Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death
Abstract
:1. Introduction
1.1. Physiological Basis
1.2. The Wavelet Continuous Transform
1.3. The Use of the Wavelet Transform for the Study of the Surface Electrocardiogram
1.4. Pan-Tompkins Algorithm for Automatic Detection of QRS Complexes
1.5. Hypothesis and Objectives
2. Materials and Methods
2.1. Study Population
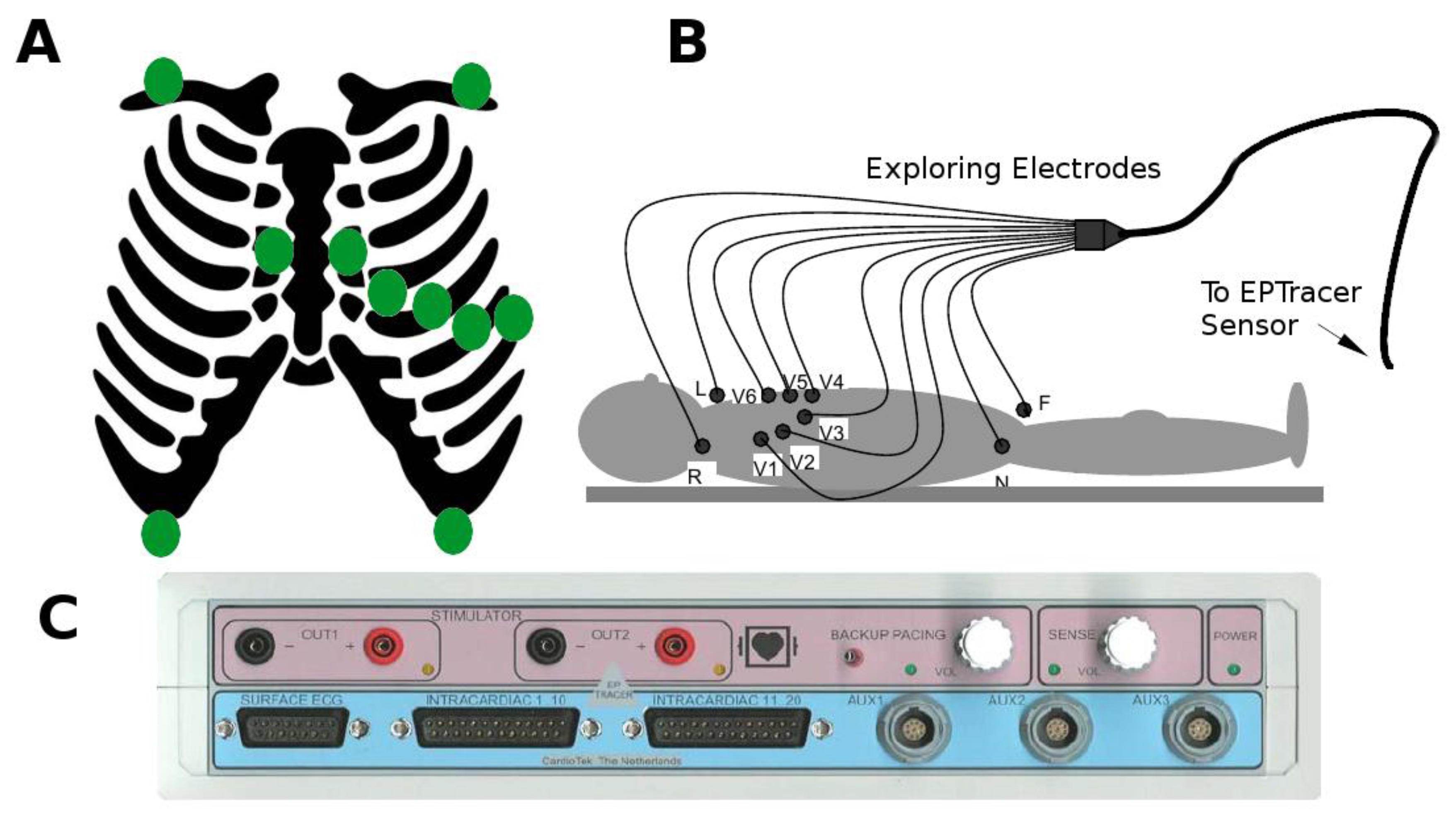
2.2. Collection of Electrocardiographic Data: The ECG Sensor
2.3. Extraction of QRS Complexes Using a Modified Pan-Tompkins Algorithm
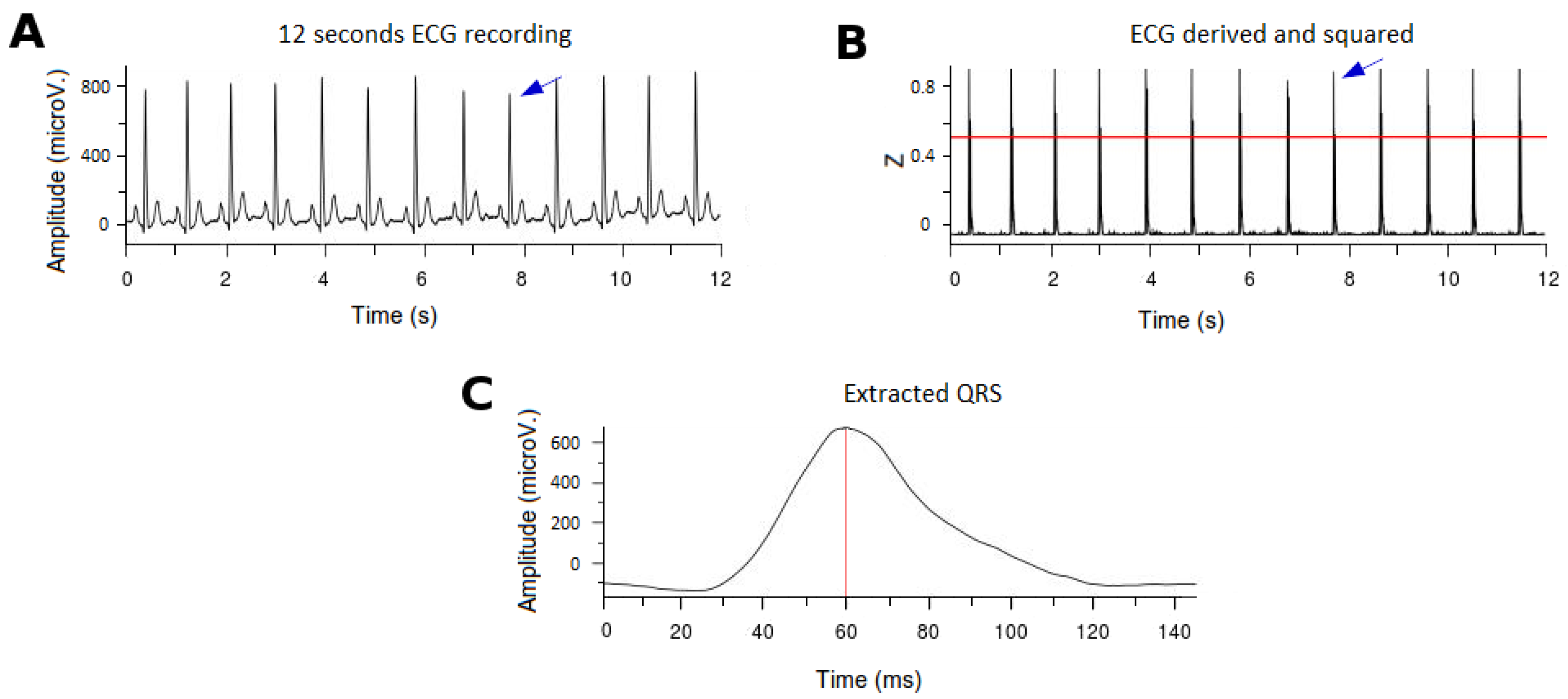
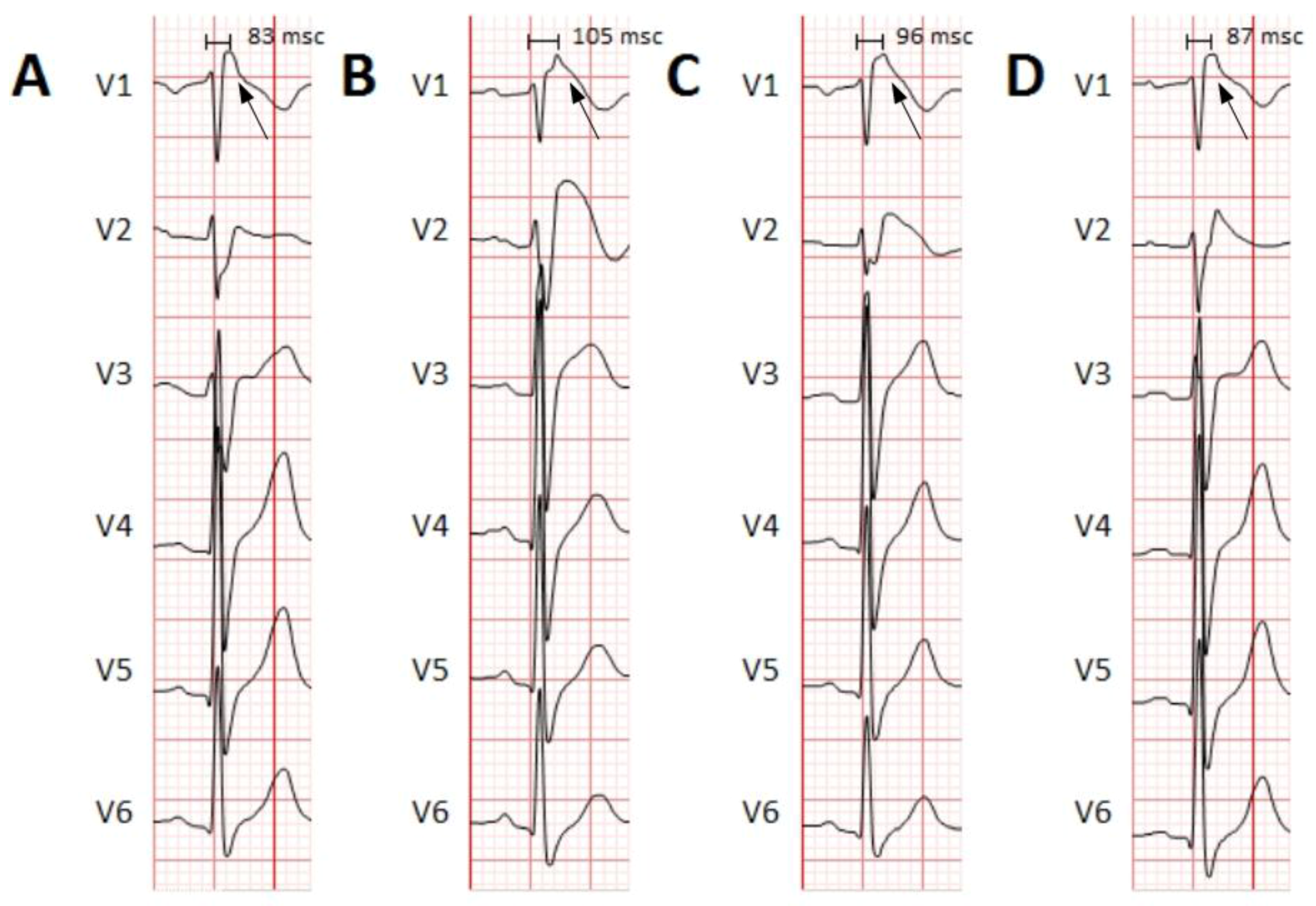
First, we normalized the ECG signal to allow appropriate comparisons between leads and patients.In order to determine the threshold for detection of the R-wave in the QRS complexes (after differentiation and squared elevation of the signal), the 99.5 percentile of the signal (P99.5) was calculated. All the extreme values (defined as those greater than P99.5) were removed and the signal was typified over the value of P99.5 (Figure 2B). A threshold of 0.6 defined the time points where there were QRS complexes (tQRS; red line in Figure 2B).A temporal