Electromagnetic–Acoustic Sensing for Biomedical Applications
Abstract
:1. Introduction
2. Principles
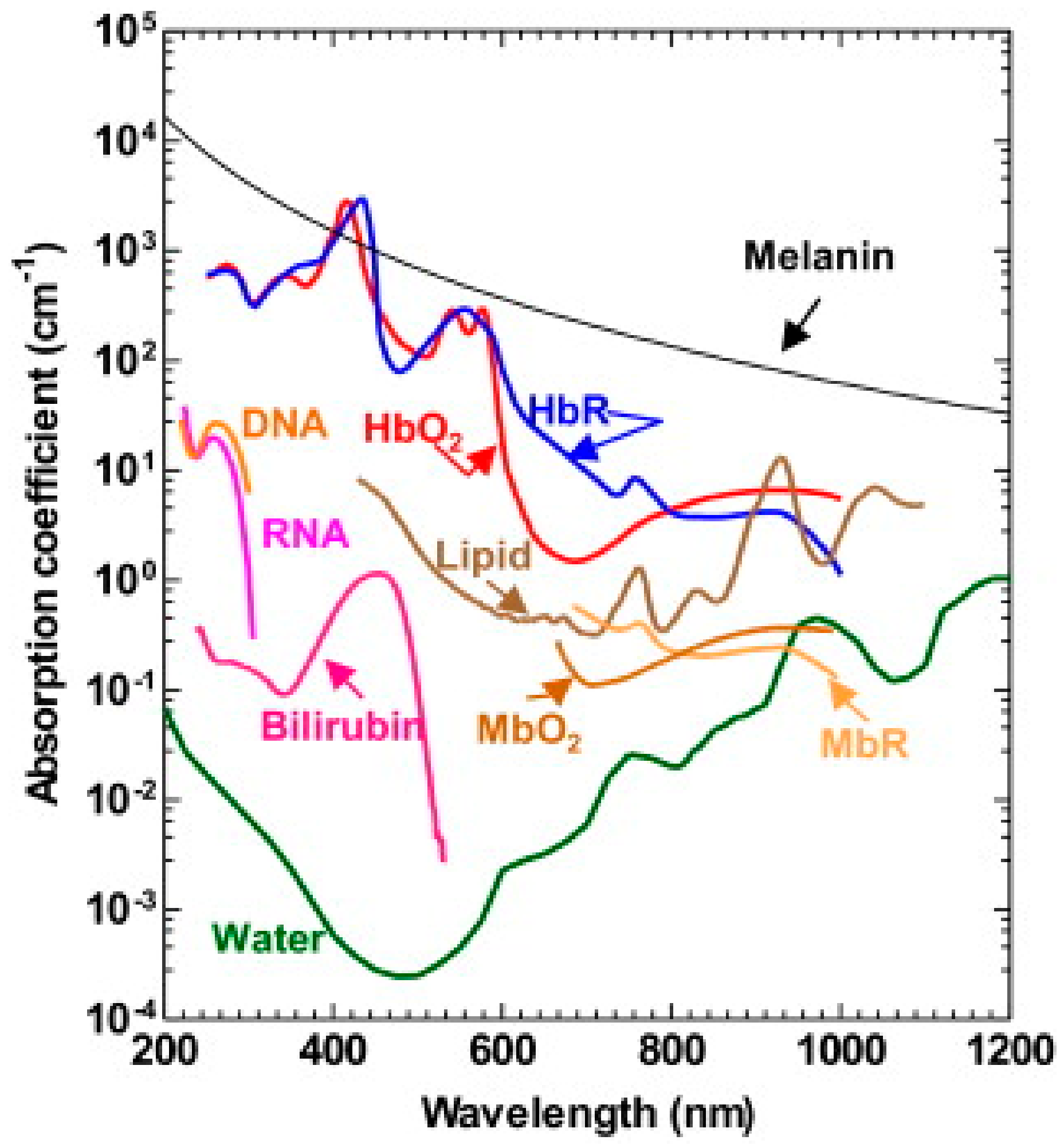
2.1. EM Absorption and Heating
2.1.1. Light-Induced Photoacoustics (PA)
2.1.2. Microwave-Induced Thermoacoustics (TA)
2.1.3. X-ray-Induced Thermoacoustics (XTA)
2.1.4. Magnetic Modulated Thermoacoustics (MMTA)
2.2. EM Acoustic Generation
3. Biomedical Sensing Application
3.1. Structural and Molecular Imaging
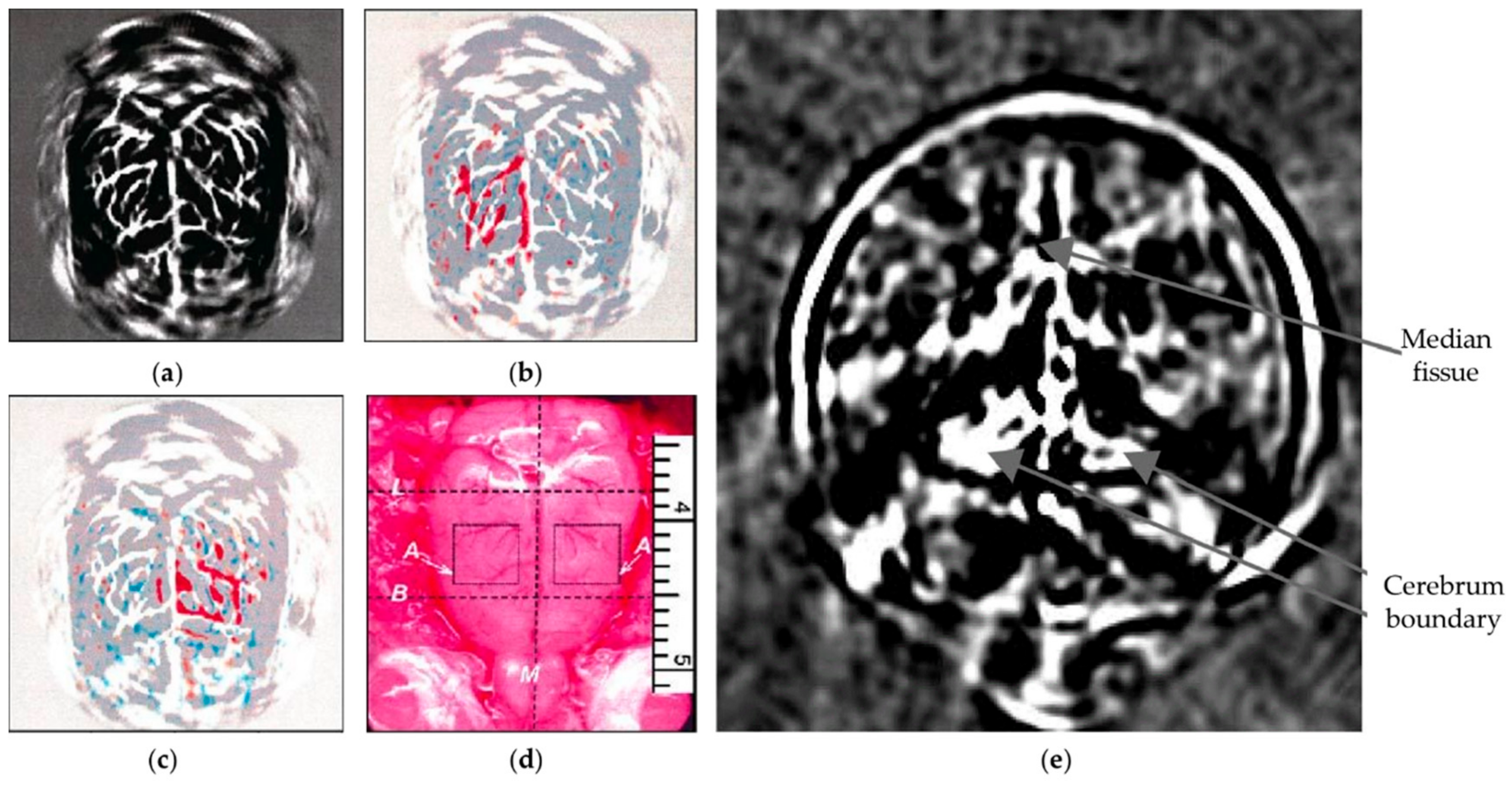
3.1.1. Brain Imaging
3.1.2. Whole-Body Imaging
3.1.3. Molecular Imaging
3.2. Flowmetry
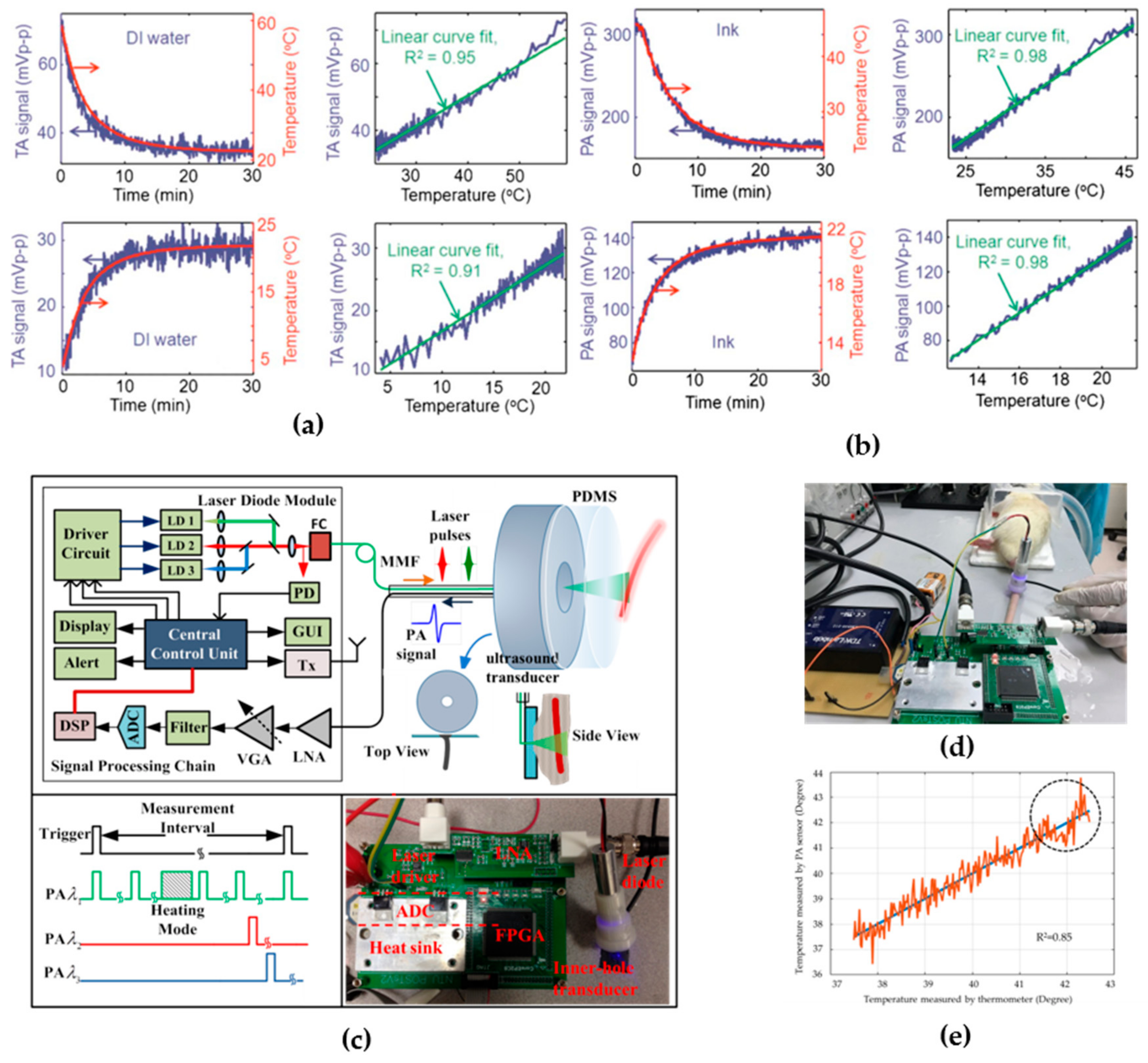
3.3. Thermometry
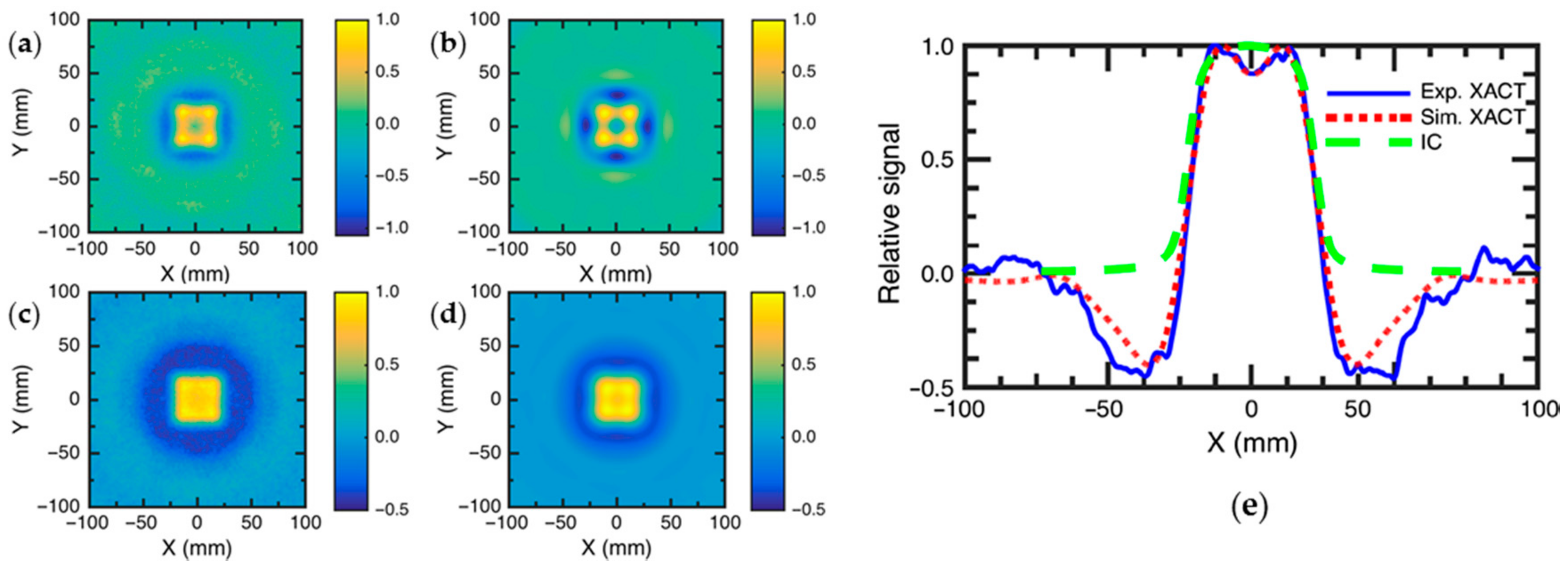
3.4. Dosimetry for Radiation Therapy
3.5. Hemoglobin Oxygen Saturation (SO2) Sensing
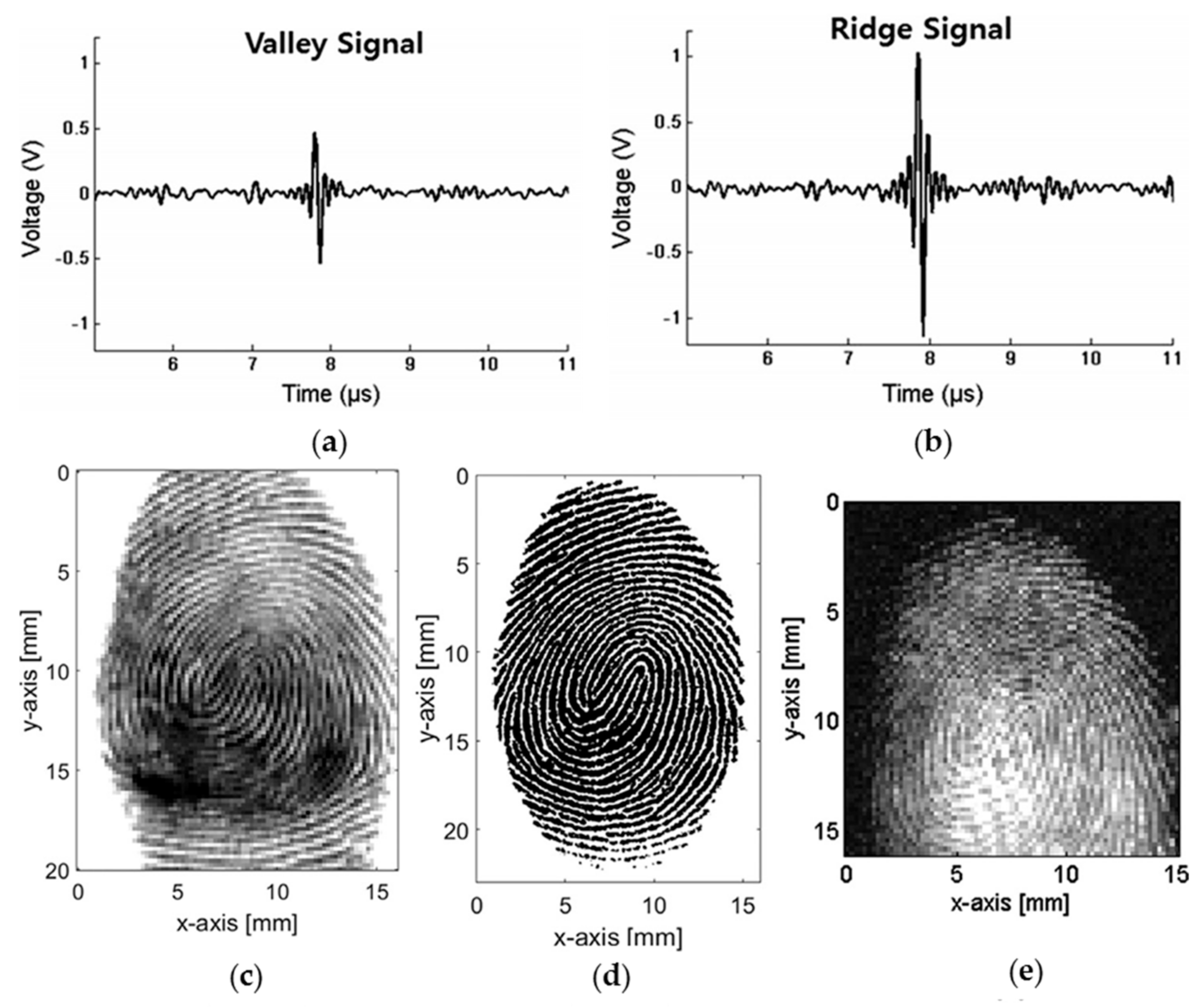
3.6. Fingerprint Sensing
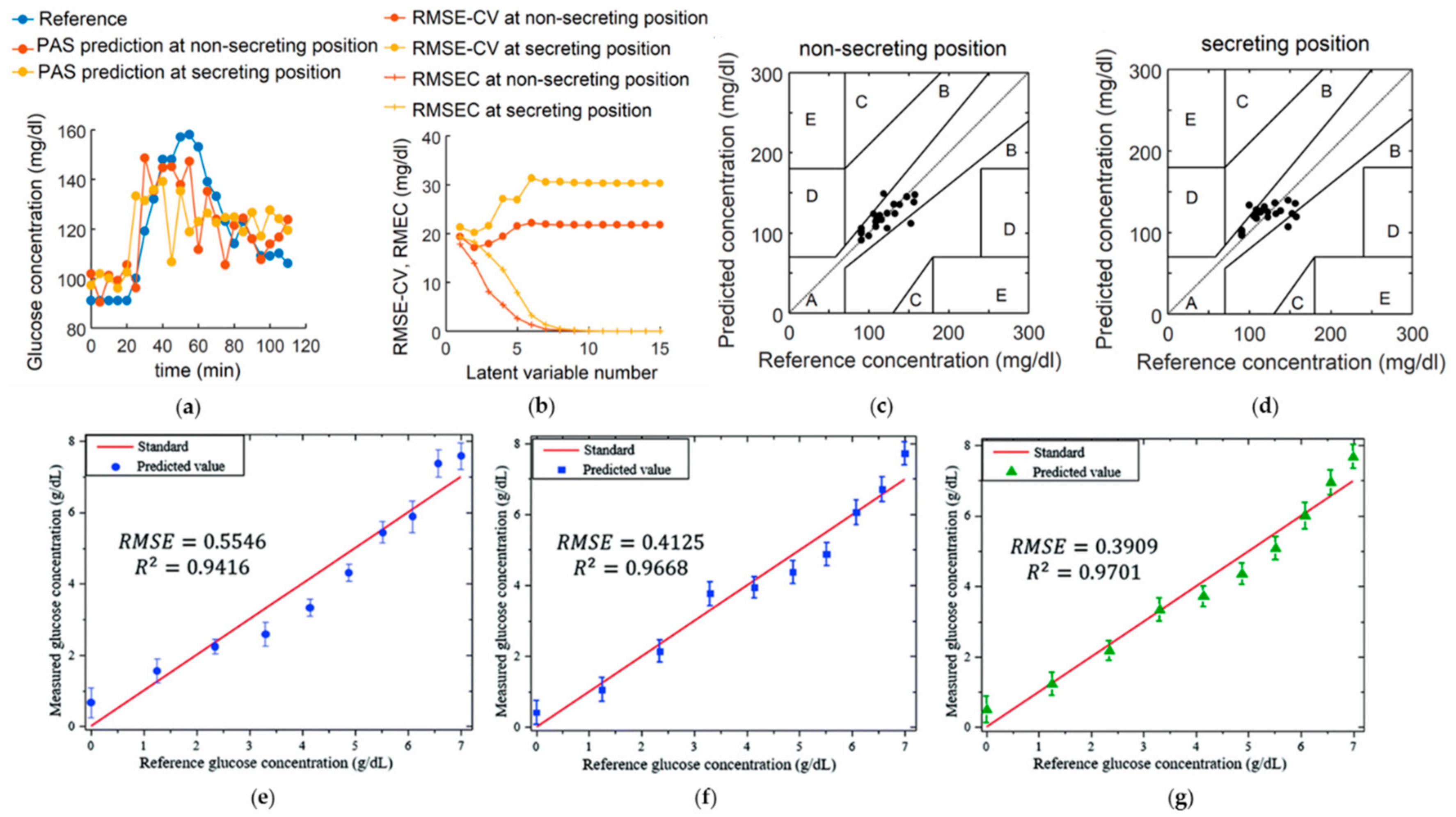
3.7. Glucose Sensing
3.8. PH Sensing
4. Prospects and Conclusions
Funding
Conflicts of Interest
References
- Hu, S.; Hoffman, E.; Reinhardt, J. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans. Med. Imaging 2001, 20, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsler, W. Optical Coherence Tomography: Technology and Applications; Springer: New York, NY, USA, 2008. [Google Scholar]
- Corlu, A.; Choe, R.; Durduran, T.; Rosen, M.A.; Schweiger, M.; Arridge, S.R.; Schnall, M.D.; Yodh, A.G. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt. Express 2007, 15, 6696. [Google Scholar] [CrossRef] [PubMed]
- Villringer, A.; Planck, J.; Hock, C.; Schleinkofer, L.; Dirnagl, U. Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci. Lett. 1993, 154, 101–104. [Google Scholar] [CrossRef]
- Heilemann, M.; Van De Linde, S.; Schüttpelz, M.; Kasper, R.; Seefeldt, B.; Mukherjee, A.; Tinnefeld, P.; Sauer, M. Subdiffraction-Resolution Fluorescence Imaging with Conventional Fluorescent Probes. Angew. Chem. Int. Ed. 2008, 47, 6172–6176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Wu, H. Biomedical Optics: Principles and Imaging; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Semenov, S.Y.; Corfield, D.R. Microwave Tomography for Brain Imaging: Feasibility Assessment for Stroke Detection. Int. J. Antennas Propag. 2008, 2008, 1–8. [Google Scholar] [CrossRef]
- Ku, G.; Wang, L.V. Scanning microwave-induced thermoacoustic tomography: Signal, resolution, and contrast. Med. Phys. 2001, 28, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Stewart, W.R.; Gough, W. Magnetic Induction Tomography: A Measuring System for Biological Tissues. Ann. N. Y. Acad. Sci. 1999, 873, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Grasland-Mongrain, P.; Mari, J.-M.; Chapelon, J.-Y.; Lafon, C. Lorentz force electrical impedance tomography. Irbm 2013, 34, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Shah, J.; Balaban, R. Hall effect imaging. IEEE Trans. Biomed. Eng. 1998, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J.; Seo, J.K. Magnetic resonance electrical impedance tomography (MREIT) for high-resolution conductivity imaging. Physiol. Meas. 2008, 29, R1. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, s3-20, 305–324. [Google Scholar] [CrossRef]
