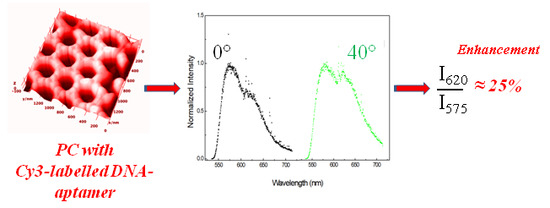
Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polystyrene Nanospheres
2.3. Preparation of the Prehydrolized Silica Sol
2.4. Deposition of Colloidal Crystal Films and Calcination
2.5. Functionalization and Immobilization of the DNA-Cy3
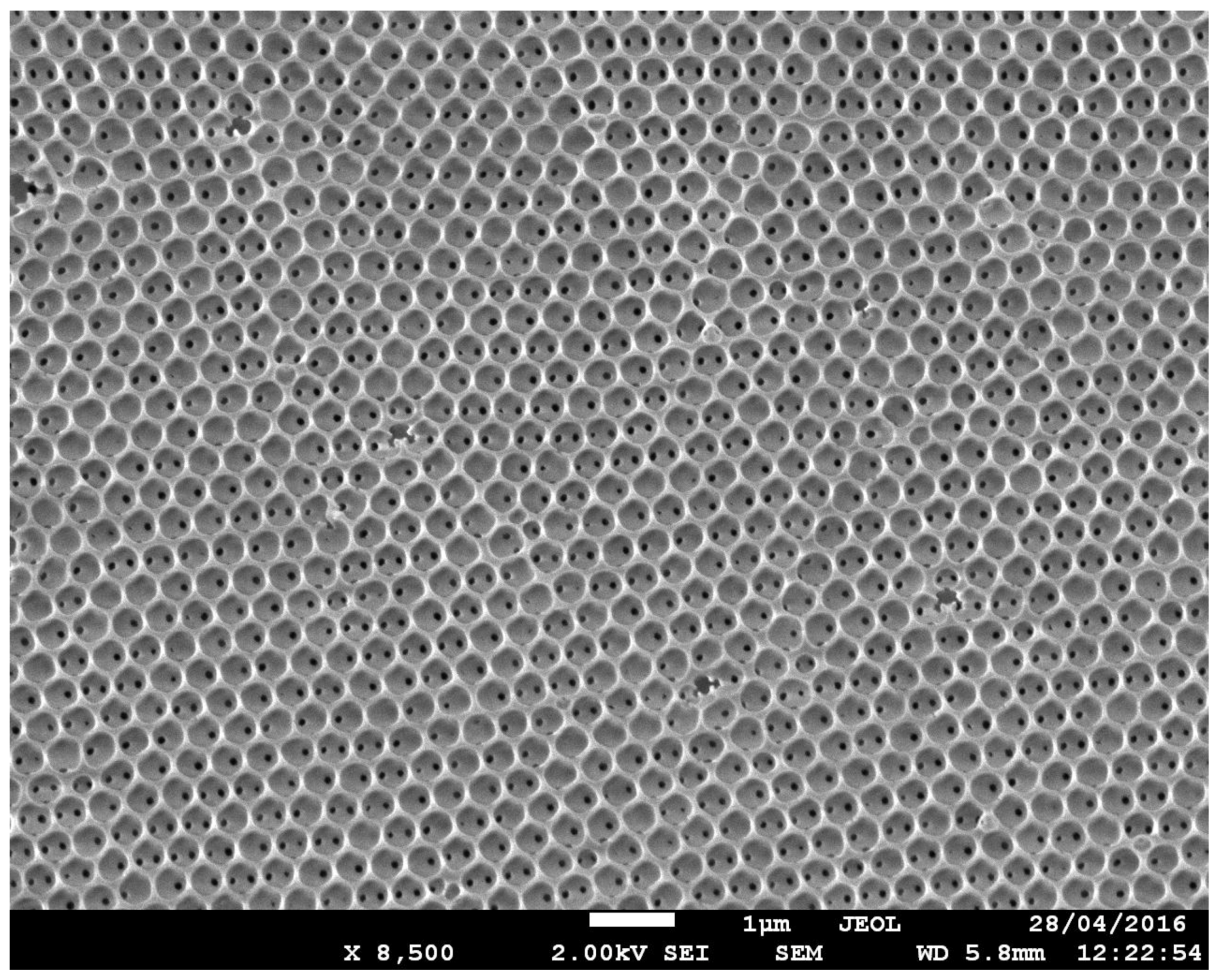
2.6. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuator B-Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Cunningham, B.T. Label-free biosensor imaging on photonic crystal surfaces. Sensors 2015, 15, 21613–21635. [Google Scholar] [CrossRef] [PubMed]
- Threm, D.; Nazirizadeh, Y.; Gerken, M. Photonic crystal biosensors towards on-chip integration. J. Biophotonics 2012, 5, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Pasquardini, L.; Berneschi, S.; Barucci, A.; Cosi, F.; Dallapiccola, R.; Insinna, M.; Lunelli, L.; Conti, G.N.; Pederzolli, C.; Salvadori, S.; et al. Whispering gallery mode aptasensors for detection of blood proteins. J. Biophotonics 2013, 6, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Keng, D.; McAnanama, S.R.; Teraoka, I.; Arnold, S. Resonance fluctuations of a whispering gallery mode biosensor by particles undergoing Brownian motion. Appl. Phys. Lett. 2007, 91, 103902. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals Molding the Flow of Light, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 1995; ISBN 9780691124568. [Google Scholar]
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.M. (Ed.) Photonic Crystals and Light Localization in the 21st Century; Springer: Dordrecht, The Netherlands, 2001; ISBN 978-94-010-0738-2. [Google Scholar]
- Fujita, M.; Takahashi, S.; Tanaka, Y.; Asano, T.; Noda, S. Simultaneous Inhibition and Redistribution of Spontaneous Light Emission in Photonic Crystals. Science 2005, 308, 1296–1298. [Google Scholar] [CrossRef]
- Huihui, X.; Jun, L.; Jinbao, G.; Jie, W. Bio-inspired thermal-responsive inverse opal films with dual structural colors based on liquid crystal elastomer. J. Mater. Chem. C 2015, 3, 4424–4430. [Google Scholar] [CrossRef]
- Chiappini, A.; Armellini, C.; Carpentiero, A.; Minati, L.; Righini, G.C.; Ferrari, M. Solvent sensitive polymer composite structures. Opt. Mater. 2014, 36, 130–134. [Google Scholar] [CrossRef]
- Frascella, F.; Ricciardi, S.; Pasquardini, L.; Potrich, C.; Angelini, A.; Chiadò, A.; Pederzolli, C.; De Leo, N.; Rivolo, P.; Pirri, C.F.; Descrovi, E. Enhanced fluorescence detection of miRNA-16 on a photonic crystal. Analyst 2015, 140, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Min, K.; Lee, M.; Kang, M.; Park, Y.; Cho, K.-S.; Roh, Y.-G.; Woo Hwang, S.; Jeon, H. Colloidal quantum dot lasers built on a passive two-dimensional photonic crystal backbone. Nanoscale 2016, 8, 6571–6576. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Besseling, T.H.; Hermes, M.; Demirörs, A.F.; Imhof, A.; Van Blaaderen, A. Switching plastic crystals of colloidal rods with electric fields. Nat. Commun. 2014, 5, 3092. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Ibisate, M.; John, S.; Leonard, S.W.; Lopez, C.; Meseguer, F.; Miguez, H.; Mondla, J.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap near 1.5 micrometres. Nature 2000, 405, 437–440. [Google Scholar] [CrossRef] [PubMed]
- João, C.F.C.; Vasconcelos, J.M.; Silva, J.C.; Borges, J.P. An overview of inverted colloidal crystal systems for tissue engineering. J. Tissue Eng. 2014, 20, 437–454. [Google Scholar] [CrossRef]