correction was performed for the morphology of the QRS complexes. To do this, the point of the QRS complex with a higher positive voltage value in the V6 derivation was selected (tMaxQRS; red line in Figure 2C), because clinically it is the lead in which the peak of the R wave is better defined.Finally, to subtract the QRS complexes for analysis a window of 145 ms around the tMaxQRS point was selected (from 60 ms before tMaxQRS to 85 ms after tMaxQRS; Figure 2C).
2.4. Wavelet Continuous Transformation for the Analysis of the High-Frequency Content
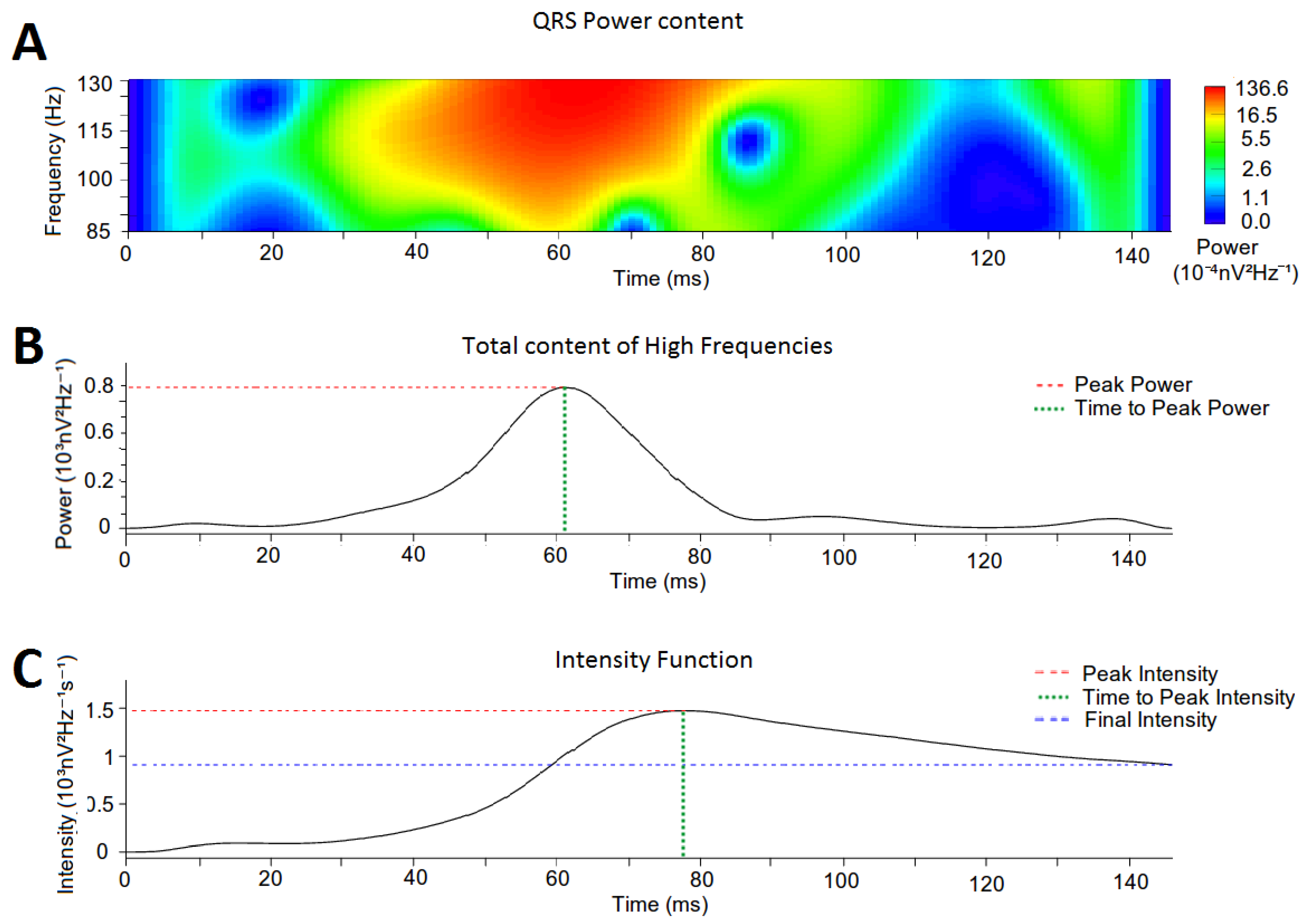
2.5. Quantification of High-Frequency Content in the QRS Complexes
2.6. Intensity Analysis
2.7. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Comparative Analysis between Different QRS Detection Algorithms
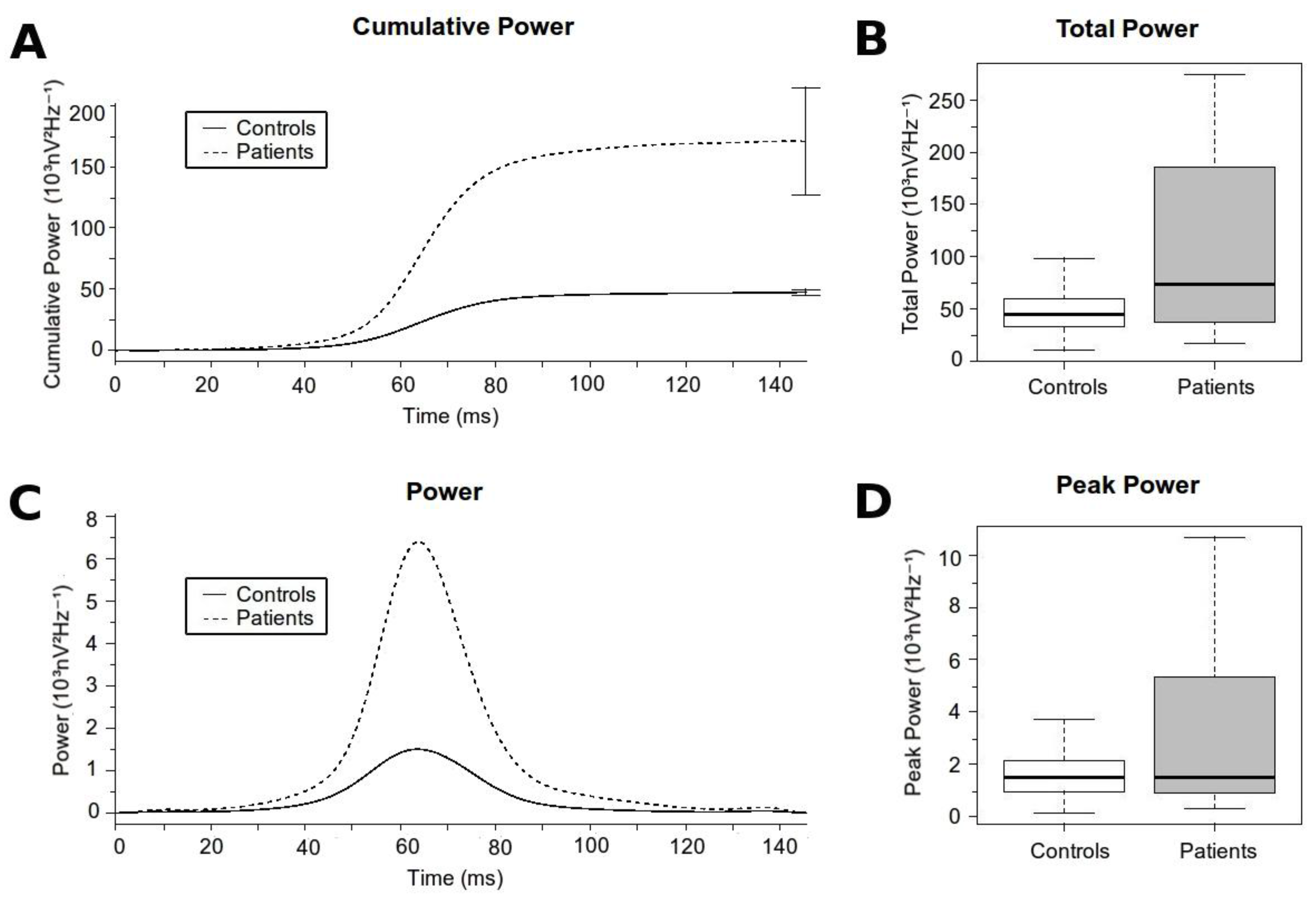
3.3. Analysis of the High-Frequency Content
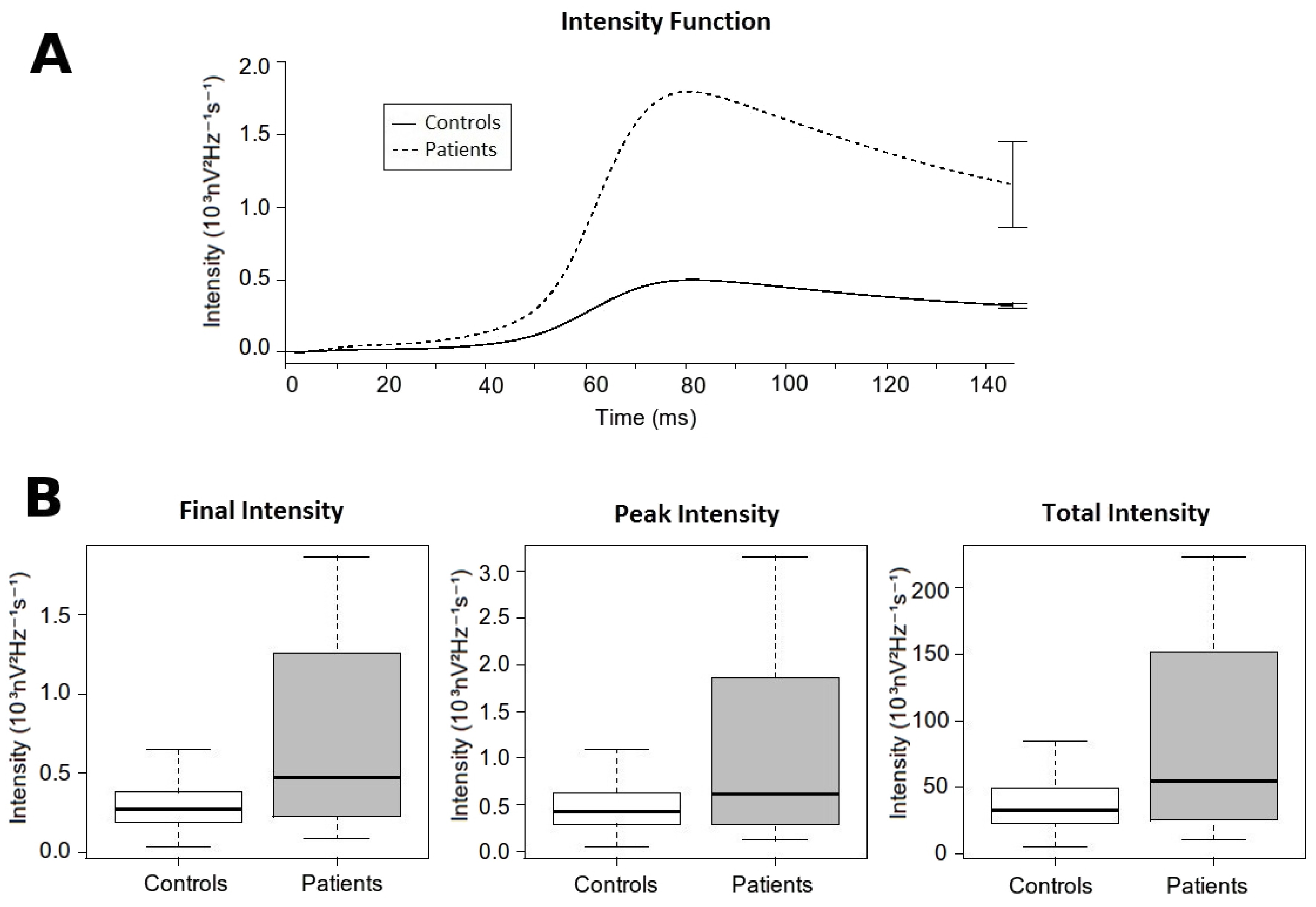
3.4. Intensity Analysis
4. Discussion
5. Conclusions
6. Limitations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- From, R.; Fishman, G.I.; Chugh, S.S.; Dimarco, J.P.; Albert, C.M.; Anderson, M.E.; Bonow, R.O.; Buxton, A.E.; Chen, P.-S.; Estes, M.; et al. Sudden Cardiac Death Prediction and Prevention. Circulation 2010, 122, 2335–2348. [Google Scholar]
- Adler, A.; Viskin, S. Risk stratification in brugada syndrome: Clinical characteristics, electrocardiographic parameters and auxiliary testing. Heart Rhythm 2016, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Kusano, K.F.; Miura, D.; Nagase, S.; Nakamura, K.; Morita, S.T.; Ohe, T.; Zipes, D.P.; Wu, J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008, 118, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Suradi, H.; Maskoun, W.; Michael, M.A.; Shen, C.; Peng, J.; Dandamudi, G.; Mahenthiran, J. Fragmented Wide QRS on a 12-Lead ECG. Clinical perspective. Circ. Arrhythm. Electrophysiol. 2008, 1, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Zipes, D.P. Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 2009, 6, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Khan, B.; Jacob, S.; Kumar, A.; Mahenthiran, J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006, 113, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Saha, C.; El Masry, H.; Peng, J.; Dandamudi, G.; Mahenthiran, J.; McHenry, P.; Zipes, D.P. Fragmented QRS on a 12-lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007, 4, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Janardhan, A.H.; Jung, K.T.; Lee, H.S.; Lee, M.H.; Hwang, H.J. Fragmented QRS as a candidate marker for high-risk assessment in hypertrophic cardiomyopathy. Heart Rhythm 2014, 11, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Tada, H.; Yamasaki, H.; Kuroki, K.; Ishizu, T.; Seo, Y.; Machino, T.; Murakoshi, N.; Sekiguchi, Y.; Noguchi, Y.; et al. Fragmented QRS Is a Novel Risk Factor for Ventricular Arrhythmic Events After Receiving Cardiac Resynchronization Therapy in Nonischemic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2017, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Flórez, J.P.; Valverde, I.; Rubín, J.; Pérez, D.; Vasserot, M.G.; Rodríguez-Reguero, J.; Avanzas, P.; De La Hera, J.M.; Gómez, J.; et al. Surveillance after cardiac arrest in patients with Brugada syndrome without an implantable defibrillator: An alarm effect of the previous syncope. Int. J. Cardiol. 2016, 218, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.P.; García, D.; Valverde, I.; Rubín, J.; Pérez, D.; González-Vasserot, M.; Reguero, J.; de la Hera, J.M.; Avanzas, P.; Gómez, J.; et al. Role of syncope in predicting adverse outcomes in patients with suspected Brugada syndrome undergoing standardized flecainide testing. Europace 2017. [Google Scholar] [CrossRef]
- Nademanee, K.; Raju, H.; De Noronha, S.V.; Papadakis, M.; Robinson, L.; Rothery, S.; Makita, N.; Kowase, S.; Boonmee, N.; Vitayakritsirikul, V.; et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J. Am. Coll. Cardiol. 2015, 66, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Atienza, F.; Saiz, J.; Martínez, L.; Ávila, P.; Rubín, J.; Herreros, B.; Arenal, Á.; García-Fernández, J.; Ferrer, A.; et al. Ventricular Tachycardia and Early Fibrillation in Patients with Brugada Syndrome and Ischemic Cardiomyopathy Show Predictable Frequency-Phase Properties on the Precordial ECG Consistent with the Respective Arrhythmogenic Substrate. Circ. Arrhythm. Electrophysiol. 2015, 8, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Couderc, J.-P.; Chevalier, P.; Fayn, J.; Rubel, P.; Touboul, P. Identification of post-myocardial infarction patients prone to ventricular tachycardia using time–frequency analysis of QRS and ST segments. EP Eur. 2000, 2, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Simson, M.B.; Untereker, W.J.; Spielman, S.R.; Horowitz, L.N.; Marcus, N.H.; Falcone, R.A.; Harken, A.H.; Josephson, M.E. Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am. J. Cardiol. 1983, 51, 105–112. [Google Scholar] [CrossRef]
- Coto, H.; Maldonado, C.; Palakurthy, P.; Flowers, N.C. Late potentials in normal subjects and in patients with ventricular tachycardia unrelated to myocardial infarction. Am. J. Cardiol. 1985, 55, 384–390. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Josephson, M.E. Frequency content and characteristics of ventricular conduction. J. Electrocardiol. 2015, 48, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Rubín, J.M.; Pérez, D.; Gómez, J.; Flórez, J.P.; Avanzas, P.; García-Ruíz, J.M.; De La Hera, J.M.; Reguero, J.; Coto, E.; et al. Time-dependent responses to provocative testing with flecainide in the diagnosis of Brugada syndrome. Heart Rhythm 2015, 12, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rosch, A.; Schmidbauer, H. WaveletComp: A Guided Tour through the R-Package. Available online: http://www.hs-stat.com/projects/WaveletComp/WaveletComp_guided_tour.pdf (accessed on 15 June 2017).
