One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration towards Functional Sensing Devices
Abstract
:1. Introduction
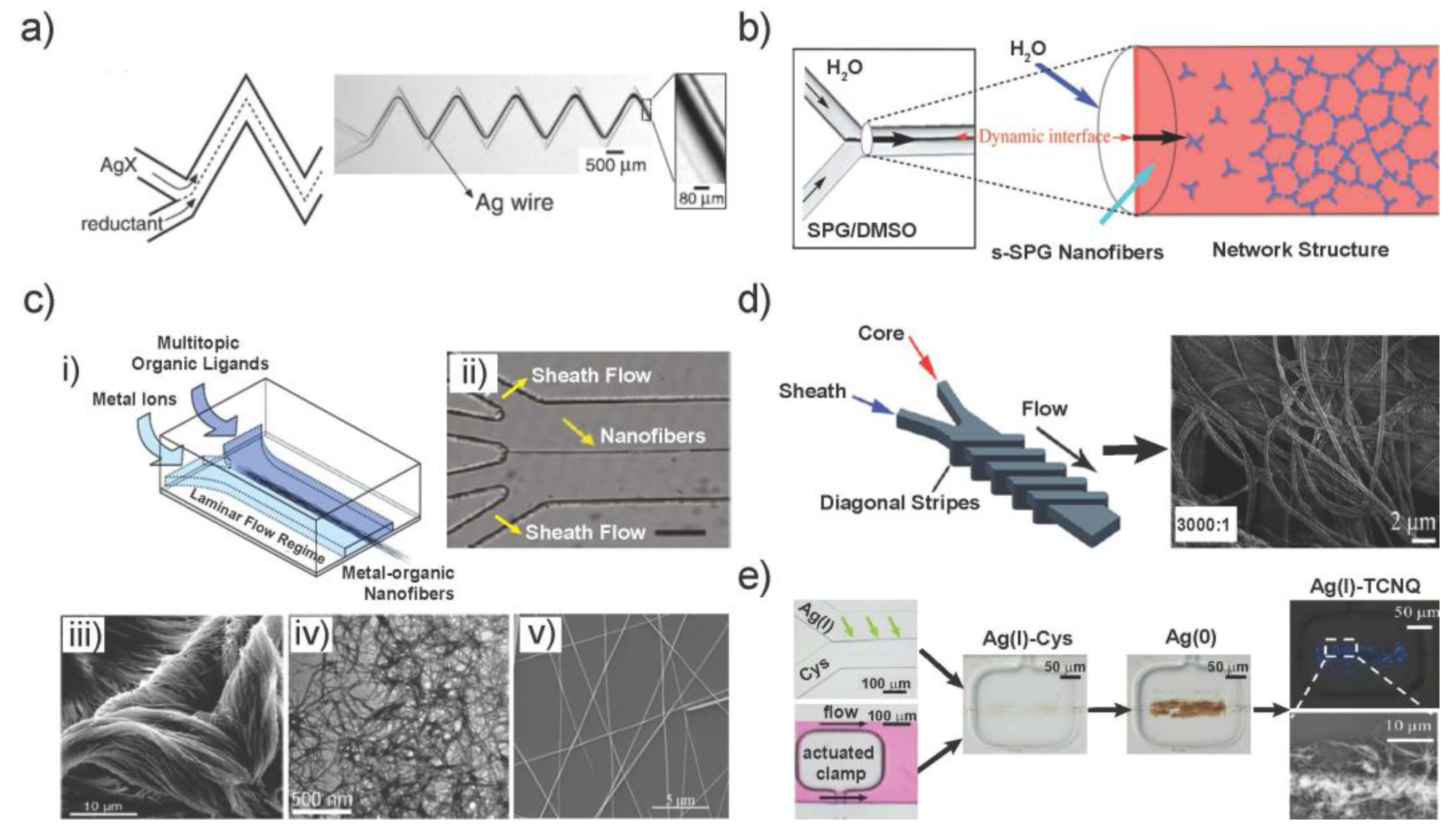
2. Microfluidic-Based Synthesis of 1D Nanostructures
2.1. Synthesis under Continuous Flow
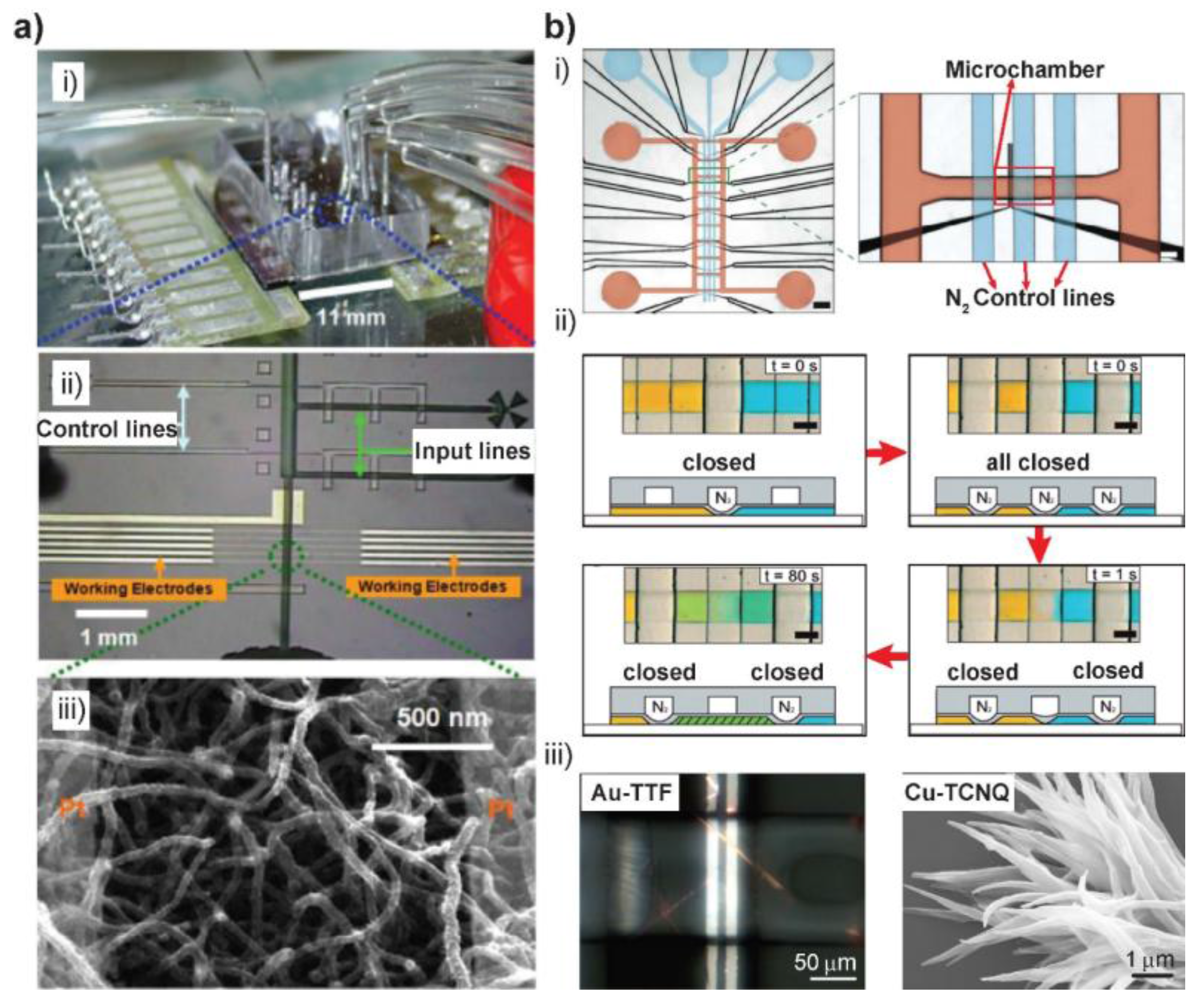
2.2. Valve-Based Synthesis
2.3. Other Microfluidic-Assisted Synthesis
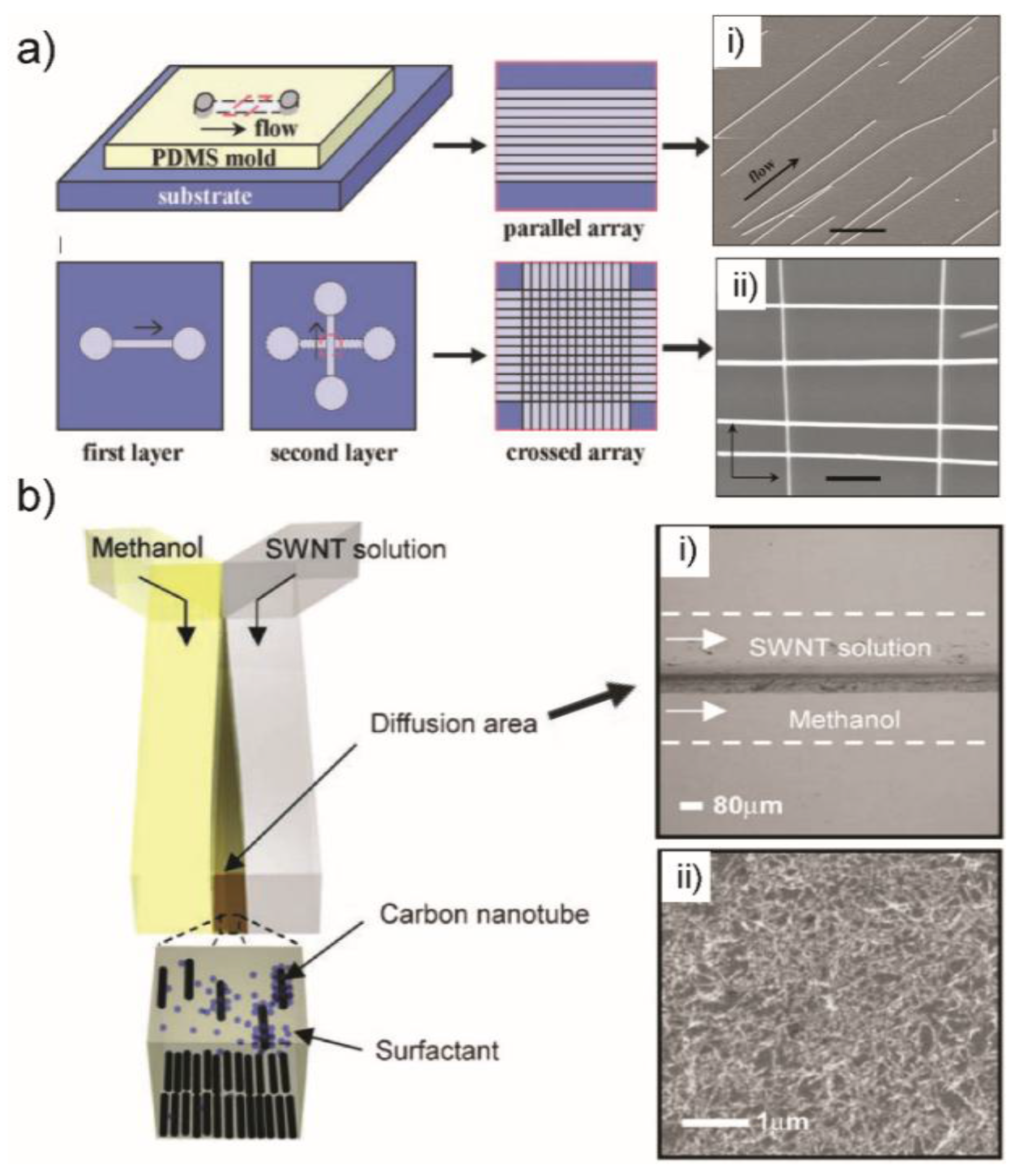
