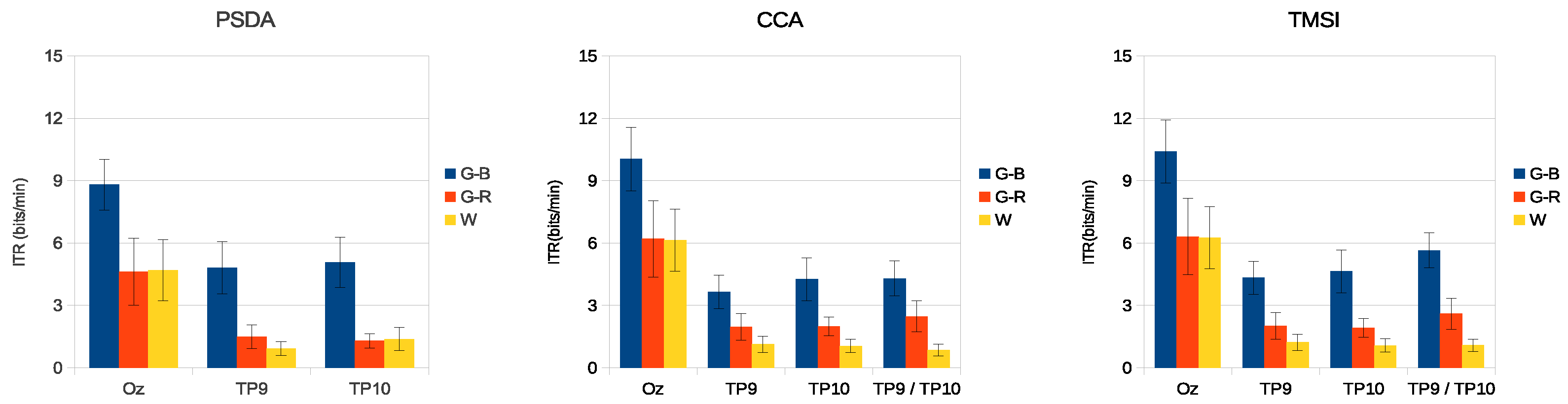
3. Results
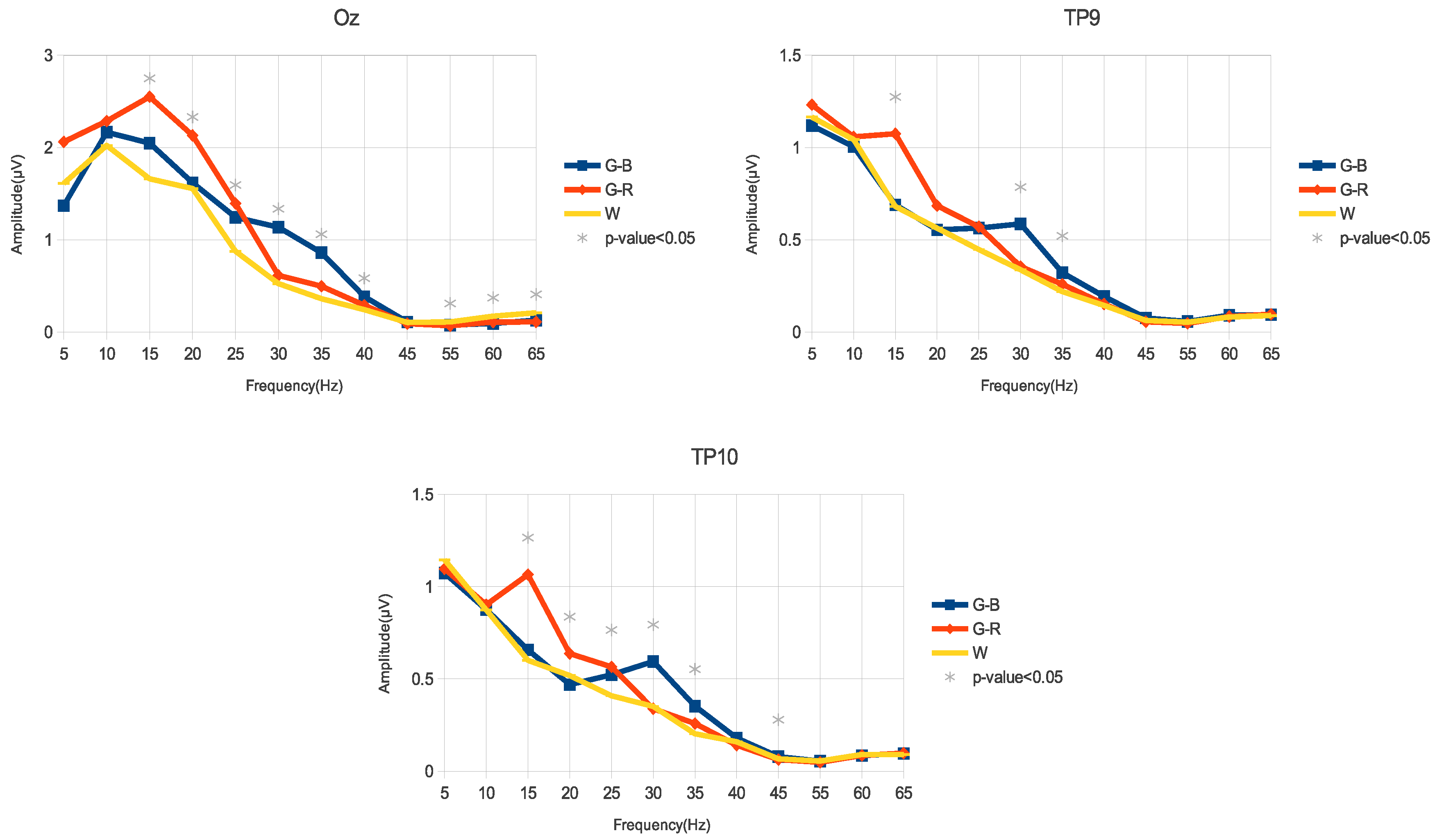
3.1. Amplitude
3.2. SNR
3.3. Simulated SSVEP Classification
4. Discussion
5. Conclusions
6. Future Works
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SSVEP | Steady-State Visual Evoked Potential |
| BCI | Brain-Computer Interface |
| SNR | Signal-to-Noise Ratio |
| REF | Reference Electrode |
| GND | Ground Electrode |
| G-R | Green-Red Stimulus |
| G-B | Green-Blue Stimulus |
| W | White Stimulus |
References
- Zhu, D.; Bieger, J.; Molina, G.G.; Aarts, R.M. A survey of stimulation methods used in SSVEP-based BCIs. Comput. Intell. Neurosci. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C. Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp. Brain Res. 2001, 137, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Diez, P.F.; Mut, V.A.; Perona, E.M.A.; Leber, E.L. Asynchronous BCI control using high-frequency SSVEP. J. Neuroeng. Rehabil. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Chabuda, A.; Durka, P.; Żygierewicz, J. High frequency SSVEP-BCI with hardware stimuli control and phase-synchronized comb filter. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Norcia, A.M.; Appelbaum, L.G.; Ales, J.M.; Cottereau, B.R.; Rossion, B. The steady-state visual evoked potential in vision research: A review. J. Vis. 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Chumerin, N.; Manyakov, N.V.; van Vliet, M.; Robben, A.; Combaz, A.; Van Hulle, M. Steady-state visual evoked potential-based computer gaming on a consumer-grade eeg device. IEEE Trans. Comput. Intell. AI Games 2013, 5, 100–110. [Google Scholar] [CrossRef]
- Vialatte, F.B.; Maurice, M.; Dauwels, J.; Cichocki, A. Steady-state visually evoked potentials: Focus on essential paradigms and future perspectives. Prog. Neurobiol. 2010, 90, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Allison, B.; Lüth, T.; Valbuena, D.; Teymourian, A.; Volosyak, I.; Gräser, A. BCI demographics: How many (and what kinds of) people can use an SSVEP BCI? IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.T.; Celeste, W.C.; Bastos-Filho, T.F.; Sarcinelli-Filho, M. Brain-computer interface based on visual evoked potentials to command autonomous robotic wheelchair. J. Med. Biol. Eng. 2010, 30, 407–415. [Google Scholar] [CrossRef]
- Diez, P.F.; Müller, S.M.T.; Mut, V.A.; Laciar, E.; Avila, E.; Bastos-Filho, T.F.; Sarcinelli-Filho, M. Commanding a robotic wheelchair with a high-frequency steady-state visual evoked potential based brain–computer interface. Med. Eng. Phys. 2013, 35, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.S.; Müller, K.R.; Lee, S.W. A lower limb exoskeleton control system based on steady state visual evoked potentials. J. Neural Eng. 2015, 12, 056009. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhu, G.; Yue, L.; Zhang, M.; Xie, S. A Feasibility Study of SSVEP-Based Passive Training on an Ankle Rehabilitation Robot. J. Healthc. Eng. 2017, 2017, 6819056. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Meng, W. SSVEP-Based BCI for Lower Limb Rehabilitation. In Biomechatronics in Medical Rehabilitation; Springer: Cham, Switzerland, 2017; pp. 69–86. [Google Scholar]