- Morlet, J.; Arens, G.; Fourgeau, E.; Giard, D. Wave propagation and sampling theory—Part II: Sampling theory and complex waves. Geophysics 1982, 47, 222–236. [Google Scholar] [CrossRef]
- Morlet, J.; Arens, G.; Fourgeau, E.; Glard, D. Wave propagation and sampling theory—Part I: Complex signal and scattering in multilayered media. Geophysics 1982, 47, 203–221. [Google Scholar] [CrossRef]
- Goupillaud, P.; Grossmann, A.; Morlet, J. Cycle-octave and related transforms in seismic signal analysis. Geoexploration 1984, 23, 85–102. [Google Scholar] [CrossRef]
- Gabor, D. Theory of communication. Part 3: Frequency compression and expansion. J. Inst. Electr. Eng. Part III Radio Commun. Eng. 1946, 93, 445–457. [Google Scholar] [CrossRef]
- Carmona, R.; Hwang, W.L.; Torrésani, B. Practical Time-Frequency Analysis: Continuous Wavelet and Gabor Transforms, with an Implementation in S; Academic Press: San Diego, CA, USA, 1998; Volume 9, ISBN 0-12-160170-6. [Google Scholar]
- Gramatikov, B.; Brinker, J.; Yi-Chun, S.; Thakor, N.V. Wavelet analysis and time-frequency distributions of the body surface ECG before and after angioplasty. Comput. Methods Programs Biomed. 2000, 62, 87–98. [Google Scholar] [CrossRef]
- Gramatikov, B.; Iyer, V. Intra-QRS spectral changes accompany ST segment changes during episodes of myocardial ischemia. J. Electrocardiol. 2015, 48, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Magrans, R.; Gomis, P.; Voss, A.; Caminal, P. Effect of acute myocardial ischemia on different high-frequency bandwidths and temporal regions of the QRS. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Boston, MA, USA, 30 August–3 September 2011; pp. 7083–7086. [Google Scholar]
- Vassilikos, V.P.; Mantziari, L.; Dakos, G.; Kamperidis, V.; Chouvarda, I.; Chatzizisis, Y.S.; Kalpidis, P.; Theofilogiannakos, E.; Paraskevaidis, S.; Karvounis, H.; et al. QRS analysis using wavelet transformation for the prediction of response to cardiac resynchronization therapy: A prospective pilot study. J. Electrocardiol. 2014, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Couderc, J.-P.; Mcnitt, S.; Zareba, W. Predicting Effectiveness of Cardiac Resynchronization Therapy based on QRS Decomposition using the Meyer Orthogonal Wavelet Transformation. Comput. Cardiol. 2010, 37, 983–986. [Google Scholar]
- Murata, H.; Ohara, T.; Kobayashi, Y.; Miyauchi, Y.; Katoh, T.; Mizuno, K. Detection of arrhythmogenic substrates in prior myocardial infarction patients with complete right bundle branch block QRS using wavelet-transformed ECG. J. Nippon Med. Sch. 2009, 76, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Takano, N.; Matsuyama, N.; Higashi, Y.; Iwasawa, K.; Nakajima, T. High-frequency powers hidden within QRS complex as an additional predictor of lethal ventricular arrhythmias to ventricular late potential in postmyocardial infarction patients. Heart Rhythm 2011, 8, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. A Digital Filter for the ORS Complex Detection. IEEE Trans. Biomed. Eng. 1979, BME-26, 700–703. [Google Scholar] [CrossRef]
- Portet, F.; Hernández, A.I.; Carrault, G. Evaluation of real-time QRS detection algorithms in variable contexts. Med. Biol. Eng. Comput. 2005, 43, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Fernandez, J.; Harris, M. Combining algorithms in automatic detection of R-peaks in ECG signals. In Proceedings of the 18th IEEE Symposium on Computer-Based Medical Systems, Dublin, Ireland, 23–24 June 2005; Volume 10. [Google Scholar] [CrossRef]
- Wilson, F.N.; Johnston, F.D.; Macleod, A.G.; Barker, P.S. Electrocardiograms that represent the potential variations of a single electrode. Am. Heart J. 1934, 9, 447–458. [Google Scholar] [CrossRef]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the Standardization and Interpretation of the Electrocardiogram. J. Am. Coll. Cardiol. 2007, 49, 1109–1127. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.; Mark, R. The impact of the MIT-BIH Arrhythmia Database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, R.; Jaleel, A.; Lim, J.; Tafreshi, L. Automated analysis of ECG waveforms with atypical QRS complex morphologies. Biomed. Signal Process. Control 2014, 10, 41–49. [Google Scholar] [CrossRef]