3. Controllable Alignment and Patterning of 1D Nanostructures
3.1. Alignment-after-Synthesis
3.2. Alignment-during-Synthesis
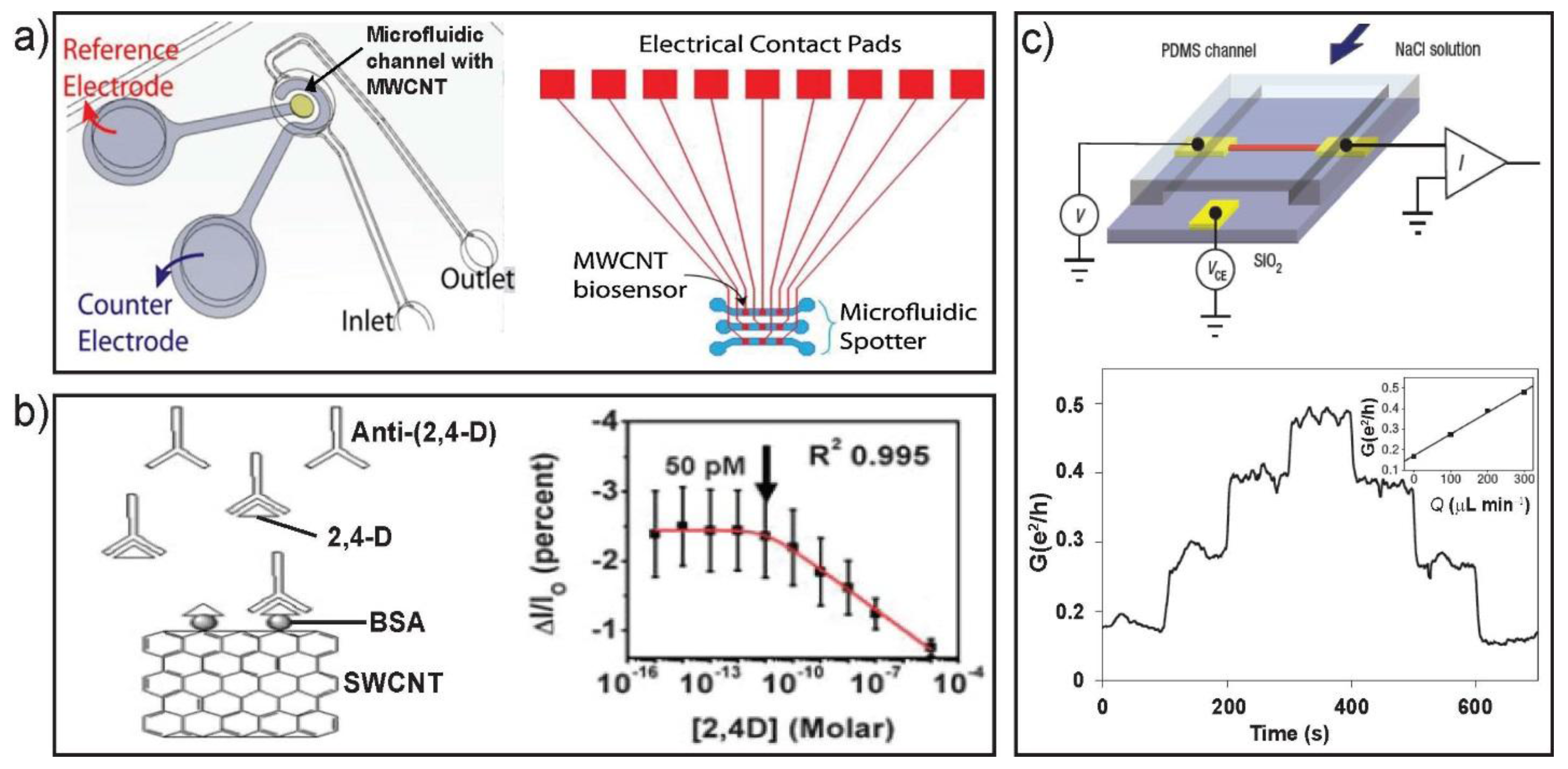
4. Microfluidic-Assisted Analytical Application of 1D Nanostructures
4.1. Nanowire Sensors
4.2. Nanotube Sensors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joachim, C.; Gimzewski, J.K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, X.; Cui, Y.; Lauhon, L.J.; Kim, K.-H.; Lieber, C.M. Logic gates and computation from assembled nanowire building blocks. Science 2001, 294, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater. 2007, 6, 841. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Lynall, D.; Savelyev, I.; Blumin, M.; Wang, S.; Ruda, H. Sensing responses based on transfer characteristics of InAs nanowire field-effect transistors. Sensors 2017, 17, 1640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; Chen, D.; Shen, G. Flexible electronics based on inorganic nanowires. Chem. Soc. Rev. 2015, 44, 161–192. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Kim, J.; Kim, S.; Lee, S.; Mensing, G.; Beebe, D.J. Hydrodynamic microfabrication via “on the fly” photopolymerization of microscale fibers and tubes. Lab Chip 2004, 4, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J.; