Study of the Weathering Process of Gasoline by eNose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. eNose Spectra Acquisition
2.3. Data Analysis
3. Results and Discussion
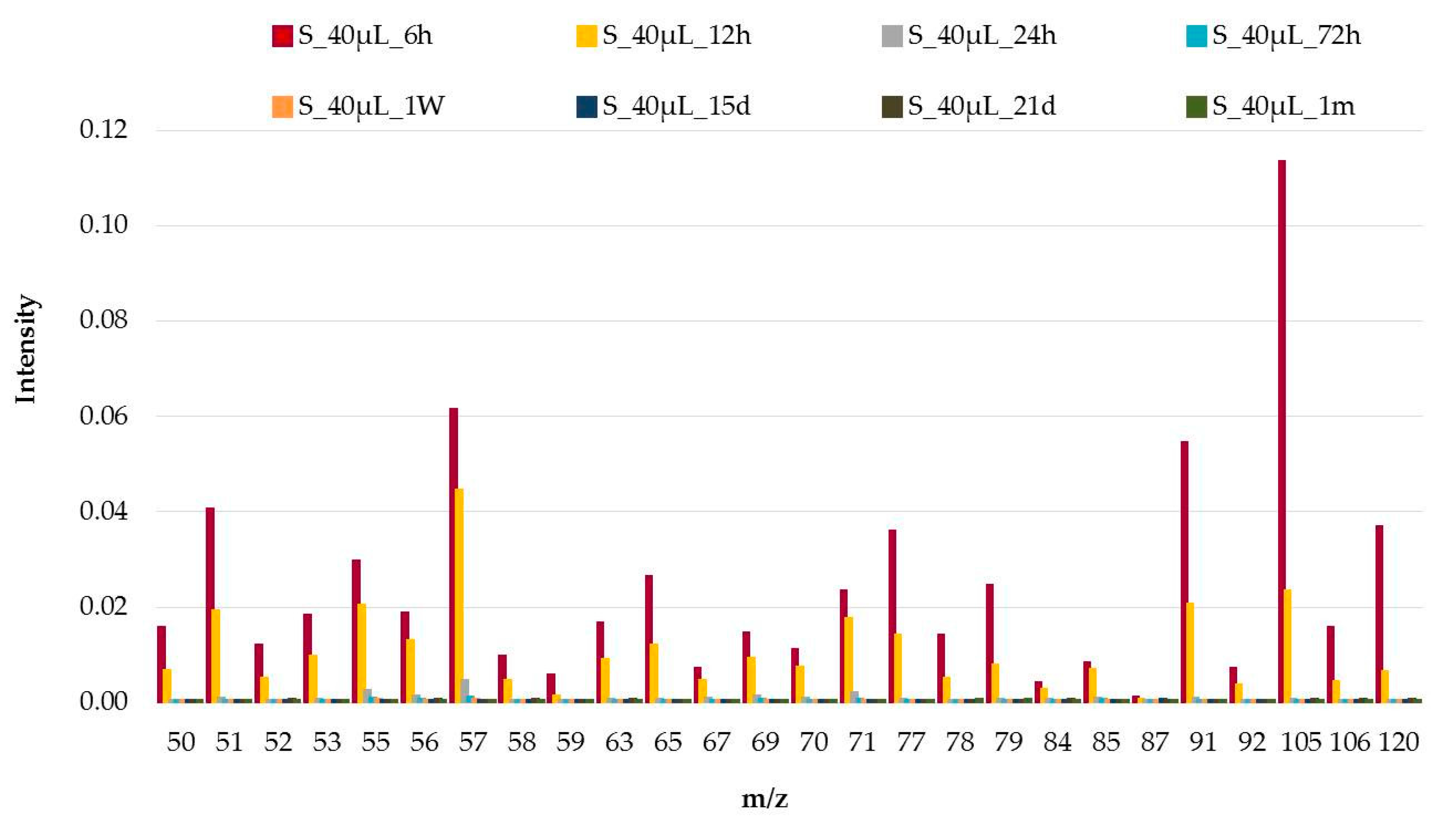
3.1. Analysis of the Weathering Process
3.2. Influence of the Volume
3.3. Influence of the Support
3.4. Study of the Kinectics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreiro-González, M.; Ayuso, J.; Álvarez, J.A.; Palma, M.; Barroso, C.G. Application of an HS-MS for the detection of ignitable liquids from fire debris. Talanta 2015, 142, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.C. Comparison of Automotive Gasolines Using Capillary Gas Chromatography II: Limitations of Automotive Gasoline Comparisons in Casework. J. Forensic Sci. 1987, 32, 616–628. [Google Scholar] [CrossRef]
- Jackowski, J.P. The Incidence of Ignitable Liquid Residues in Fire Debris as Determined by a Sensitive and Comprehensive Analytical Scheme. J. Forensic Sci. 1997, 42, 828–832. [Google Scholar] [CrossRef]
- Martin-Alberca, C.; Ortega-Ojeda, F.E.; Garcia-Ruiz, C. Analytical tools for the analysis of fire debris. A review: 2008–2015. Anal. Chim. Acta 2016, 928, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sturaro, A.; Vianello, A.; Denti, P.; Rella, R. Fire debris analysis and scene reconstruction. Sci. Justice 2013, 53, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Rella, R.; Sturaro, A.; Parvoli, G.; Ferrara, D.; Casellato, U.; Vadalà, G. A brush fire forensic case. Justice 2005, 45, 29–34. [Google Scholar] [CrossRef]
- Birks, H.L.; Cochrana, A.R.; Williamsa, T.J.; Jacksonab, G.P. The surprising effect of temperature on the weathering of gasoline. Forensic Chem. 2017, 4, 32–40. [Google Scholar] [CrossRef]
- Turner, D.A.; Goodpaster, J.V. Comparing the Effects of Weathering and Microbial Degradation on Gasoline Using Principal Components Analysis. J. Forensic Sci. 2012, 57, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Zorzetti, B.M.; Shaver, J.M.; Harynuk, J.J. Estimation of the age of a weathered mixture of volatile organic compounds. Anal. Chim. Acta 2011, 694, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Lee, K.S. Carbon isotope fractionation of benzene and toluene by progressive evaporation. Rapid Commun. Mass Spectrom. 2010, 24, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Sunac, Y.; Zhanga, Y.; Chaia, P. Stable carbon isotope fractionation of individual light hydrocarbons in the C6–C8 range in crude oil as induced by natural evaporation: Experimental results and geological implications. Org. Geochem. 2012, 50, 44–56. [Google Scholar] [CrossRef]
- Zorzetti, B.M.; Harynuk, J.J. Using GC x GC-FID profiles to estimate the age of weathered gasoline samples. Anal. Bioanal. Chem. 2011, 40, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Falkova, M.; Vakh, C.; Shishov, A.; Zubakina, E.; Moskvin, A.; Moskvin, L.; Bulatov, A. Automated IR determination of petroleum products in water based on sequential injection analysis. Talanta 2016, 148, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Sampat, A.A.; Lopatka, M.; Vivó-Truyols, G.; Schoenmakers, P.J.; Van Asten, A.C. Towards chemical profiling of ignitable liquids with comprehensive two-dimensional gas chromatography: Exploring forensic application to neat white spirits. Forensic Sci. Int. 2016, 267, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Moss, P.J.; Buchanan, A.H.; Seputro, J.; Wastney, C. Effect of support conditions on the fire behaviour of steel and composite beams. Fire Mater. 2004, 28, 159–175. [Google Scholar] [CrossRef]
- Ohlemiller, T.J.; Shields, J.R. The effect of surface coatings on fire growth over composite materials in a corner configuration. Fire Saf. J. 1999, 32, 173–193. [Google Scholar] [CrossRef]
- ASTM, A. Standard Test Method for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography–Mass Spectrometry. ASTM Int. 2014. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Sissons, N.; Robinson, S. Fire debris analysis by Raman spectroscopy and chemometrics. J. Anal. Appl. Pyrol. 2011, 91, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Pert, A.D.; Baron, M.G.; Birkett, J.W. Review of analytical techniques for arson residues. J. Forensic Sci. 2006, 51, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Tzing, S.H.; Chang, J.Y.; Ghule, A.; Chang, J.J.; Lo, B.; Ling, Y.C. A simple and rapid method for identifying the source of spilled oil using an electronic nose: Confirmation by gas chromatography with mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz, M.; Morrison, C.; Biziuk, M. Application and optimization of headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–flame-ionization detector (GC–FID) to determine products of the petroleum industry in aqueous samples. Microchem. J. 2013, 108, 117–123. [Google Scholar] [CrossRef]
- Martin-Alberca, C.; Garcia-Ruiz, C.; Delemont, O. Study of acidified ignitable liquid residues in fire debris by solid-phase microextraction with gas chromatography and mass spectrometry. J. Sep. Sci. 2015, 38, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; An, Y.; Konstantynova, K.I.; Jackson, G.P. Analysis of household ignitable liquids and their post-combustion weathered residues using compound-specific gas chromatography-combustion-isotope ratio mass spectrometry. Forensic Sci. Int. 2013, 233, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Orecchio, S.; Fiore, M.; Barreca, S.; Vara, G. Volatile Profiles of Emissions from Different Activities Analyzed Using Canister Samplers and Gas Chromatography-Mass Spectrometry (GC/MS) Analysis: A Case Study. Int. J. Environ. Res. Public Health 2017, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Hupp, A.M.; Marshall, L.J.; Campbell, D.I.; Smith, R.W.; McGuffin, V.L. Chemometric analysis of diesel fuel for forensic and environmental applications. Anal. Chim. Acta 2008, 606, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.M.L.; Du Pasquier, E. Chemical fingerprinting of unevaporated automotive gasoline samples. Forensic Sci. Int. 2003, 134, 1–10. [Google Scholar] [CrossRef]
- Stauffer, É.; Lentini, J.J. ASTM standards for fire debris analysis: A review. Forensic Sci. Int. 2003, 132, 63–67. [Google Scholar] [CrossRef]
- Williams, M.R.; Sigman, M.E.; Lewis, J.; Pitan, K.M. Combined target factor analysis and Bayesian soft-classification of interference-contaminated samples: Forensic fire debris analysis. Forensic Sci. Int. 2012, 222, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Del Nogal Sanchez, M.; Pérez Pavón, J.L.; Fernández Laespada, M.E.; García Pinto, C.; Moreno Cordero, B. Factors affecting signal intensity in headspace mass spectrometry for the determination of hydrocarbon pollution in beach sands. Anal. Bioanal. Chem. 2005, 382, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.E.; Williams, M.R.; Castelbuono, J.A.; Colca, J.G.; Clark, C.D. Ignitable Liquid Classification and Identification Using the Summed-Ion Mass Spectrum. Instrum. Sci. Technol. 2008, 36, 375–393. [Google Scholar] [CrossRef]
- Lopatka, M.; Sigman, M.E.; Sjerps, M.J.; Williams, M.R.; Vivó-Truyols, G. Class-conditional features modeling for ignitable liquid classification with substantial substrate contribution in fire debris analysis. Forensic Sci. Int. 2015, 252, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.E.; Williams, M.R. Assessing evidentiary value in fire debris analysis by chemometric and likelihood ratio approaches. Forensic Sci. Int. 2016, 264, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Del Nogal Sanchez, M.; García, E.H.; Pérez Pavón, J.; Cordero, B.M. Fast analytical methodology based on mass spectrometry for the determination of volatile biomarkers in saliva. Anal. Chem. 2012, 84, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Pérez Pavón, J.; del Nogal Sánchez, M.; Pinto, C.G.; Fernández Laespada, M.E.; Cordero, B.M.; Peña, A.G. Strategies for qualitative and quantitative analyses with mass spectrometry-based electronic noses. Trac-Trends Anal. Chem. 2006, 25, 257–266. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; GholamHosseini, H.; Li, Z.; He, J. Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges. Sensors 2017, 17, 1073. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lü, E.; Lu, H.; Zhou, Z.; Wang, Y.; Yang, J.; Wang, Y. Quality Detection of Litchi Stored in Different Environments Using an Electronic Nose. Sensors 2016, 16, 852. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-González, M.; Barbero, G.F.; Ayuso, J.; Álvarez, J.A.; Palma, M.; Barroso, C.G. Validation of an HS-MS method for directs determination and classification of ignitable liquids. Microchem. J. 2017, 132, 358–364. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Ayuso, J.; Álvarez, J.A.; Barroso, C.G. Determination of Ignitable Liquids in Fire Debris: Direct Analysis by Electronic Nose. Sensors 2016, 16, 695. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-González, M.; Ayuso, J.; Álvarez, J.A.; Palma, M.; Barroso, C.G. Validation New Headspace-Mass Spectrometry Method for the Discrimination of Commercial Gasoline Samples with Different Research Octane Numbers. Energy Fuels 2014, 28, 6249–6254. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Ayuso, J.; Álvarez, J.A.; Palma, M.; Barroso, C.G. Gasoline analysis by headspace mass spectrometry and near infrared spectroscopy. Fuel 2015, 153, 402–407. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Ayuso, J.; Álvarez, J.A.; Barroso, C.G. Characterization and Differentiation of Petroleum-Derived Products by E-Nose Fingerprints. Sensors 2017, 17, 2544. [Google Scholar] [CrossRef] [PubMed]



| Support | Volume Used/µL | Groups |
|---|---|---|
| Wood | 40 | Group 0: 6–24 h |
| Group 1: 72 h–1 month | ||
| 80 | Group 0: 6–24 h | |
| Group 1: 72 h–1 month | ||
| Cork | 40 | Group 0: 6–12 h |
| Group 1: 24 h–1 month | ||
| 80 | Group 0: 6–24 h | |
| Group 1: 72 h–1 month | ||
| Paper | 40 | Group 0: 6–12 h |
| Group 1: 24 h–1 month | ||
| 80 | Group 0: 6–24 h | |
| Group 1: 72 h–1 month | ||
| Sheet | 40 | Group 0: 6–12 h |
| Group 1: 24 h–1 month | ||
| 80 | Group 0: 6–24 h | |
| Group 1: 72 h–1 month |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Ayuso, J.; Palma, M.; Barroso, C.G. Study of the Weathering Process of Gasoline by eNose. Sensors 2018, 18, 139. https://doi.org/10.3390/s18010139
Aliaño-González MJ, Ferreiro-González M, Barbero GF, Ayuso J, Palma M, Barroso CG. Study of the Weathering Process of Gasoline by eNose. Sensors. 2018; 18(1):139. https://doi.org/10.3390/s18010139
Chicago/Turabian StyleAliaño-González, María José, Marta Ferreiro-González, Gerardo F. Barbero, Jesús Ayuso, Miguel Palma, and Carmelo G. Barroso. 2018. "Study of the Weathering Process of Gasoline by eNose" Sensors 18, no. 1: 139. https://doi.org/10.3390/s18010139