- Zidelmal, Z.; Amirou, A.; Adnane, M.; Belouchrani, A. QRS detection based on wavelet coefficients. Comput. Methods Programs Biomed. 2012, 107, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Yochum, M.; Renaud, C.; Jacquir, S. Automatic detection of P, QRS and T patterns in 12 leads ECG signalbased on CWT. Biomed. Signal Process. Control 2016, 25, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.; Almeida, R.; Olmos, S.; Rocha, A.; Laguna, P. A wavelet-based ECGdelineator: Evaluation on standard databases. IEEE Trans. Biomed. Eng. 2004, 51, 570–581. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Amit, G.; Granot, Y.; Abboud, S. Quantifying QRS changes during myocardial ischemia: Insights from high frequency electrocardiography. J. Electrocardiol. 2014, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Okamoto, Y.; Takano, N.; Wakatsuki, D.; Tomaru, T.; Nakajima, T. High-frequency power within the QRS complex in ischemic cardiomyopathy patients with ventricular arrhythmias: Insights from a clinical study and computer simulation of cardiac fibrous tissue. Comput. Biol. Med. 2017, 87, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kelen, G.J.; Henkin, R.; Starr, A.M.; Caref, E.B.; Bloomfield, D.; El-Sherif, N. Spectral turbulence analysis of the signal-averaged electrocardiogram and its predictive accuracy for inducible sustained monomorphic ventricular tachycardia. Am. J. Cardiol. 1991, 67, 965–975. [Google Scholar] [CrossRef]
| Variables | Controls (n = 120) | Patients (n = 42) |
|---|---|---|
| Age (years) | 53.3 ± 14.37 | 59.6 ± 18.26 |
| Male (N/%) | 67 (55.8) | 34 (80.95) |
| Cardiomyopathy (N/%) | 0 (0) | 42 (100) |
| Ischemic | NA | 12 (28.57) |
| Idiopathic | NA | 4 (9.52) |
| BrS * | NA | 11 (26.19) |
| Others | NA | 15 (35.71) |
| Arrhythmic Events (N/%) | 0 (0) | 42 (100) |
| SCA * | NA | 5 (11.9) |
| MA * | NA | 42 (100) |
| Algorithm | Database | Se (%) | PPV (%) | Er | TB | FP | FN |
|---|---|---|---|---|---|---|---|
| J. Pan et al. (1985) [32] | MITDB | 99.75 | 99.53 | 0.675 | 116137 | 507 | 277 |
| J.P. Martinez et al. (2004) [42] | MITDB | 99.8 | 99.86 | 0.34 | 107567 | 153 | 220 |
| Z. Zidelmal et al. (2012) [39] | MITDB | 99.64 | 99.82 | 0.54 | 109494 | 193 | 393 |
| R. Tafresi et al. (2014) [40] | PTBDB | 99.06 | 98.9 | N/A | N/A | N/A | N/A |
| M. Yochum et al. (2016) [41] | CinCC11 | 99.87 | 91.71 | N/A | N/A | N/A | N/A |
| Present Work | MITDB | 98.45 | 96.67 | 3.53 | 114654 | 2567 | 1125 |
| Variables | Controls (n = 120) | Patients (n = 42) | p |
|---|---|---|---|
| Peak Power (103nV2Hz−1) | 1.709 (± 1.13) | 7.033 (± 15.09) | 0.028 |
| Time to Peak Power (ms) | 64.768 (± 5.868) | 68.952 (± 7.609) | 0.002 |
| Total Power (103nV2Hz−1) | 47.298 (± 26.129) | 170.782 (± 282.714) | 0.007 |
| Initial High Frequency Contribution (103nV2Hz−1) | 2.012 (± 1.21) | 5.409 (± 4.97) | <0.001 |
| Final High Frequency Contribution (103nV2Hz−1) | 45.287 (± 25.601) | 165.463 (± 280.608) | 0.008 |
| High Frequency Contribution Ratio | 0.053 (± 0.034) | 0.069 (± 0.049) | 0.059 |
| Variables | Controls (n = 120) | Patients (n = 42) | p |
|---|---|---|---|
| Peak Intensity (103nV2Hz−1s−1) | 0.506 (± 0.296) | 1.854 (± 3.389) | 0.014 |
| Time to Peak Intensity (ms) | 82.116 (± 5.103) | 88.738 (± 9.461) | <0.001 |
| Total Intensity (103nV2Hz−1s−1) | 39.024 (± 21.574) | 137.128 (± 233.521) | 0.001 |
| Final Intensity (103nV2Hz−1s−1) | 0.32 (± 0.177) | 1.155 (± 1.91) | 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Iglesias, D.; Roqueñi Gutiérrez, N.; De Cos, F.J.; Calvo, D. Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death. Sensors 2018, 18, 560. https://doi.org/10.3390/s18020560
García Iglesias D, Roqueñi Gutiérrez N, De Cos FJ, Calvo D. Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death. Sensors. 2018; 18(2):560. https://doi.org/10.3390/s18020560
Chicago/Turabian StyleGarcía Iglesias, Daniel, Nieves Roqueñi Gutiérrez, Francisco Javier De Cos, and David Calvo. 2018. "Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death" Sensors 18, no. 2: 560. https://doi.org/10.3390/s18020560