- Nakanishi, M.; Wang, Y.; Wang, Y.T.; Mitsukura, Y.; Jung, T.P. A high-speed brain speller using steady-state visual evoked potentials. Int. J. Neural Syst. 2014, 24, 1450019. [Google Scholar] [CrossRef] [PubMed]
- Won, D.O.; Hwang, H.J.; Dähne, S.; Müller, K.R.; Lee, S.W. Effect of higher frequency on the classification of steady-state visual evoked potentials. J. Neural Eng. 2015, 13, 016014. [Google Scholar] [CrossRef] [PubMed]
- Diez, P.F.; Mut, V.A.; Laciar, E.; Perona, E.M.A. Mobile robot navigation with a self-paced brain-computer interface based on high-frequency SSVEP. Robotica 2014, 32, 695–709. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, H.C.; Lee, P.L.; Li, K.S.; Sie, J.J.; Sun, C.W.; Yang, C.Y.; Li, P.H.; Deng, H.T.; Shyu, K.K. Frequency recognition in an SSVEP-based brain computer interface using empirical mode decomposition and refined generalized zero-crossing. J. Neurosci. Methods 2011, 196, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Lalor, E.C.; Kelly, S.P.; Finucane, C.; Burke, R.; Smith, R.; Reilly, R.B.; Mcdarby, G. Steady-state VEP-based brain-computer interface control in an immersive 3D gaming environment. EURASIP J. Appl. Signal Process. 2005, 2005, 706906. [Google Scholar] [CrossRef]
- Shyu, K.K.; Lee, P.L.; Lee, M.H.; Lin, M.H.; Lai, R.J.; Chiu, Y.J. Development of a low-cost FPGA-based SSVEP BCI multimedia control system. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Wang, Y.; Cheng, C.K.; Jung, T.P. Measuring steady-state visual evoked potentials from non-hair-bearing areas. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 1806–1809. [Google Scholar]
- Wei, C.S.; Wang, Y.T.; Lin, C.T.; Jung, T.P. Toward non-hair-bearing brain-computer interfaces for neurocognitive lapse detection. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6638–6641. [Google Scholar]
- Di Russo, F.; Pitzalis, S.; Aprile, T.; Spitoni, G.; Patria, F.; Stella, A.; Spinelli, D.; Hillyard, S.A. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum. Brain Mapp. 2007, 28, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.A.; Artieda, J.; Arbizu, J.; Valencia, M.; Masdeu, J.C. Human cerebral activation during steady-state visual-evoked responses. J. Neurosci. 2003, 23, 11621–11627. [Google Scholar] [PubMed]
- Srinivasan, R.; Fornari, E.; Knyazeva, M.G.; Meuli, R.; Maeder, P. fMRI responses in medial frontal cortex that depend on the temporal frequency of visual input. Exp. Brain Res. 2007, 180, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.A.; Valencia, M.; Artieda, J.; Alegre, M.; Masdeu, J. Topography of cortical activation differs for fundamental and harmonic frequencies of the steady-state visual-evoked responses. An EEG and PET H215O study. Cereb. Cortex 2007, 17, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Bibi, F.A.; Nunez, P.L. Steady-state visual evoked potentials: Distributed local sources and wave-like dynamics are sensitive to flicker frequency. Brain Topogr. 