Motion Tracking System for Robust Non-Contact Blood Perfusion Sensor
Abstract
:1. Introduction
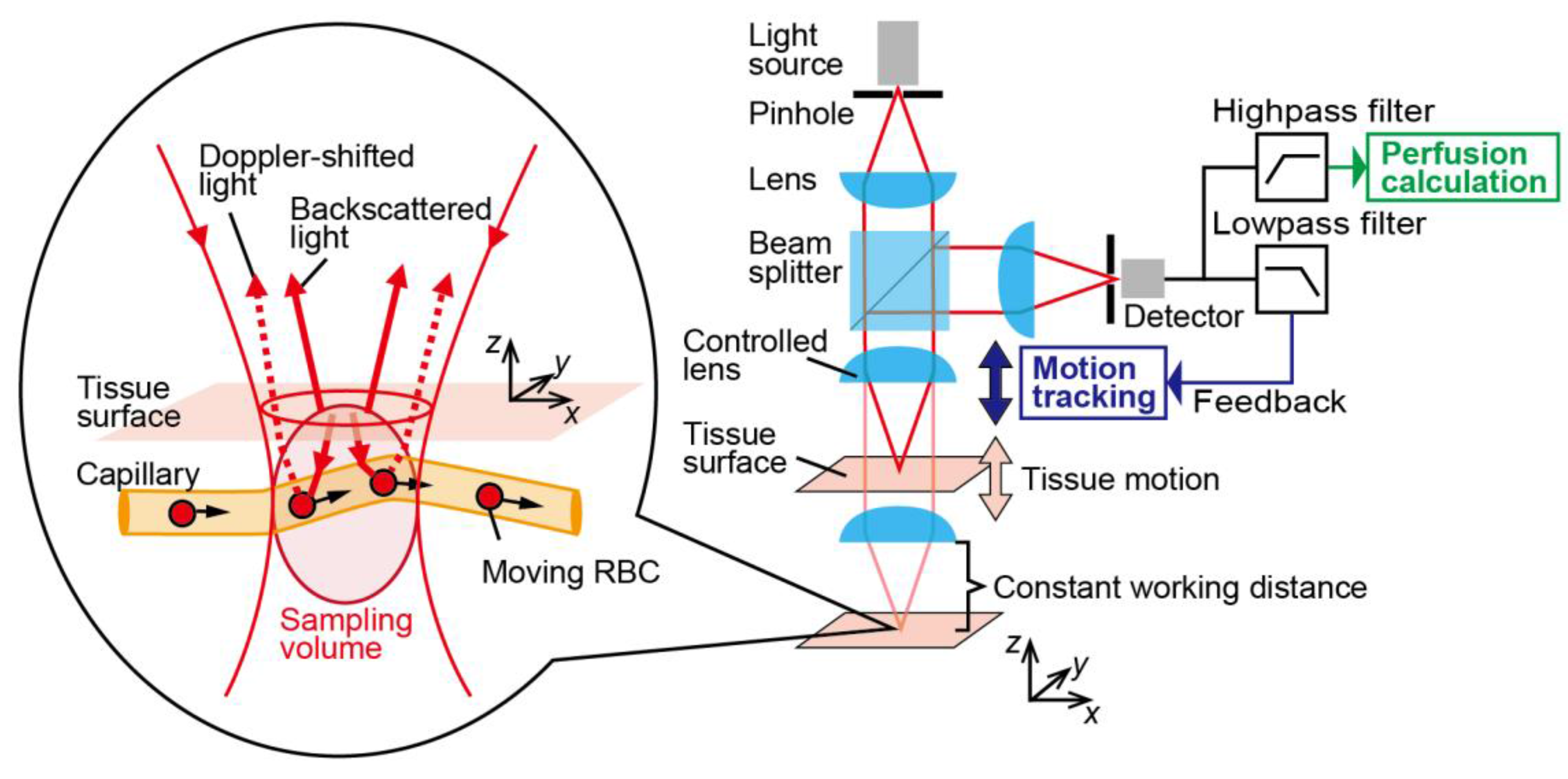
2. Principle
3. Experimental Setup
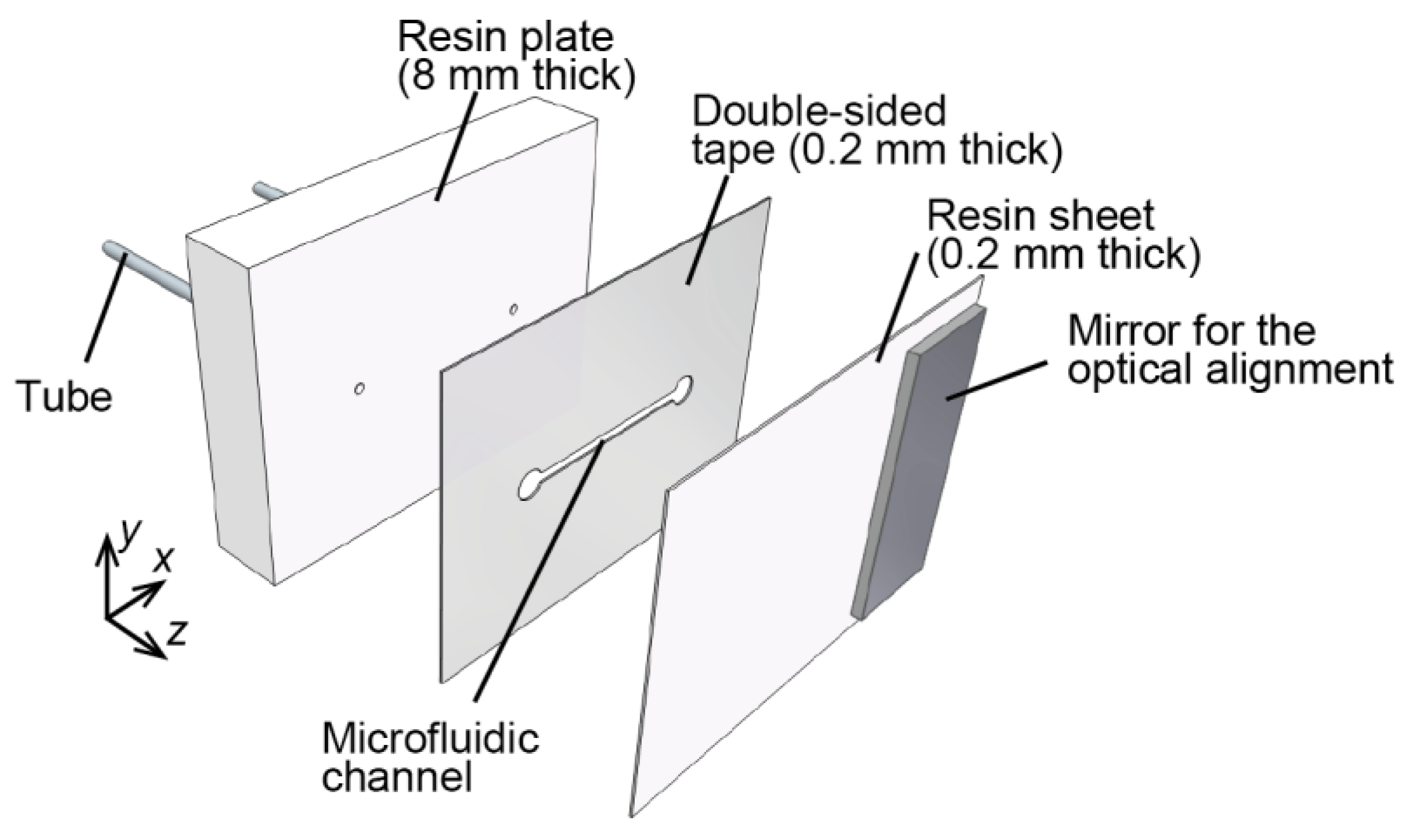
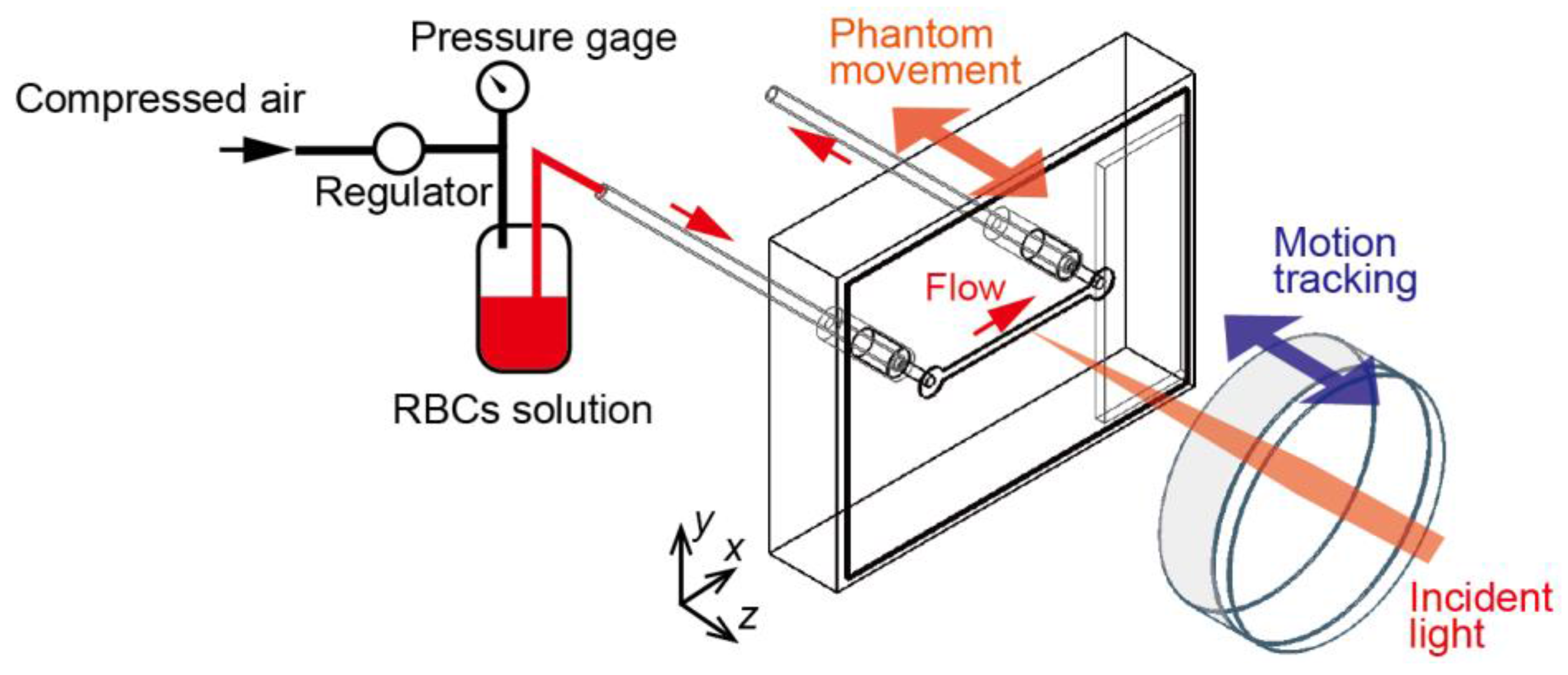
3.1. Microfluidic Phantom
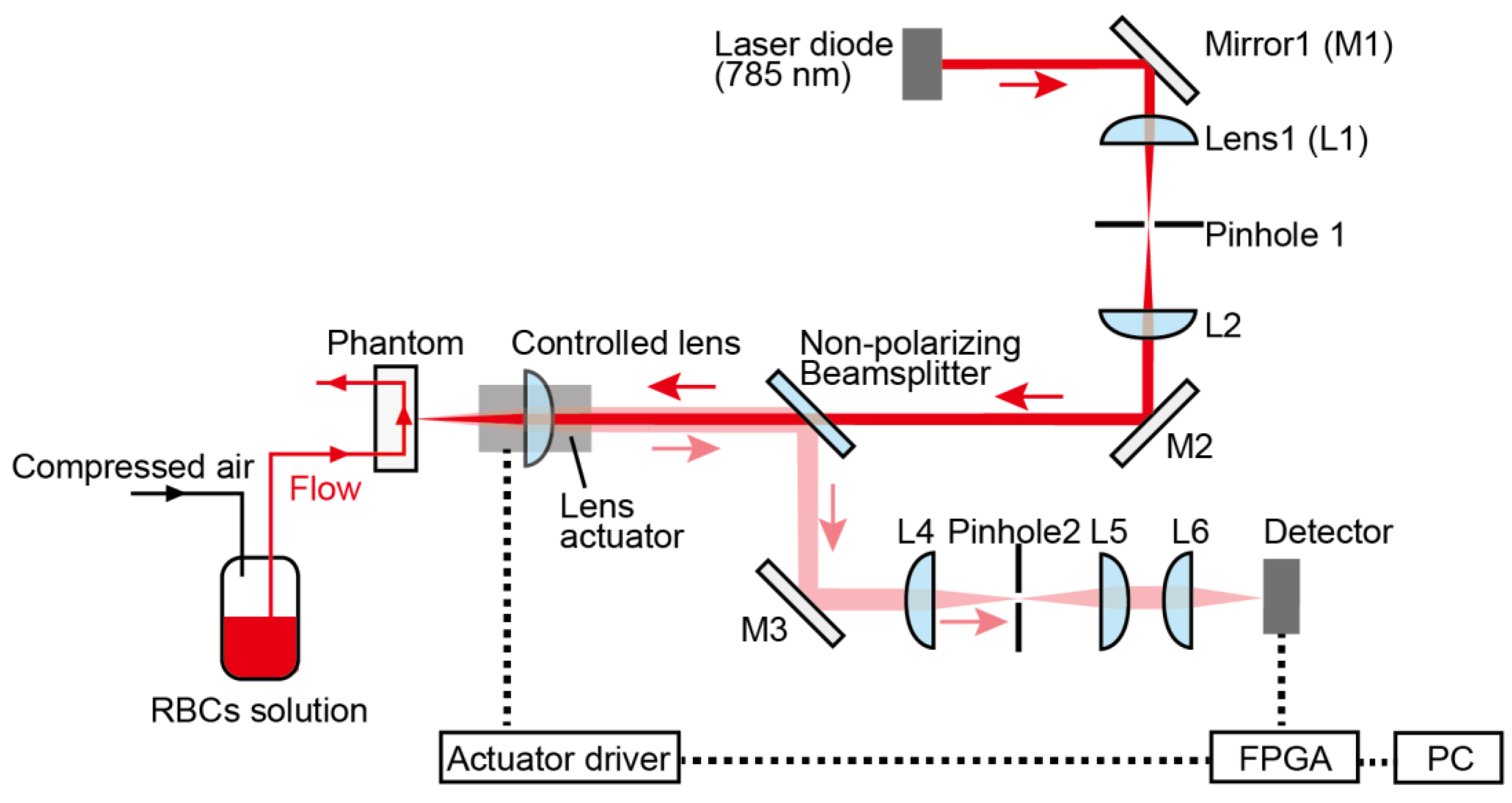
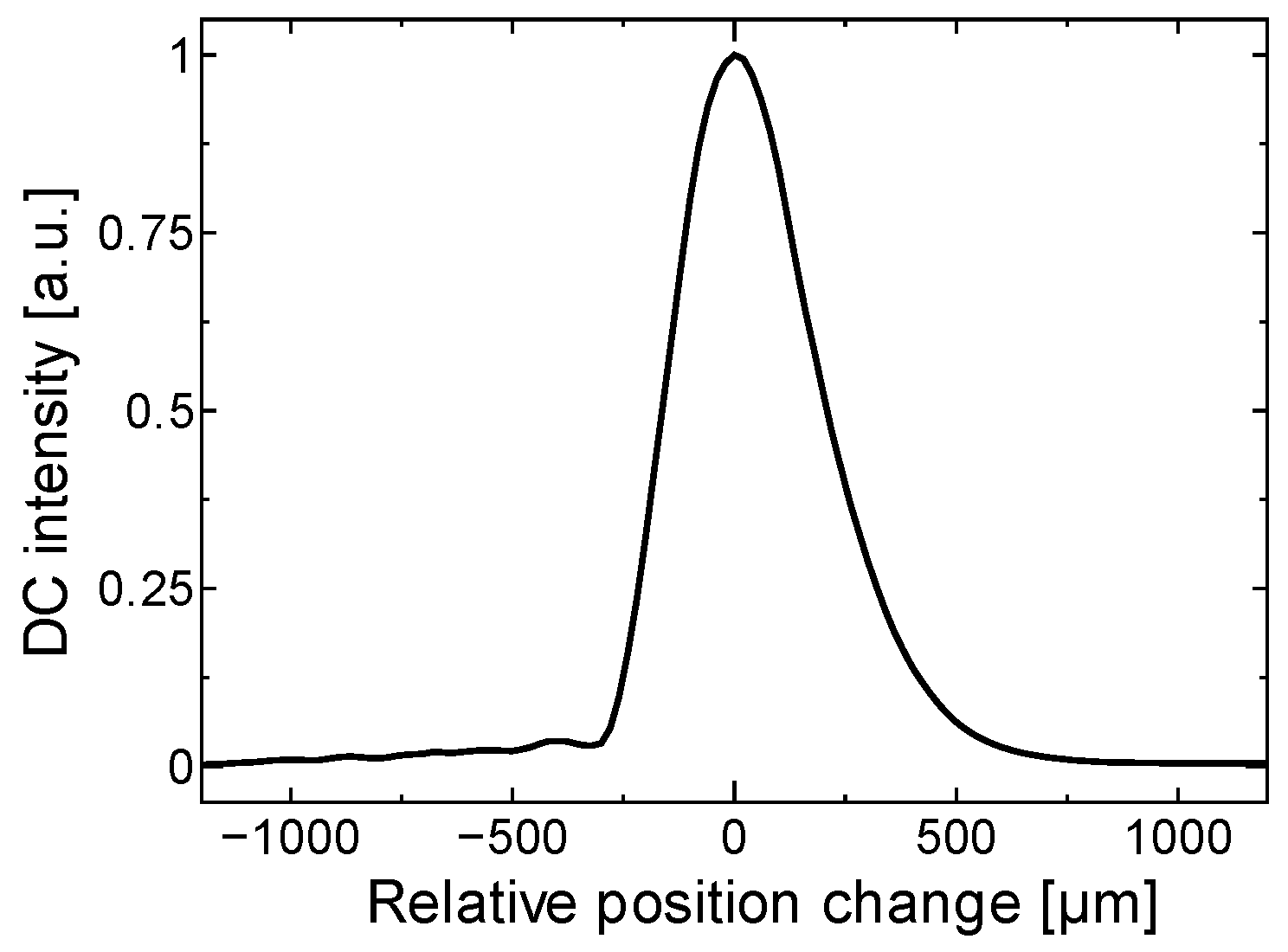
3.2. Motion-Tracking LDF System
4. Results and Discussion
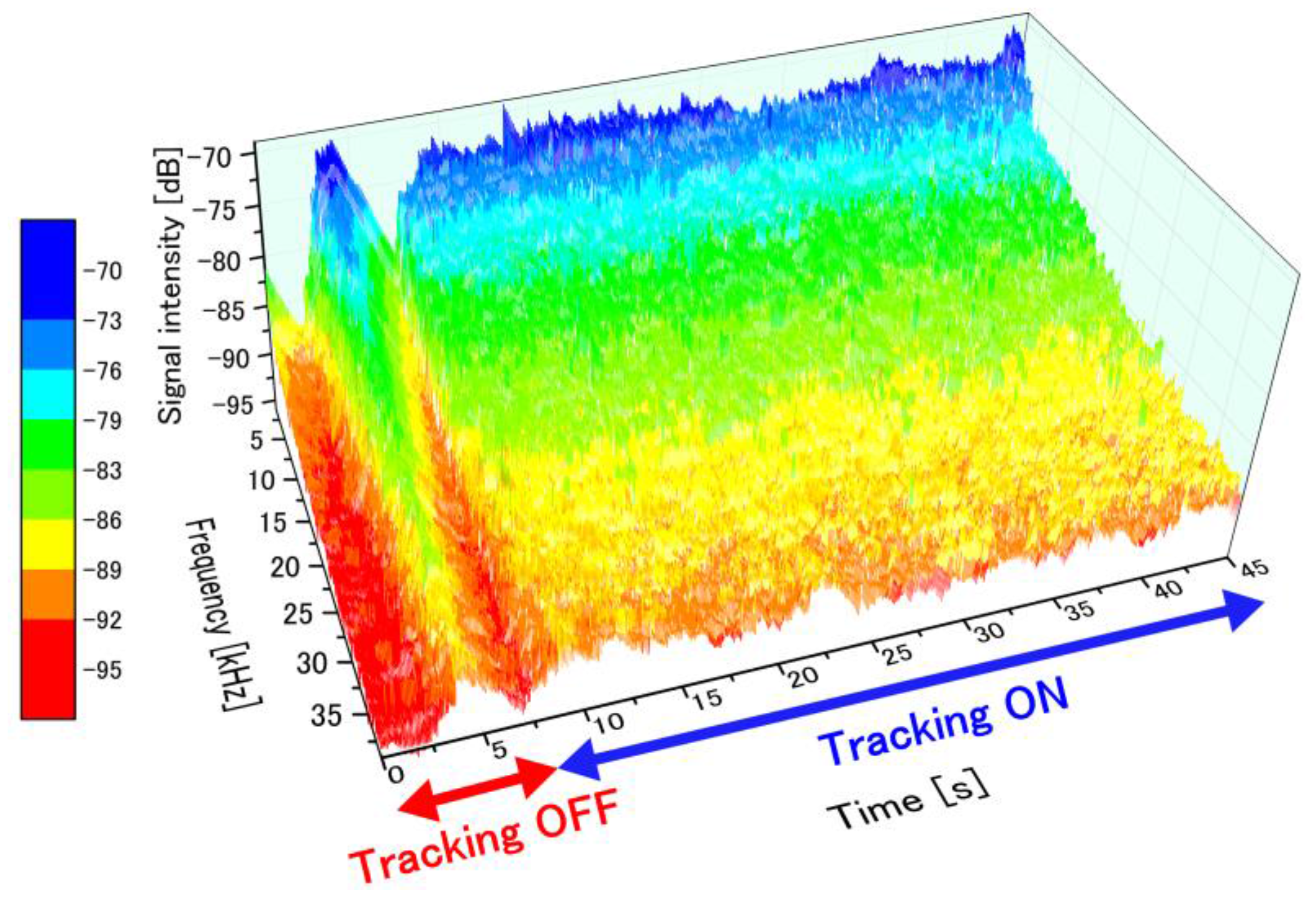
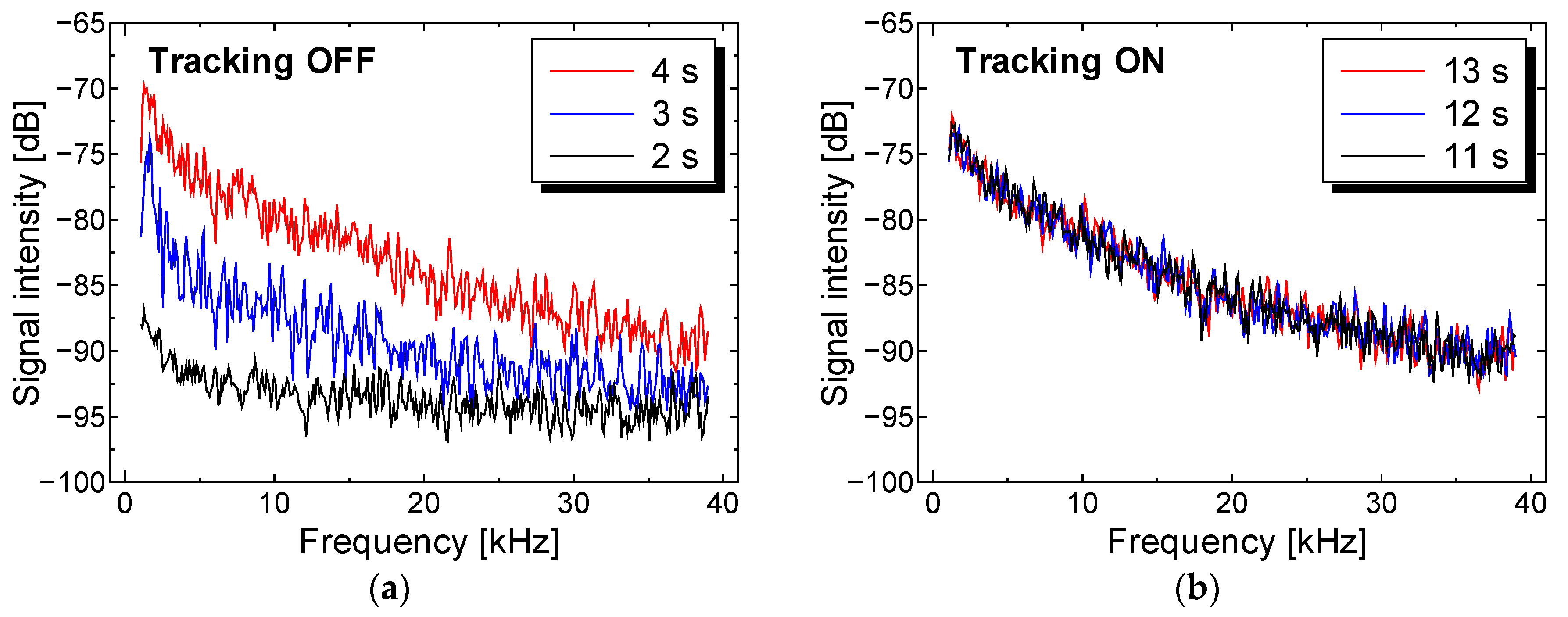
4.1. Transient Perfusion Spectra Variability with and without Tracking
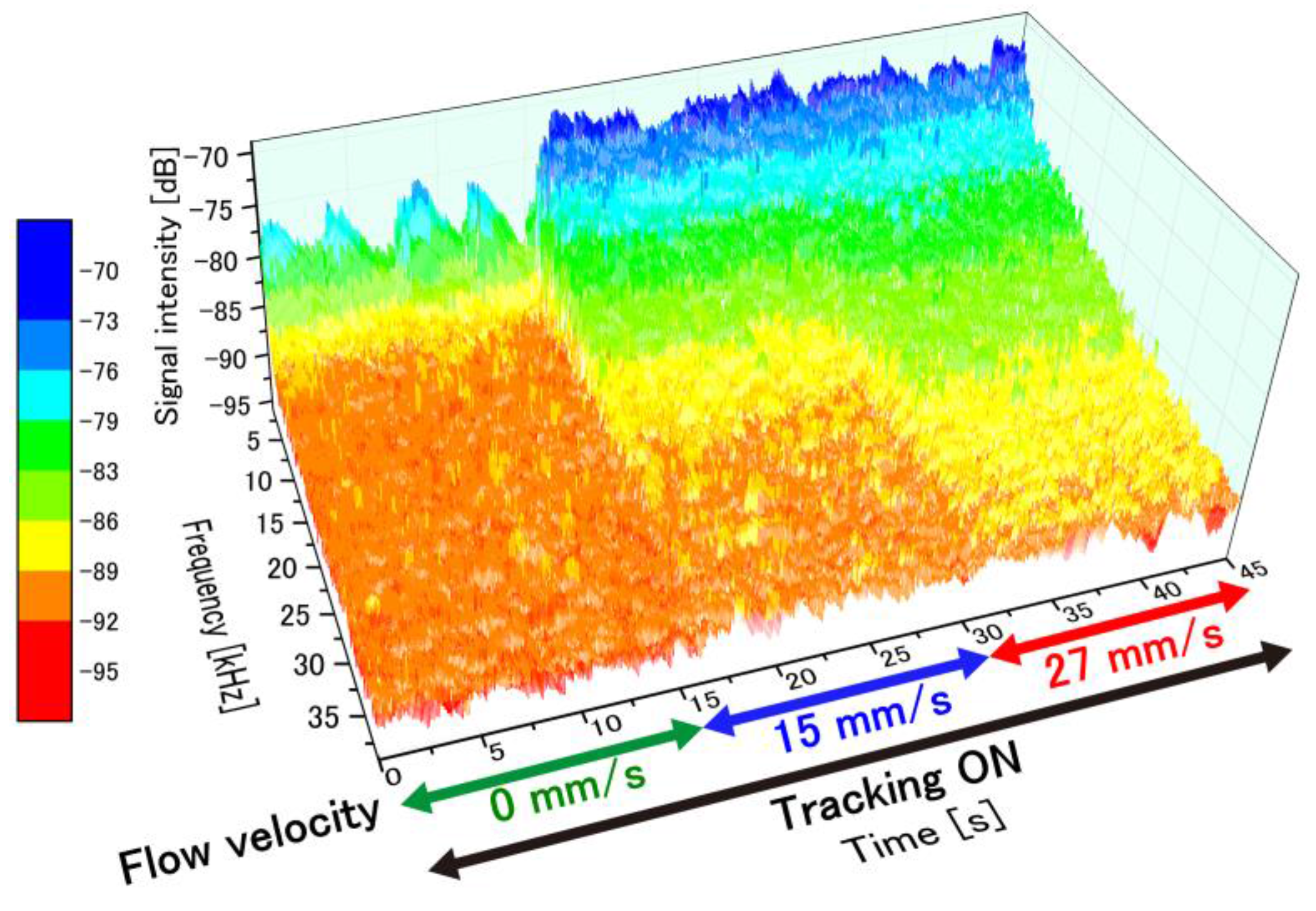
4.2. Temporal Perfusion Spectra with Motion Tracking
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riva, C.; Bennedek, G.B.; Ross, B. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Investig. Ophthalmol. Vis. Sci. 1972, 11, 936–944. [Google Scholar]
- Stern, M.D. In vivo evaluation of microcirculation by coherent light scattering. Nature 1975, 254, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Nieuwenhoff, M.D.; Hygen, F.J.P.M.; van der Helm, F.C.T.; Niehof, S.; Schouten, A.C. Characterizing human skin blood flow regulation in response to different local skin temperature perturbations. Microvasc. Res. 2017, 111, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhoff, M.D.; Wu, Y.; Huygen, F.J.P.M.; Schouten, A.C.; van der Helm, F.C.T.; Niehof, S.P. Reproducibility of axon reflex-related vasodilation assessed by dynamic thermal imaging in healthy subjects. Microvasc. Res. 2016, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kloppenberg, F.W.H.; Beerthuizen, G.I.J.M.; ten Duis, H.J. Perfusion of burn wounds assessed by Laser Doppler Imaging is related to burn depth and healing time. Burns 2001, 27, 359–363. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, Y.H.; Lee, H.S.; Moon, D.J.; Kim, S.G.; Lee, J.H.; Cho, J.K.; Yoon, C.J. The impact of laser Doppler imaging on the early decision-making process for surgical intervention in adults with indeterminate burns. Burns 2013, 39, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; AnderssonEngels, S.; Nilsson, G.E.; Wardell, K.; Svanberg, K. Superficial blood flow following photodynamic therapy of malignant non–melanoma skin tumours measured by laser Doppler perfusion imaging. Br. J. Dermatol. 1997, 136, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Stucker, M.; Horstmann, I.; Nuchel, C.; Rochling, A.; Hoffmann, K.; Altmeyer, P. Blood flow compared in benign melanocytic naevi, malignant melanomas and basal cell carcinomas. Clin. Exp. Dermatol. 1999, 24, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Sato, E.; Hayashi, H.; Fujimoto, Y.; Nasashima, T. Vascular evaluation in laryngeal diseases: Comparison between contact endoscopy and laser Doppler flowmetry. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Davis, J.P.; Birchall, M.A. Laser Doppler flux-metry in laryngeal squamous cell carcinoma. Clin. Otolaryngol. 2003, 28, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Mahe, G.; Durand, S.; Humeau-Heurtier, A.; Leftheriotis, G.; Abraham, P. Impact of experimental conditions on noncontact laser recordings in microvascular studies. Microcirculation 2012, 19, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashima, S. Non-contact laser tissue blood flow measurement using polarization to reduce the specular reflection artefact. Opt. Laser Technol. 1994, 26, 169–175. [Google Scholar] [CrossRef]
- Karlsson, M.G.D.; Wardell, K. Polarized laser Doppler perfusion imaging—reduction of movement-induced artifacts. J. Biomed. Opt. 2005, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Humeau, A.; Trzepizur, W.; Rousseau, D.; Chapeau-Blondeau, F.; Abraham, P. Fisher information and Shannon entropy for on-line detection of transient signal high-values in laser Doppler flowmetry signals of healthy subjects. Phys. Med. Biol. 2008, 53, 5061–5076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humeau, A.; Trzepizur, W.; Rousseau, D.; Chapeau-Blondeau, F.; Abraham, P. Localization of transient signal high-values in laser Doppler flowmetry signals with an empirical mode decomposition. Med. Phys. 2009, 36, 18–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunde, O.C.; Kvernebo, K.; Larsen, S. Evaluation of endoscopic laser Doppler flowmetry for measurement of human gastric blood flow: Methodologic aspects. Scand. J. Gastroenterol. 1988, 23, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Krohg-Sorensen, K.; Lunde, O.C. Perfusion of the Human Distal Colon and Rectum Evaluated with Endoscopic Laser Doppler Flowmetry: Methodologic Aspects. Scand. J. Gastroenterol. 1993, 28, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.S.; Kim, J.H.; Lee, H.Y.; Park, H.; Lee, S.I. Endoscopic Evaluation of Gastric Emptying and Effect of Mosapride Citrate on Gastric Emptying. Yonsei Med. J. 2010, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, M.A.; Steingoetter, A.; Pal, A.; Menne, D.; Brasseur, J.G.; Hebbard, G.S.; Boesiger, P.; Thumshim, M.; Fried, M.; Schwizer, W. Quantification of Distal Antral Contractile Motility in Healthy Human Stomach with Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2006, 24, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, C.K.; Yang, Q.; Arathorn, D.W.; Tiruveedhula, P.; de Boer, J.F.; Roorda, A. High-speed, image-based eye tracking with a scanning laser ophthalmoscope. Biomed. Opt. Express. 2012, 3, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hwang, R.C.; Chen, Y.J. A passive auto-focus camera control system. Appl. Soft Comput. 2010, 10, 296–303. [Google Scholar] [CrossRef]
- Fan, K.C.; Chu, C.L.; Mou, J.I. Development of a low-cost autofocusing probe for profile measurement. Meas. Sci. Technol. 2001, 12, 2137–2146. [Google Scholar] [CrossRef]
- Li, Q.; Bai, L.; Xue, S.; Chen, L. Autofocus system for microscope. Opt. Eng. 2002, 41, 1289–1294. [Google Scholar] [CrossRef]
- Schlangen, S.; Ihme, M.; Rahlves, M.; Roth, B. Autofocusing system for spatial light modulator-based maskless lithography. Appl. Opt. 2016, 55, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Kino, G.S.; Corle, T.R. Confocal Scanning Optical Microscopy and Related Imaging Systems; Academic Press: San Diego, CA, USA, 1996; pp. 31–40. [Google Scholar]
- Nillson, G.E.; Tenland, T.; Oberg, P.A. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans. Biomed. Eng. 1980, 27, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.J.; Wegner, M.; Tiziani, H. Highly accurate non-contact characterization of engineering surfaces using confocal microscopy. Meas. Sci. Technol. 