- Kruger, R.A.; Liu, P.; Fang, Y.R.; Appledorn, C.R. Photoacoustic ultrasound (PAUS)-Reconstruction tomography. Med. Phys. 1995, 22, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Hoelen, C.G.A.; Mul, F.F.M.D.; Pongers, R.; Dekker, A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Opt. Lett. 1998, 23, 648. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A.; Reinecke, D.R.; Kruger, G.A. Thermoacoustic computed tomography-technical considerations. Med. Phys. 1999, 26, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A.; Kiser, W.L.; Reinecke, D.R.; Kruger, G.A.; Miller, K.D. Thermoacoustic Molecular Imaging of Small Animals. Mol. Imaging 2003, 2, 153535002003031. [Google Scholar] [CrossRef]
- Wang, L.V.; Zhao, X.; Sun, H.; Ku, G. Microwave-induced acoustic imaging of biological tissues. Rev. Sci. Instrum. 1999, 70, 3744–3748. [Google Scholar] [CrossRef] [Green Version]
- Ku, G.; Wang, L.V. Scanning thermoacoustic tomography in biological tissue. Med. Phys. 2000, 27, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Ku, G.; Wang, L.V. Microwave-induced thermoacoustic tomography using multi-sector scanning. Med. Phys. 2001, 28, 1958–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.; Han, B.; Carpenter, C.; Pratx, G.; Kuang, Y.; Xing, L. X-ray acoustic computed tomography with pulsed X-ray beam from a medical linear accelerator. Med. Phys. 2012, 40, 010701. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Tang, S.; Ahmad, M.; Xing, L. High Resolution X-ray-Induced Acoustic Tomography. Sci. Rep. 2016, 6, 26118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Gao, F.; Zheng, Y. Modulatable magnetically mediated thermoacoustic imaging with magnetic nanoparticles. Appl. Phys. Lett. 2015, 106, 153702. [Google Scholar] [CrossRef]
- Kellnberger, S.; Rosenthal, A.; Myklatun, A.; Westmeyer, G.G.; Sergiadis, G.; Ntziachristos, V. Magnetoacoustic Sensing of Magnetic Nanoparticles. Phys. Rev. Lett. 2016, 116, 108103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Maslov, K.I.; Puckett, E.R.; Rowland, K.J.; Warner, B.W.; Wang, L.V. Double-illumination photoacoustic microscopy. Opt. Lett. 2012, 37, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Wang, L.V. Grueneisen Relaxation Photoacoustic Microscopy. Phys. Rev. Lett. 2014, 113. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, B.; Ji, L.; Jiang, H. 4-D Photoacoustic Tomography. Sci. Rep. 2013, 3, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Coleman, J.E.; Dai, X.; Jiang, H. Wearable 3-D Photoacoustic Tomography for Functional Brain Imaging in Behaving Rats. Sci. Rep. 2016, 6, 25470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Wang, L. Time-domain reconstruction for thermoacoustic tomography in a spherical geometry. IEEE Trans. Med. Imaging 2002, 21, 814–822. [Google Scholar] [PubMed] [Green Version]
- Xu, Y.; Feng, D.; Wang, L. Exact frequency-domain reconstruction for thermoacoustic tomography. I. Planar geometry. IEEE Trans. Med. Imaging 2002, 21, 823–828. [Google Scholar] [PubMed]
- Xu, Y.; Xu, M.; Wang, L. Exact frequency-domain reconstruction for thermoacoustic tomography. II. Cylindrical geometry. IEEE Trans. Med. Imaging 2002, 21, 829–833. [Google Scholar] [PubMed] [Green Version]
- Mohajerani, P.; Kellnberger, S.; Ntziachristos, V. Fast Fourier backprojection for frequency-domain optoacoustic tomography. Opt. Lett. 2014, 39, 5455–5458. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, X.; Gao, F.; Jin, H.; Zhang, R.; Luo, Y.; Zheng, Y. GPU-accelerated two dimensional synthetic aperture focusing for photoacoustic microscopy. APL Photonics 2018, 3, 026101. [Google Scholar] [CrossRef]
- Fang, H.; Maslov, K.; Wang, L.V. Photoacoustic Doppler Effect from Flowing Small Light-Absorbing Particles. Phys. Rev. Lett. 2007, 99, 184501. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Wang, L.V. Thermoacoustic and photoacoustic sensing of temperature. J. Biomed. Opt. 2009, 14, 054024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xie, X.; Ku, G.; Wang, L.V.; Stoica, G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J. Biomed. Opt. 2006, 11, 024015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, G.; Fornage, B.D.; Jin, X.; Xu, M.; Hunt, K.K.; Wang, L.V. Thermoacoustic and Photoacoustic Tomography of Thick Biological Tissues toward Breast Imaging. Technol. Cancer Res. Treat. 2005, 4, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Xing, W.; Maslov, K.I.; Cornelius, L.A.; Wang, L.V. Handheld photoacoustic microscopy to detect melanoma depth in vivo. Opt. Lett. 2014, 39, 4731–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.Y.; Park, K.K. Fingerprint imaging of dry finger using photoacoustics. J. Acoust. Soc. Am. 2017, 141, EL205–EL209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickling, S.; Hobson, M.; Naqa, I.E. Characterization of X-ray Acoustic Computed Tomography for Applications in Radiotherapy Dosimetry. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 337–344. [Google Scholar] [CrossRef]
- Kottmann, J.; Rey, J.; Sigrist, M. Mid-Infrared Photoacoustic Detection of Glucose in Human Skin: Towards Non-Invasive Diagnostics. Sensors 2016, 16, 1663. [Google Scholar] [CrossRef] [PubMed]
- Chatni, M.R.; Yao, J.; Danielli, A.; Favazza, C.P.; Maslov, K.I.; Wang, L.V. Functional photoacoustic microscopy of pH. J. Biomed. Opt. 2011, 16, 100503. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 2012, 18, 1297–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, Z.; Carney, P.R.; Yuan, Z.; Chen, H.; Roper, S.N.; Jiang, H. Non-invasive imaging of epileptic seizuresin vivousing photoacoustic tomography. Phys. Med. Biol. 2008, 53, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.-K.; Maslov, K.; Shung, K.K.; Zhou, Q.; Wang, L.V. In vivo label-free photoacoustic microscopy of cell nuclei by excitation of DNA and RNA. Opt. Lett. 2010, 35, 4139–4141. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Y.; Wang, L.V.; Fang, Y.R.; Zanelli, C.I.; Howard, S.M. Imaging of high-intensity focused ultrasound-induced lesions in soft biological tissue using thermoacoustic tomography. Med. Phys. 2004, 32, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chamberland, D.L.; Carson, P.L.; Fowlkes, J.B.; Bude, R.O.; Jamadar, D.A.; Roessler, B.J. Imaging of joints with laser-based photoacoustic tomography: An animal study. Med. Phys. 2006, 33, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pramanik, M.; Senpan, A.; Ghosh, S.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials 2010, 31, 4088–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemp, R.; Song, L.; Bitton, R.; Shung, K.; Wang, L. Realtime Photoacoustic Microscopy of Murine Cardiovascular Dynamics. Opt. Express 2008, 16, 18551–18556. [Google Scholar] [CrossRef] [PubMed]
- Barnes, F.S.; Greenebaum, B. Handbook of Biological Effects of Electromagnetic Fields; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Taroni, P.; Pifferi, A.; Torricelli, A.; Comelli, D.; Cubeddu, R. In vivo absorption and scattering spectroscopy of biological tissues. Photochem. Photobiol. Sci. 2003, 2, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Adey, W.R. Tissue interactions with nonionizing electromagnetic fields. Physiol. Rev. 1981, 61, 435–514. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 5007. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Biomedical Photonics Handbook; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wang, L.V. Tutorial on Photoacoustic Microscopy and Computed Tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Danielli, A.; Maslov, K.; Favazza, C.P.; Xia, J.; Wang, L.V. Nonlinear photoacoustic spectroscopy of hemoglobin. Appl. Phys. Lett. 2015, 106, 203701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhang, Y.S.; Yao, D.-K.; Xia, Y.; Wang, L.V. Label-free photoacoustic microscopy of cytochromes. J. Biomed. Opt. 2013, 18, 020504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.-K. Optimal ultraviolet wavelength for in vivo photoacoustic imaging of cell nuclei. J. Biomed. Opt. 2012, 17, 056004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Yao, J.; Li, L.; Wang, L.V. In vivophotoacoustic tomography of myoglobin oxygen saturation. J. Biomed. Opt. 2015, 21, 061002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, G.S.; Phillips, E.H.; Goergen, C.J. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomed. Opt. Express 2017, 8, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kole, A.; Hui, J.; Zhang, Y.; Mai, J.; Alloosh, M.; Sturek, M.; Cheng, J.-X. Fast assessment of lipid content in arteries in vivo by intravascular photoacoustic tomography. Sci. Rep. 2018, 8, 2400. [Google Scholar] [CrossRef] [PubMed]
- Christison, G.B.; Mackenzie, H.A. Laser photoacoustic determination of physiological glucose concentrations in human whole blood. Med. Biol. Eng. Comput. 1993, 31, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Duck, F.A. Physical Properties of Tissue: A Comprehensive Reference Book; Institute of Physics and Engineering in Medicine: York, UK, 2012. [Google Scholar]
- Bowen, T.; Chen, C.X.; Liew, S.C.; Lutz, W.R.; Nasoni, R.L. Observation of ultrasonic emission from edges of therapeutic X-ray beams. Phys. Med. Biol. 1991, 36, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.E.; Pastor, G.M.; Bennemann, K.H. Theory for the Photoacoustic Response to X-ray Absorption. Phys. Rev. Lett. 1988, 61, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Xing, D.; Zhou, Q.; Yang, D.; Guo, H. Microwave-induced thermoacoustic scanning CT for high-contrast and noninvasive breast cancer imaging. Med. Phys. 2008, 35, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Piao, D.; Towner, R.A.; Smith, N.; Chen, W.R. Magnetothermoacoustics from magnetic nanoparticles by short bursting or frequency chirped alternating magnetic field: A theoretical feasibility analysis. Med. Phys. 2013, 40, 063301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Gusev, V.E.; Karabutov, A.A. Laser Optoacoustics; American Institute of Physics: New York, NY, USA, 1993. [Google Scholar]
- Kinsler, L.E. Fundamentals of Acoustics; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Hu, S.; Maslov, K.; Tsytsarev, V.; Wang, L.V. Functional transcranial brain imaging by optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2009, 14, 040503. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xia, J.; Li, G.; Garcia-Uribe, A.; Sheng, Q.; Anastasio, M.A.; Wang, L.V. Label-free photoacoustic tomography of whole mouse brain structures ex vivo. Neurophotonics 2016, 3, 035001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, H.; Huang, X.; Rebling, J.; Zwack, M.; Gottschalk, S.; Razansky, D. Virtual craniotomy for high-resolution optoacoustic brain microscopy. Sci. Rep. 2018, 8, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, L. Rhesus monkey brain imaging through intact skull with thermoacoustic tomography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 542–548. [Google Scholar] [PubMed]
- Yao, J.; Wang, L.V. Photoacoustic brain imaging: from microscopic to macroscopic scales. Neurophotonics 2014, 1, 011003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brecht, H.-P.; Su, R.; Fronheiser, M.; Ermilov, S.A.; Conjusteau, A.; Oraevsky, A.A. Whole-body three-dimensional optoacoustic tomography system for small animals. J. Biomed. Opt. 2009, 14, 064007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Gong, L.; Xu, X.; Hai, P.; Shen, Y.; Suzuki, Y.; Wang, L.V. Motionless volumetric photoacoustic microscopy with spatially invariant resolution. Nat. Commun. 2017, 8, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, M.; Kim, J.; Kim, C. Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput. 2014, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Su, R. Three-dimensional optoacoustic imaging as a new noninvasive technique to study long-term biodistribution of optical contrast agents in small animal models. J. Biomed. Opt. 2012, 17, 101506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Distel, M.; Deán-Ben, X.L.; Ntziachristos, V.; Razansky, D. Non-invasive whole-body imaging of adult zebrafish with optoacoustic tomography. Phys. Med. Biol. 2012, 57, 7227–7237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Wang, L.V. Small-Animal Whole-Body Photoacoustic Tomography: A Review. IEEE Trans. Biomed. Eng. 2014, 61, 1380–1389. [Google Scholar] [PubMed] [Green Version]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, R.; Tabata, T.; Yoshizawa, S.; Umemura, S.-I.; Saijo, Y. Visualization of murine lymph vessels using photoacoustic imaging with contrast agents. Photoacoustics 2018, 9, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zerda, A.D.L.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, H.; Yang, S.; Xing, D. Microwave-induced thermoacoustic computed tomography with a clinical contrast agent of NMG2[Gd(DTPA)]. Appl. Phys. Lett. 2012, 100, 033701. [Google Scholar] [CrossRef]
- Nie, L.; Ou, Z.; Yang, S.; Xing, D. Thermoacoustic molecular tomography with magnetic nanoparticle contrast agents for targeted tumor detection. Med. Phys. 2010, 37, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Yoon, S.J.; Frey, W.; Dockery, M.; Emelianov, S. Dynamic contrast-enhanced photoacoustic imaging using photothermal stimuli-responsive composite nanomodulators. Nat. Commun. 2017, 8, 15782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Huang, L.; Jiang, M.; Jiang, H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallenbeck, J.M.; Dutka, A.J.; Tanishima, T.; Kochanek, P.M.; Kumaroo, K.K.; Thompson, C.B.; Obrenovitch, T.P.; Contreras, T.J. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke 1986, 17, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Brunker, J.; Beard, P. Erratum: Erratum: Acoustic resolution photoacoustic Doppler velocimetry in blood-mimicking fluids. Sci. Rep. 2016, 6, 23881. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Maslov, K.I.; Wang, L.V. In vivo Photoacoustic Tomography of Total Blood Flow and Potential Imaging of Cancer Angiogenesis and Hypermetabolism. Technol. Cancer Res. Treat. 