2006, 18, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Sammer, G.; Blecker, C.; Gebhardt, H.; Kirsch, P.; Stark, R.; Vaitl, D. Acquisition of typical EEG waveforms during fMRI: SSVEP, LRP, and frontal theta. Neuroimage 2005, 24, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, I.P.; Barnes, G.R.; Hillebrand, A.; Singh, K.D. The temporal frequency tuning of human visual cortex investigated using synthetic aperture magnetometry. Neuroimage 2004, 21, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Nakanishi, M.; Kappel, S.L.; Kidmose, P.; Mandic, D.P.; Wang, Y.; Cheng, C.K.; Jung, T.P. Developing an online steady-state visual evoked potential-based brain-computer interface system using EarEEG. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 2271–2274. [Google Scholar]
- Norton, J.J.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.Y.; Cheng, H.; Jeong, J.W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Lee, I.H.; Tsai, H.T.; Chang, H.C.; Shyu, K.K.; Hsu, C.C.; Chang, H.H.; Yeh, T.K.; Chang, C.Y.; Lee, P.L. Evaluate the Feasibility of Using Frontal SSVEP to Implement an SSVEP-Based BCI in Young, Elderly and ALS Groups. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Nakanishi, M.; Wang, Y.; Wei, C.S.; Cheng, C.K.; Jung, T.P. An Online Brain-Computer Interface Based on SSVEPs Measured From Non-Hair-Bearing Areas. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Komatsu, T.; Hata, N.; Nakajima, Y.; Kansaku, K. Visual stimuli for the P300 brain-computer interface: A comparison of white/gray and green/blue flicker matrices. Clin. Neurophysiol. 2009, 120, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Takano, K.; Wada, M.; Saeki, N.; Kansaku, K. Effect of the green/blue flicker matrix for P300-based brain–computer interface: An EEG–fMRI study. Front. Neurol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aminaka, D.; Makino, S.; Rutkowski, T.M. Chromatic ssvep bci paradigm targeting the higher frequency eeg responses. In Proceedings of the 2014 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA), Siem Reap, Cambodia, 9–12 December 2014; pp. 1–7. [Google Scholar]
- Sakurada, T.; Kawase, T.; Komatsu, T.; Kansaku, K. Use of high-frequency visual stimuli above the critical flicker frequency in a SSVEP-based BMI. Clin. Neurophysiol. 2015, 126, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhang, S.; Gao, S.; Hu, Y.; Gao, X. A novel stimulation method for multi-class SSVEP-BCI using intermodulation frequencies. J. Neural Eng. 2017, 14, 026013. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.R.; Moeller, S.; Tsao, D.Y. Specialized color modules in macaque extrastriate cortex. Neuron 2007, 56, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Koida, K.; Komatsu, H. Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat. Neurosci. 2007, 10, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, M.; Ungerleider, L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982, 6, 57–77. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Parra, J.; Lopes da Silva, F.H.; Stroink, H.; Kalitzin, S. Is colour modulation an independent factor in human visual photosensitivity? Brain 2007, 130, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Gao, X.; Hong, B.; Gao, S. A practical VEP-based brain-computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Gao, S.; Gao, X. A high-itr ssvep-based bci speller. Brain-Comput. Interfaces 2014, 1, 181–191. [Google Scholar] [CrossRef]