1998, 9, 1142–1152. [Google Scholar] [CrossRef]
- Yang, L.S.; Wang, G.Y.; Wang, J.G.; Xu, Z.Z. Surface profilometry with a fibre optical confocal scanning microscope. Meas. Sci. Technol. 2000, 11, 1786–1792. [Google Scholar] [CrossRef]
- Cohen-Sabban, J.; Gaillard-Groleas, J.; Crepin, P.J. Extended-field confocal imaging for 3D surface sensing. In Proceedings of the SPIE Optical Fabrication, Testing, And Metrology Conference, St Etienne, France, 30 September–3 October 2003; pp. 366–371. [Google Scholar]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Hasler, W.L.; Barnett, J.L.; Eaker, E.Y. Electrogastrography: A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol. Motil. 2003, 15, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Strege, P.R.; Ou, Y.J.; Sha, L.; Rich, A.; Gibbons, S.J.; Szurszewski, J.H.; Sarr, M.G.; Farrugia, G. Sodium current in human intestinal interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Whittet, D.C.B.; Dayawansa, I.J.; Dickinson, P.M.; Marsden, J.P.; Thomas, B. The optical constants of polyoxymethylene. Mon. Not. R. Astron. Soc. 1976, 175, 197–207. [Google Scholar] [CrossRef]
- Jang, H.; Singh, K.; Wang, H.W.; Pfefer, T.J.; Chen, Y. Oximetry system performance assessment with POM (acetal) phantoms incorporating hemoglobin calibration standards and customized saturation levels. In Proceedings of the Conference on Design and Quality for Biomedical Technologies VIII, San Francisco, CA, USA, 7–8 February 2015. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, M.; Taguchi, Y. Motion Tracking System for Robust Non-Contact Blood Perfusion Sensor. Sensors 2018, 18, 277. https://doi.org/10.3390/s18010277
Hashimoto M, Taguchi Y. Motion Tracking System for Robust Non-Contact Blood Perfusion Sensor. Sensors. 2018; 18(1):277. https://doi.org/10.3390/s18010277
Chicago/Turabian StyleHashimoto, Masaaki, and Yoshihiro Taguchi. 2018. "Motion Tracking System for Robust Non-Contact Blood Perfusion Sensor" Sensors 18, no. 1: 277. https://doi.org/10.3390/s18010277