- Lash, M.H.; Fedorchak, M.V.; McCarthy, J.J.; Little, S.R. Scaling up self-assembly: Bottom-up approaches to macroscopic particle organization. Soft Matter 2015, 11, 5597–5609. [Google Scholar] [CrossRef]
- Tan, T.; Xie, W.; Zhu, G.; Shan, J.; Xu, P.; Li, L.; Wang, J. Fabrication and photocatalysis of BiFeO3 with inverse opal structure. J. Porous Mater. 2015, 22, 659–663. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Yuan, X.; Huo, M.; Zhao, Y.; Lu, Y.; Qiu, Y. Incorporation of Cu2O nanocrystals into TiO2 photonic crystal for enhanced UV-visible light driven photocatalysis. J. Alloys Comp. 2015, 644, 734–741. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.-L.; Kim, B.; Kwon, K.; Park, J.; Han, K.; Lee, H.; Lee, W. Rapid on-chip integration of opal films and photonic gel sensor array via directed enhanced water evaporation for colloidal assembly. Sens. Actuators B 2016, 231, 256–264. [Google Scholar] [CrossRef]
- Galisteo-Lõpez, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Pérez, L.S.; Blanco, Ú.; Lõpez, C. Self-assembled photonic structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.S. Fabrication of Crack-Free Colloidal Crystals Using a Modified Vertical Deposition Method. J. Phys. Chem. C 2007, 111, 8538–8542. [Google Scholar] [CrossRef]
- Cai, Z.; Xiong, Z.; Lu, X.; Teng, J. In situ gold-loaded titania photonic crystals with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 545–553. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.J.; Lu, X.; Teng, J. In Situ “Doping” Inverse Silica Opals with Size-Controllable Gold Nanoparticles for Refractive Index Sensing. J. Phys. Chem. C 2013, 117, 9440–9445. [Google Scholar] [CrossRef]
- Cai, Z.; Teng, J.; Wan, Y.; Zhao, X.S. An improved convective self-assembly method for the fabrication of binary colloidal crystals and inverse structures. J. Colloid Interface Sci. 2012, 380, 42–50. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Fortes, L.M.; Almeida, R.M.; Chiasera, A.; Chiappini, A.; Ferrari, M. 3-D rare earth-doped colloidal photonic crystals. Opt. Mater. 2009, 31, 1315–1318. [Google Scholar] [CrossRef]
- Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354–10359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishchenko, L.; Hatton, B.; Kolle, M.; Aizenberg, J. Patterning hierarchy in direct and inverse opal crystals. Small 2012, 8, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.J. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Anal. Chem. 1999, 45, 1628–1650. [Google Scholar]
- Song, S.; Wang, L.; Li, J.; Zhao, J.; Fan, C. Aptamer-based biosensors. Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Orava, E.W.; Jarvik, N.; Shek, Y.L.; Sidhu, S.S.; Garié, J. A Short DNA Aptamer That Recognizes TNFα and Blocks Its Activity. ACS Chem. Biol. 2013, 8, 170–178. [Google Scholar] [CrossRef]
- Ye, B.F.; Zhao, Y.J.; Cheng, Y.; Li, T.T.; Xie, Z.Y.; Zhao, X.W.; Gu, Z.Z. Colorimetric photonic hydrogel aptasensor for the screening of heavy metal ions. Nanoscale 2012, 4, 5998–6003. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kang, T.; Kim, S.H.; Jeong, J. An Antibody-Immobilized Silica Inverse Opal Nanostructure for Label-Free Optical Biosensors. Sensors 2018, 18, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Wen, L.; Heng, L.; Song, Y. Highly sensitive, selective and reusable mercury(II) ion sensor based on a ssDNA-functionalized photonic crystal film. Phys. Chem. Chem. Phys. 2013, 15, 11943–11949. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, E.; Park, J. Highly sensitive protein detection using quenching effects from aptamer-functionalized photonic crystals. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 839–841. [Google Scholar]
- Zhou, J.; Rossi, J.J. Cell-type-specific, Aptamer-functionalized Agents for Targeted Disease Therapy. Mol. Nucleic Acids 2014, 3, e169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, H.; Luan, C.; Liu, Y.; Chen, B.; Zhao, Y. Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens. Bioelectron. 2018, 100, 404–410. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Won, S.; Tae, H.; Lim, G.; Koh, W.G. Preparation of photolithographically patterned inverse opal hydrogel microstructures and its application to protein patterning. Biosens. Bioelectron. 2012, 35, 243–250. [Google Scholar] [CrossRef]
- Nikolaev, I.S.; Vos, W.L.; Koenderink, A.F. Accurate calculation of the local density of optical states in inverse-opal photonic crystals. J. Opt. Soc. Am. B Opt. Phys. B 2009, 26, 987–997. [Google Scholar] [CrossRef]
- Chiappini, A.; Armellini, C.; Chiasera, A.; Ferrari, M.; Fortes, L.; Gonçalves, M.C.; Guider, R.; Jestin, Y.; Retoux, R.; Nunzi Conti, G.; Pelli, S.; et al. An alternative method to obtain direct opal photonic crystal structures. J. Non-Cryst. Solids 2009, 355, 1167–1170. [Google Scholar] [CrossRef]
- Reculusa, S.; Ravaine, S. Synthesis of Colloidal Crystals of Controllable Thickness through the Langmuir-Blodgett Technique. Chem. Mater. 2003, 15, 598–605. [Google Scholar] [CrossRef]
- Cunningham, B.T. Photonic Crystal Surfaces as a General Purpose Platform for Label-Free and Fluorescent Assays. JALA 2010, 15, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Busch, K.; John, S. Photonic band gap formation in certain self-organizing systems. Phys. Rev. E 1998, 58, 3896–3908. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Bai, X.; Liu, Q.; Zhub, Y. Modified spontaneous emissions of europium complex in weak PMMA opals. Phys. Chem. Chem. Phys. 2011, 13, 18023–18030. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, P.; Xu, W.; Xu, S.; Jiang, Y.; Chen, X.; Song, H. NaYF4:Yb3+,Tm3+ inverse opal photonic crystals and NaYF4:Yb3+,Tm3+/TiO2 composites: Synthesis, highly improved upconversion properties and NIR photoelectric response. J. Mater. Chem. C 2016, 4, 659–662. [Google Scholar] [CrossRef]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio-sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, A.; Pasquardini, L.; Nodehi, S.; Armellini, C.; Bazzanella, N.; Lunelli, L.; Pelli, S.; Ferrari, M.; Pietralunga, S.M. Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals. Sensors 2018, 18, 4326. https://doi.org/10.3390/s18124326
Chiappini A, Pasquardini L, Nodehi S, Armellini C, Bazzanella N, Lunelli L, Pelli S, Ferrari M, Pietralunga SM. Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals. Sensors. 2018; 18(12):4326. https://doi.org/10.3390/s18124326
Chicago/Turabian StyleChiappini, Andrea, Laura Pasquardini, Somayeh Nodehi, Cristina Armellini, Nicola Bazzanella, Lorenzo Lunelli, Stefano Pelli, Maurizio Ferrari, and Silvia M. Pietralunga. 2018. "Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals" Sensors 18, no. 12: 4326. https://doi.org/10.3390/s18124326