- Oikonomou, V.P.; Liaros, G.; Georgiadis, K.; Chatzilari, E.; Adam, K.; Nikolopoulos, S.; Kompatsiaris, I. Comparative evaluation of state-of-the-art algorithms for SSVEP-based BCIs. arXiv Preprint, 2016; arXiv:1602.00904. [Google Scholar]
- Chien, Y.Y.; Lin, F.C.; Zao, J.K.; Chou, C.C.; Huang, Y.P.; Kuo, H.Y.; Wang, Y.; Jung, T.P.; Shieh, H.P.D. Polychromatic SSVEP stimuli with subtle flickering adapted to brain-display interactions. J. Neural Eng. 2017, 14, 016018. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, C.; Wu, W.; Gao, X. Frequency recognition based on canonical correlation analysis for SSVEP-based BCIs. IEEE Trans. Biomed. Eng. 2007, 54, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, D.; Xu, P.; Zhang, Y.; Yao, D. Robust frequency recognition for SSVEP-based BCI with temporally local multivariate synchronization index. Cogn. Neurodynamics 2016, 10, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Harding, G.; Erba, G.; Barkley, G.L.; Wilkins, A. Photic-and Pattern-induced Seizures: A Review for the Epilepsy Foundation of America Working Group. Epilepsia 2005, 46, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Volosyak, I.; Valbuena, D.; Lüth, T.; Malechka, T.; Gräser, A. BCI demographics II: How many (and what kinds of) people can use a high-frequency SSVEP BCI? IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Yijun, W.; Ruiping, W.; Xiaorong, G.; Shangkai, G. Brain-computer interface based on the high-frequency steady-state visual evoked potential. In Proceedings of the IEEE 2005 First International Conference on Neural Interface and Control, Wuhan, China, 26–28 May 2005; pp. 37–39. [Google Scholar]
- Lin, F.C.; Chien, Y.Y.; Zao, J.K.; Huang, Y.P.; Ko, L.W.; Shieh, H.P.D.; Wang, Y.; Jung, T.P. High-frequency polychromatic visual stimuli for new interactive display systems. SPIE Newsroom 2015, 1–4. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floriano, A.; F. Diez, P.; Freire Bastos-Filho, T. Evaluating the Influence of Chromatic and Luminance Stimuli on SSVEPs from Behind-the-Ears and Occipital Areas. Sensors 2018, 18, 615. https://doi.org/10.3390/s18020615
Floriano A, F. Diez P, Freire Bastos-Filho T. Evaluating the Influence of Chromatic and Luminance Stimuli on SSVEPs from Behind-the-Ears and Occipital Areas. Sensors. 2018; 18(2):615. https://doi.org/10.3390/s18020615
Chicago/Turabian StyleFloriano, Alan, Pablo F. Diez, and Teodiano Freire Bastos-Filho. 2018. "Evaluating the Influence of Chromatic and Luminance Stimuli on SSVEPs from Behind-the-Ears and Occipital Areas" Sensors 18, no. 2: 615. https://doi.org/10.3390/s18020615