2012, 11, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Zhou, Y.; Maslov, K.I.; Wang, L.V. Cross-correlation-based transverse flow measurements using optical resolution photoacoustic microscopy with a digital micromirror device. J. Biomed. Opt. 2013, 18, 096004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.-C.; Huang, S.-W.; Wei, C.-W.; Chiou, Y.-C.; Chen, C.-D.; Wang, C.-R.C. Photoacoustic flow measurements by use of laser-induced shape transitions of gold nanorods. Opt. Lett. 2005, 30, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Yao, J. Transverse flow imaging based on photoacoustic Doppler bandwidth broadening. J. Biomed. Opt. 2010, 15, 021304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Ke, H.; Tai, S.; Zhou, Y.; Wang, L.V. Absolute photoacoustic thermometry in deep tissue. Opt. Lett. 2013, 38, 5228–5231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhamami, M.; Kolios, M.C.; Tavakkoli, J. Photoacoustic detection and optical spectroscopy of high-intensity focused ultrasound-induced thermal lesions in biologic tissue. Med. Phys. 2014, 41, 053502. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Park, S.; Aglyamov, S.; Larson, T.; Ma, L.; Sokolov, K.; Johnston, K.; Milner, T.; Emelianov, S.Y. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008, 13, 034024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landa, F.J.O.; Deán-Ben, X.L.; Sroka, R.; Razansky, D. Volumetric Optoacoustic Temperature Mapping in Photothermal Therapy. Sci. Rep. 2017, 7, 9695. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, X.; Ruochong, Z.; Zheng, Y. Portable photoacoustic system for noninvasive blood temperature measurement. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018. [Google Scholar]
- Hossain, M.; Su, M. Nanoparticle Location and Material-Dependent Dose Enhancement in X-ray Radiation Therapy. J. Phys. Chem. C 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijnheer, B.; Beddar, S.; Izewska, J.; Reft, C. In vivo dosimetry in external beam radiotherapy. Med. Phys. 2013, 40, 070903. [Google Scholar] [CrossRef] [PubMed]
- Hickling, S.; Lei, H.; Hobson, M.; Léger, P.; Wang, X.; Naqa, I.E. Experimental evaluation of X-ray acoustic computed tomography for radiotherapy dosimetry applications. Med. Phys. 2017, 44, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Nguyen, D.H.; Zarafshani, A.; Ramseyer, C.; Zheng, B.; Liu, H.; Xiang, L. X-ray-induced acoustic computed tomography with an ultrasound transducer ring-array. Appl. Phys. Lett. 2017, 110, 103504. [Google Scholar] [CrossRef]
- Hickling, S.; Hobson, M.; Naqa, I.E. Feasibility of X-ray Acoustic Computed Tomography as a Tool for Noninvasive Volumetric In Vivo Dosimetry. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S843. [Google Scholar] [CrossRef]
- Maslov, K.; Zhang, H.F.; Wang, L.V. Effects of wavelength-dependent fluence attenuation on the noninvasive photoacoustic imaging of hemoglobin oxygen saturation in subcutaneous vasculature in vivo. Inverse Probl. 2007, 23, S113. [Google Scholar] [CrossRef]
- Colak, S.; Mark, M.V.D.; Hooft, G.T; Hoogenraad, J.; Linden, E.V.D.; Kuijpers, F. Clinical optical tomography and NIR spectroscopy for breast cancer detection. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1143–1158. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of hemoglobin oxygen saturation variations in single vesselsin vivousing photoacoustic microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.; Yang, J.-M.; Maslov, K.I.; Wong, T.T.W.; Li, L.; Huang, C.-H.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Peng, Q.; Feng, X.; Gao, B.; Zheng, Y. Single-Wavelength Blood Oxygen Saturation Sensing With Combined Optical Absorption and Scattering. IEEE Sensors J. 2016, 16, 1943–1948. [Google Scholar] [CrossRef]
