Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
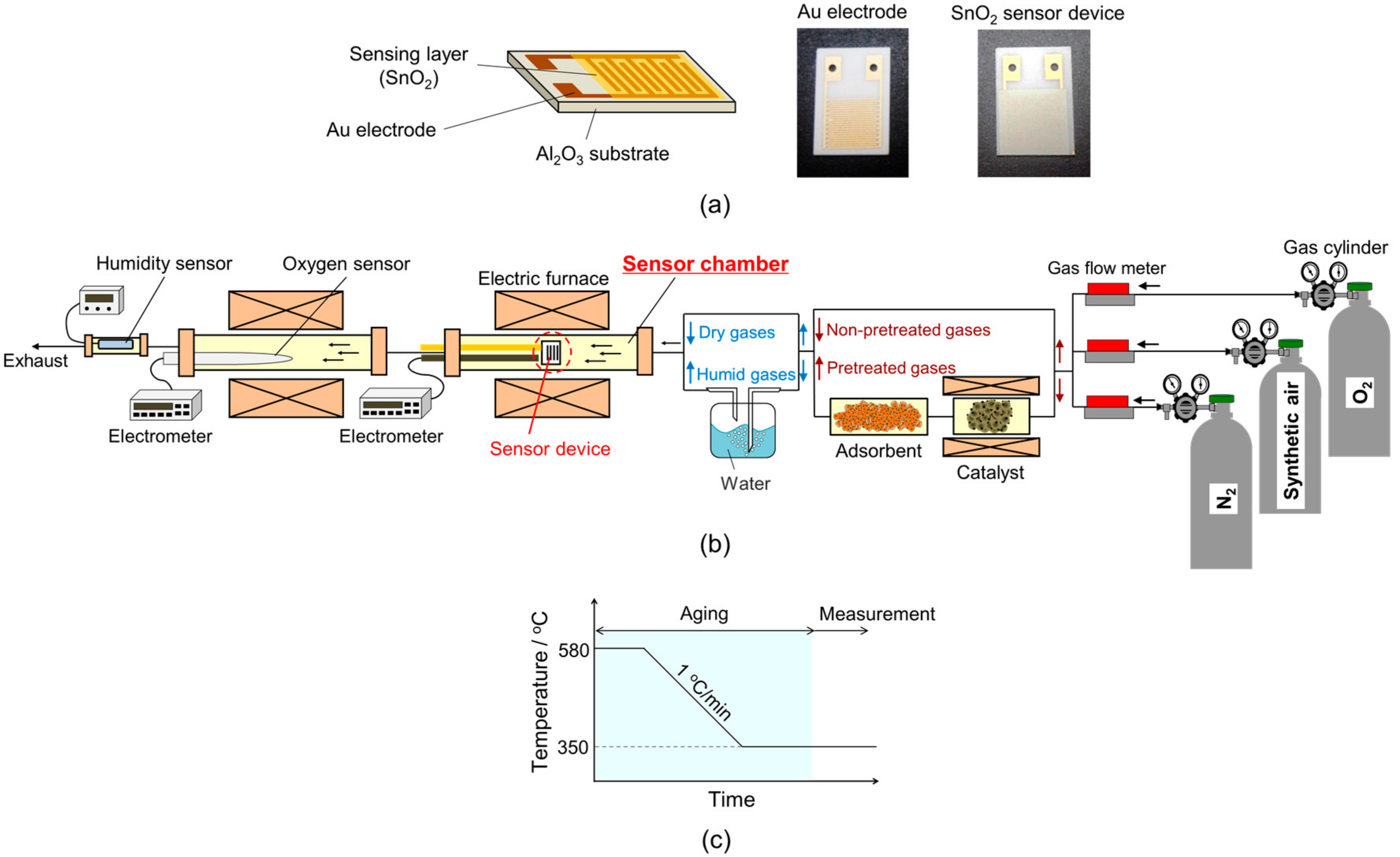
2.1. Preparation and Characterization of SnO2 Nanoparticles and Sensor Fabrication
2.2. Evaluation of the Electrical Properties
3. Results and Discussion
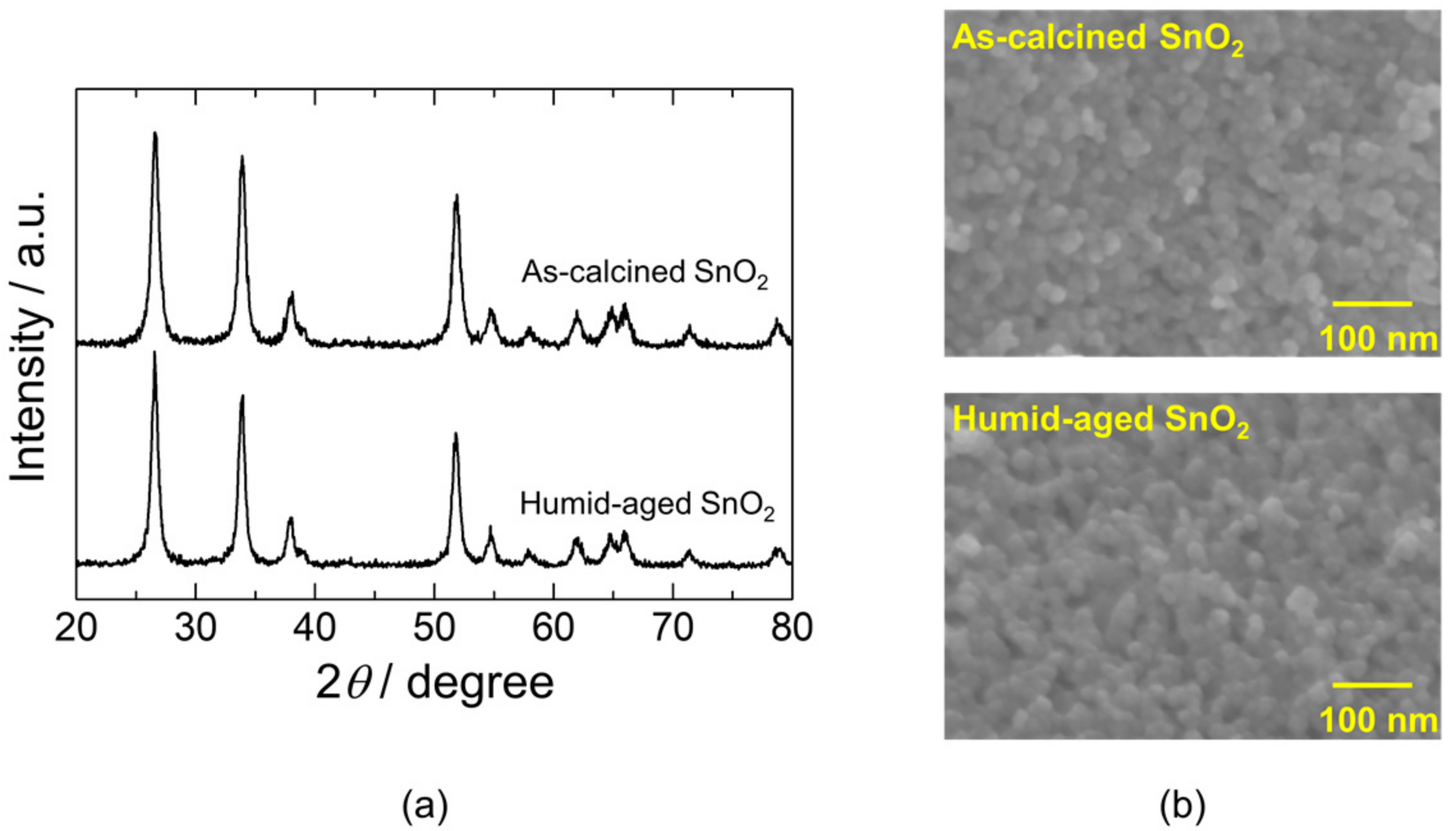
3.1. Effect of Humid Aging on the Material Characteristics
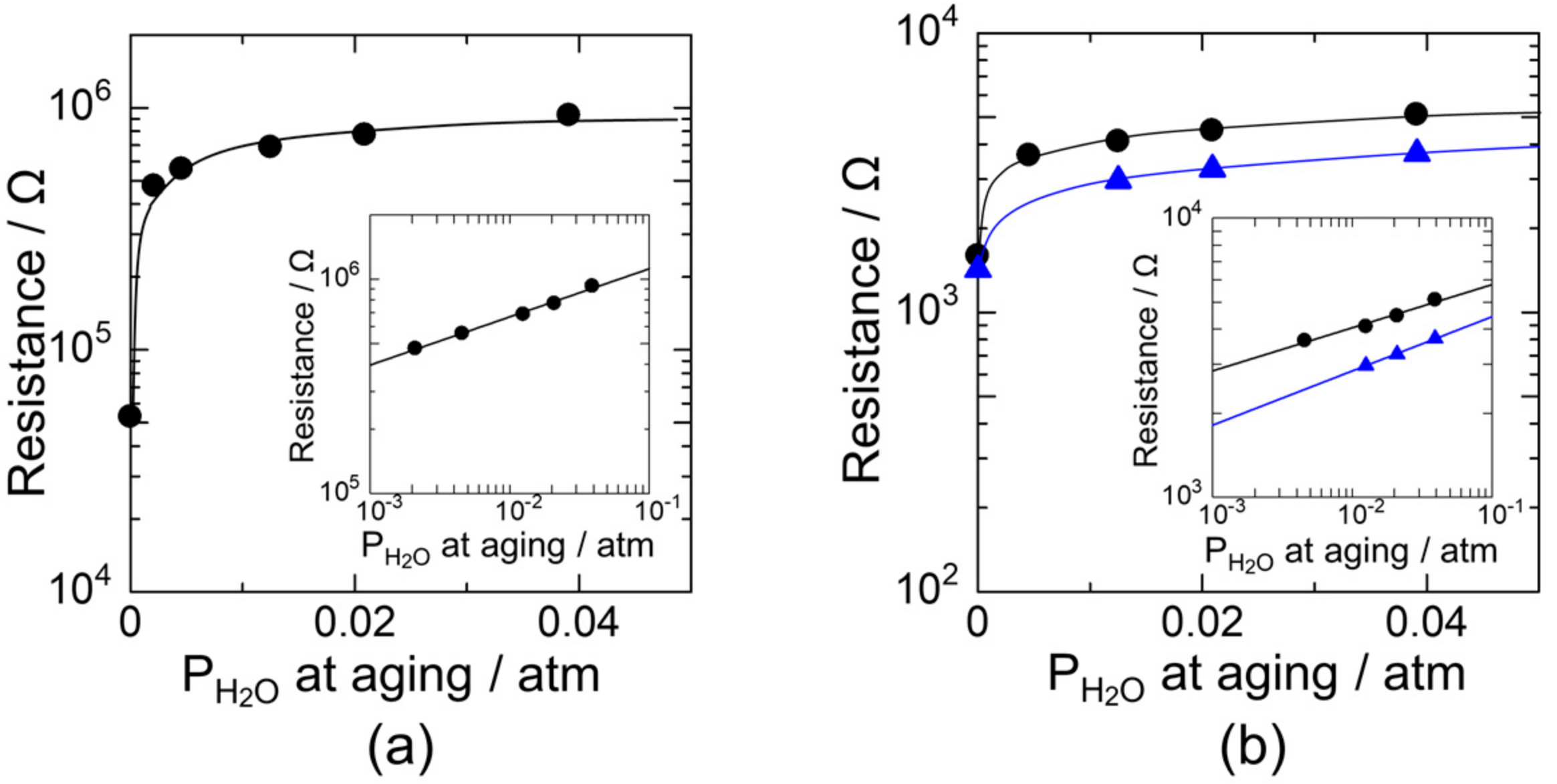
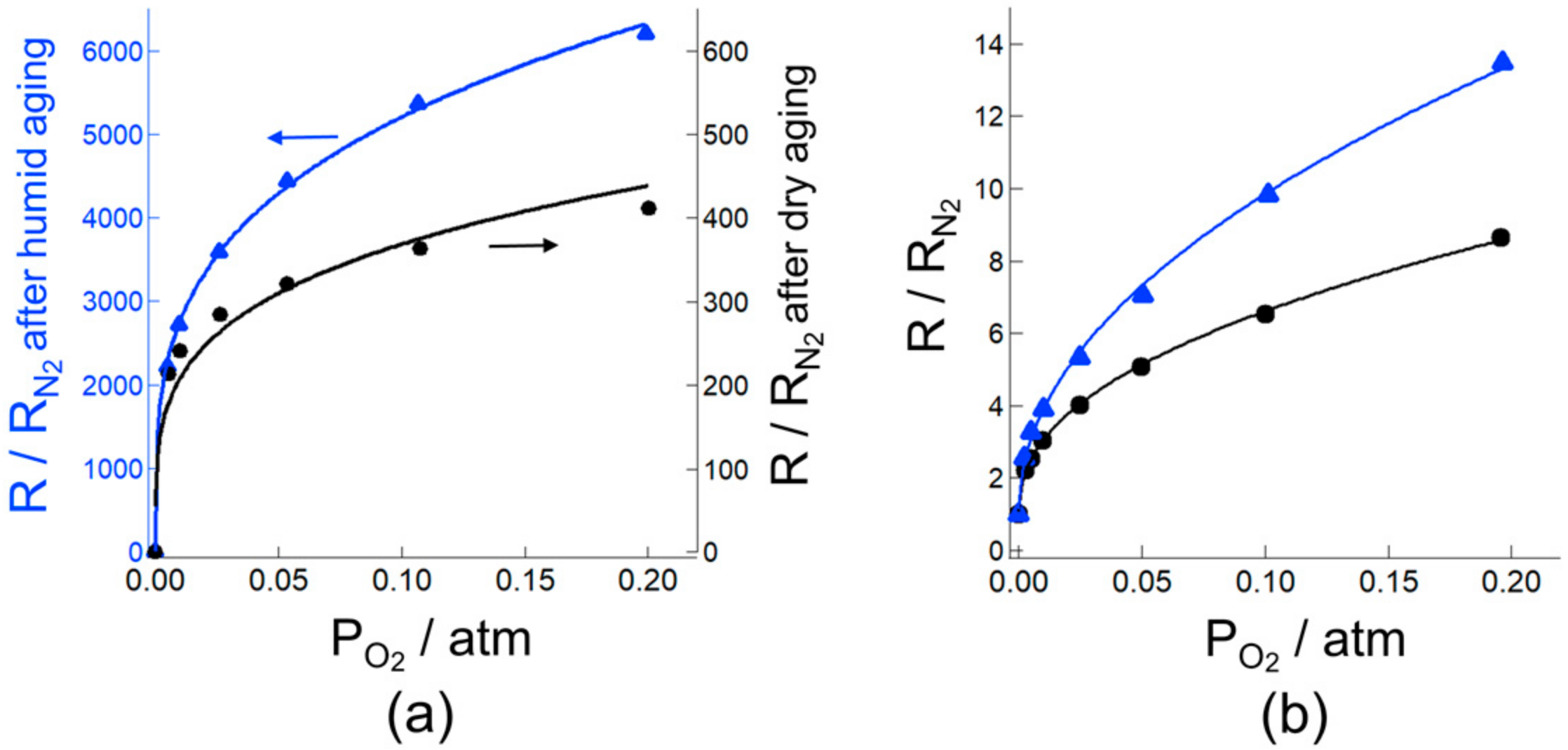
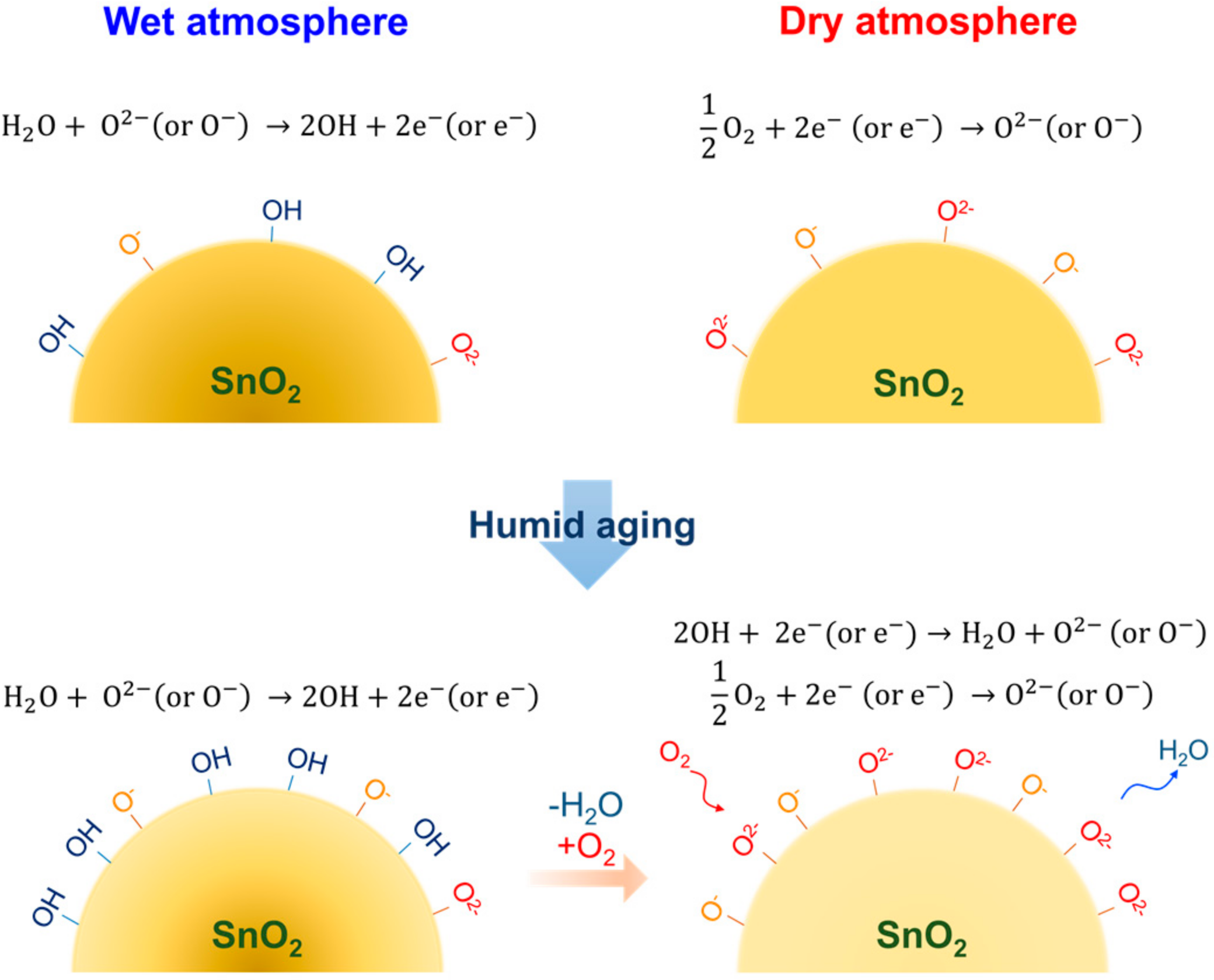
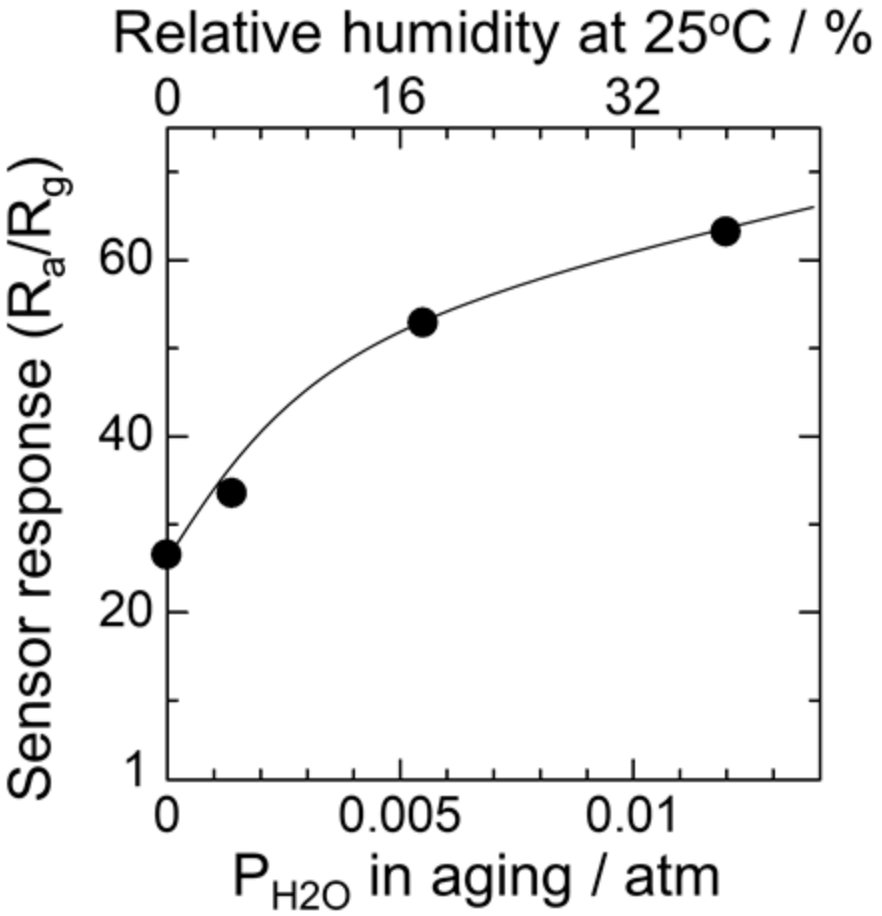
3.2. Effect of Humid Aging on the Oxygen Adsorption
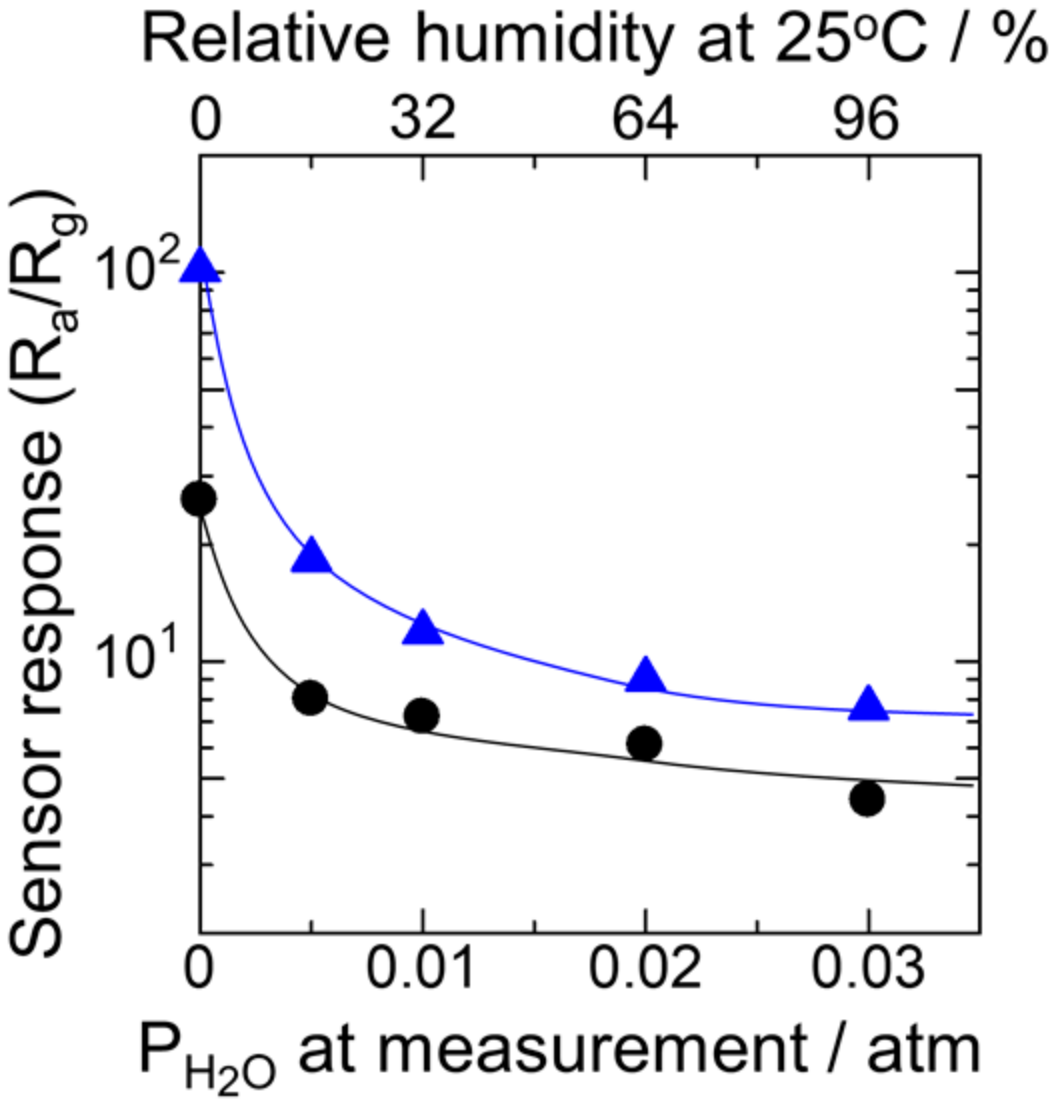
3.3. Effects of Humid Aging on the Sensor Response to Hydrogen
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taguchi, N. Metal Oxide Gas Sensor. Japan Patent No. S45-38200, 1962. [Google Scholar]
- Ihokura, K.; Tanaka, K.; Murakami, N. Use of tin dioxide sensor to control a domestic gas heater. Sens. Actuat. 1983, 4, 607–612. [Google Scholar] [CrossRef]
- Homepage of Figaro Engineering Inc. Available online: http://www.figaro.co.jp/en/ (accessed on 10 January 2018).
- Homepage of Nissha FIS Inc. Available online: http://www.fisinc.co.jp/en/products/ (accessed on 10 January 2018).
- Clifford, P.K.; Tuma, D.T. Characteristics of semiconductor gas sensors I. Steady state gas response. Sens. Actuat. 1982/1983, 3, 233–254. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Tamaki, J.; Nagaishi, M.; Teraoka, Y.; Miura, N.; Yamazoe, N.; Moriya, K.; Nakamura, Y. Adsorption behavior of CO and Interfering gases on SnO2. Surf. Sci. 1989, 221, 183–196. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Yuasa, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. Determination of Oxygen Adsorption on SnO2: Exact Analysis of Gas Sensing Properties Using a Sample Gas Pretreatment System. J. Electrochem. Soc. 2014, 161, B123–B128. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Tin dioxide gas sensors. J. Chem. Soc. Faraday Trans. I 1987, 83, 1323–1346. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Nakamura, S. ESR and Electric Conductance Studies of Fine-Powdered SnO2. Jpn. J. Appl. Phys. 1975, 14, 779–788. [Google Scholar] [CrossRef]
- Kocemba, I.; Rynkowski, J.M. The effect of oxygen adsorption on catalytic activity of SnO2 in CO oxidation. Catal. Today 2011, 169, 192–199. [Google Scholar] [CrossRef]
- Ogawa, H.; Nishikawa, M.; Abe, A. Hall measurement studies and electrical conduction model of tin oxide ultrafine particle films. J. Appl. Phys. 1982, 53, 4448–4455. [Google Scholar] [CrossRef]
- Oprea, A.; Moretton, E.; Barsan, N.; Becker, W.J.; Wöllenstein, J.; Weimar, U. Conduction model of SnO2 thin films based on conductance and Hall effect measurement. J. Appl. Phys. 2006, 100, 033716. [Google Scholar] [CrossRef]
- Koziej, D.; Bârsan, N.; Weimar, U.; Szuber, J.; Shimanoe, K.; Yamazoe, N. Water-oxygen interplay on tin dioxide surface: Implication on gas sensing. Chem. Phys. Lett. 2005, 410, 321–323. [Google Scholar] [CrossRef]
- Pavelko, R.G.; Daly, H.; Hardacre, C.; Vasiliev, A.A.; Llobet, E. Interaction of water, hydrogen and their mixtures with SnO2 based materials: the role of surface hydroxyl groups in detection mechanisms. Phys. Chem. Chem. Phys. 2010, 12, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Ma, N.; Yuasa, M.; Kida, T.; Shimanoe, K. Surface-modification of SnO2 nanoparticles by incorporation of Al for the detection of combustible gases in a humid atmosphere. RSC Adv. 2015, 5, 86347–86354. [Google Scholar] [CrossRef]
- Tanaka, K.; Morimoto, T.; Sonoda, S.; Matsuura, S.; Moriya, K.; Egashira, M. Combustion monitoring sensor using tin dioxide semiconductor. Sens. Actuat. B 1991, 3, 247–253. [Google Scholar] [CrossRef]
- Ansari, S.G.; Ansari, Z.A.; Kadam, M.R.; Karekar, R.N.; Aiyer, R.C. The effect of humidity on an SnO2 thick-film planar resistor. Sens. Actuat. B 1994, 21, 159–163. [Google Scholar] [CrossRef]
- Nicoletti, S.; Dori, L.; Corticelli, F. Tin Oxide Thin-Films for Aromatic Hydrocarbons Detection: Effect of Aging Time on Film Microstructure. J. Am. Ceram. Soc. 1999, 82, 1201–1206. [Google Scholar] [CrossRef]
- Nelli, P.; Faglia, G.; Sberveglieri, G.; Cereda, E.; Gabetta, G.; Dieguez, A.; Romano-Rodriguez, A.; Morante, J.R. The aging on SnO2-Au thin film sensors: electrical and structural characterization. Thin Solid Films 2000, 371, 249–253. [Google Scholar] [CrossRef]
- Ito, T.; Matsubara, I.; Kadosaki, M.; Sakai, Y.; Shin, W.; Izu, N.; Nishibori, M. Effects of High-Humidity Aging on Platinum, Palladium, and Golad Loaded Tin Oxide—Volatile Organic Compound Sensors. Sensors 2010, 2010, 6513–6521. [Google Scholar] [CrossRef] [PubMed]
- Skariah, B.; Naduvath, J.; Thomas, B. Effect of long term ageing under humid environment on the LPG sensing properties and the surface composition of Mg-doped SnO2 thin films. Ceram. Int. 2016, 42, 7490–7498. [Google Scholar] [CrossRef]
- Yamazoe, N.; Suematsu, K.; Shimanoe, K. Extension of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuat.B 2012, 163, 128–135. [Google Scholar] [CrossRef]
- Suematsu, K.; Yamada, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Evaluation of Oxygen Adsorption Based on the Electric Properties of SnO2 Semiconductor Gas Sensors. Sens. Mater. 2016, 28, 1211–1217. [Google Scholar]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Water Vapor on Pd-Loaded SnO2 Nanoparticles Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Lee, D.H.; Sakaguchi, I.; Nomura, K.; Kamiya, T.; Haneda, H.; Hosono, H.; Ohashi, N. Surface reactivity and oxygen migration in amorphous indium-gallium-zinc oxide films annealed in humid atmosphere. Appl. Phys. Lett. 2013, 103, 201904. [Google Scholar] [CrossRef]
| PH2O in Aging Atmosphere/atm | Measurement Atmosphere | K1 (cm2∙atm−1) | K2 (cm8∙atm−1) |
|---|---|---|---|
| dry | dry | 8 × 10−11 | 8 × 10−41 |
| 0.04 (wet) | dry | 5 × 10−7 | 3 × 10−36 |
| PH2O in Aging Atmosphere/atm | Measurement Atmosphere | K1/cm2∙atm−1 | K2/cm8∙atm−1 |
|---|---|---|---|
| dry | 0.012 (wet) | 1 × 10−11 | 3 × 10−49 |
| 0.05 (wet) | 0.012 (wet) | 4 × 10−11 | 7 × 10−50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors 2018, 18, 254. https://doi.org/10.3390/s18010254
Suematsu K, Ma N, Watanabe K, Yuasa M, Kida T, Shimanoe K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors. 2018; 18(1):254. https://doi.org/10.3390/s18010254
Chicago/Turabian StyleSuematsu, Koichi, Nan Ma, Ken Watanabe, Masayoshi Yuasa, Tetsuya Kida, and Kengo Shimanoe. 2018. "Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors" Sensors 18, no. 1: 254. https://doi.org/10.3390/s18010254