- Cao, F.; Qiu, Z.; Li, H.; Lai, P. Photoacoustic Imaging in Oxygen Detection. Appl. Sci. 2017, 7, 1262. [Google Scholar] [CrossRef]
- Quan, K.M.; Christison, G.B.; Mackenzie, H.A.; Hodgson, P. Glucose determination by a pulsed photoacoustic technique: An experimental study using a gelatin-based tissue phantom. Phys. Med. Biol. 1993, 38, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.Y.; Ahn, C.-G.; Jeong, E.-J.; Kim, B.K. In vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Gao, F.; Feng, X.; Liu, S.; Kishor, R.; Luo, Y.; Zheng, Y. Noninvasive photoacoustic measurement of glucose by data fusion. Analyst 2017, 142, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Williams, G.; Li, H.; Wang, D.; Wu, H.; Li, S.; Zhu, L.-M. Glucose- and temperature-sensitive nanoparticles for insulin delivery. Int. J. Nanomed. 2017, 12, 4037–4057. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Yoon, H.K.; Lee, Y.E.K.; Kopelman, R.; Wang, X. Sonophoric nanoprobe aided pH measurement in vivo using photoacoustic spectroscopy. Analyst 2013, 138, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Chen, J.; Zeng, J.; Cheng, Z.; Liu, Z. A Self-Assembled Albumin-Based Nanoprobe for In Vivo Ratiometric Photoacoustic pH Imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Pu, K. Semiconducting oligomer nanoparticle as activatable photoacoustic probe with amplified brightness for in vivo imaging of pH. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1856. [Google Scholar] [CrossRef]
- Jo, J.; Lee, C.H.; Kopelman, R.; Wang, X. In vivo quantitative imaging of tumor pH by nanosonophore assisted multispectral photoacoustic imaging. Nat. Commun. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Seeger, M.; Jiang, Y.; Mishra, A.; Sigmund, F.; Stelzl, A.; Lauri, A.; Symvoulidis, P.; Rolbieski, H.; Preller, M.; et al. Calcium Sensor for Photoacoustic Imaging. J. Am. Chem. Soc. 2017, 140, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Folz, J.; Zhang, W.; Jo, J.; Tan, J.W.Y.; Wang, X.; Kopelman, R. Correction to Ion-Selective Nanosensor for Photoacoustic and Fluorescence Imaging of Potassium. Anal. Chem. 2017, 89, 13674–13674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrmohammadi, M.; Yoon, S.J.; Yeager, D.; Emelianov, S.Y. Photoacoustic Imaging for Cancer Detection and Staging. Curr. Mol. Imaging 2013, 2, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Hysi, E.; Wirtzfeld, L.A.; May, J.P.; Undzys, E.; Li, S.-D.; Kolios, M.C. Photoacoustic signal characterization of cancer treatment response: Correlation with changes in tumor oxygenation. Photoacoustics 2017, 5, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Lou, C.; Yang, S.; Xing, D. Three-dimensional thermoacoustic imaging for early breast cancer detection. Med. Phys. 2012, 39, 6738–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bauer, D.R.; Witte, R.; Xin, H. Microwave-Induced Thermoacoustic Imaging Model for Potential Breast Cancer Detection. IEEE Trans. Biomed. Eng. 2012, 59, 2782–2791. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-L.; Wang, J.C.; Schwartz, J.A.; Gill-Sharp, K.L.; Stoica, G.; Wang, L.V. In-vivo photoacoustic microscopy of nanoshell extravasation from solid tumor vasculature. J. Biomed. Opt. 2009, 14, 010507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viator, J.A.; Gupta, S.; Goldschmidt, B.S.; Bhattacharyya, K.; Kannan, R.; Shukla, R.; Dale, P.S.; Boote, E.; Katti, K. Gold Nanoparticle Mediated Detection of Prostate Cancer Cells Using Photoacoustic Flowmetry with Optical Reflectance. J. Biomed. Nanotechnol. 2010, 6, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.; Zharov, V. Circulating Tumor Cell Detection and Capture by Photoacoustic Flow Cytometry in Vivo and ex Vivo. Cancers 2013, 5, 1691–1738. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.-W.; Yang, L.; Zharov, V.P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, W.J.; Kim, C.; Berezin, M.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X.; et al. Noninvasive Photoacoustic and Fluorescence Sentinel Lymph Node Identification using Dye-Loaded Perfluorocarbon Nanoparticles. ACS Nano 2010, 5, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhang, R.; Luo, Y.; Zheng, Y. Magnetoacoustic microscopic imaging of conductive objects and nanoparticles distribution. J. Appl. Phys. 2017, 122, 124502. [Google Scholar] [CrossRef]
- Emerson, J.F.; Chang, D.B.; Mcnaughton, S.; Jeong, J.S.; Shung, K.K.; Cerwin, S.A. Electromagnetic acoustic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, L.; Li, X.; He, B. B-Scan Based Acoustic Source Reconstruction for Magnetoacoustic Tomography with Magnetic Induction (MAT-MI). IEEE Trans. Biomed. Eng. 2011, 58, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Grasland-Mongrain, P.; Souchon, R.; Cartellier, F.; Zorgani, A.; Chapelon, J.Y.; Lafon, C.; Catheline, S. Imaging of Shear Waves Induced by Lorentz Force in Soft Tissues. Phys. Rev. Lett. 2014, 113. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohammadi, M.; Oh, J.; Mallidi, S.; Emelianov, S.Y. Pulsed Magneto-motive Ultrasound Imaging Using Ultrasmall Magnetic Nanoprobes. Mol. Imaging 2011, 10, 7290–2010. [Google Scholar] [CrossRef]
- Qu, M.; Mallidi, S.; Mehrmohammadi, M.; Truby, R.; Homan, K.; Joshi, P.; Chen, Y.-S.; Sokolov, K.; Emelianov, S. Magneto-photo-acoustic imaging. Biomed. Opt. Express 2011, 2, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Duan, T.; Lan, H.; Zhou, M.; Gao, F. Review of Low-Cost Photoacoustic Sensing and Imaging Based on Laser Diode and Light-Emitting Diode. Sensors 2018, 18, 2264. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.V. Sensitivity of photoacoustic microscopy. Photoacoustics 2014, 2, 87–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upputuri, P.K.; Periyasamy, V.; Kalva, S.K.; Pramanik, M. A High-performance Compact Photoacoustic Tomography System for In Vivo Small-animal Brain Imaging. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, M.W.; Hunt, H.K. Hand-held optoacoustic imaging: A review. Photoacoustics 2018, 11, 14–27. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, R.; Zheng, Z.; Zheng, Y. Electromagnetic–Acoustic Sensing for Biomedical Applications. Sensors 2018, 18, 3203. https://doi.org/10.3390/s18103203
Liu S, Zhang R, Zheng Z, Zheng Y. Electromagnetic–Acoustic Sensing for Biomedical Applications. Sensors. 2018; 18(10):3203. https://doi.org/10.3390/s18103203
Chicago/Turabian StyleLiu, Siyu, Ruochong Zhang, Zesheng Zheng, and Yuanjin Zheng. 2018. "Electromagnetic–Acoustic Sensing for Biomedical Applications" Sensors 18, no. 10: 3203. https://doi.org/10.3390/s18103203




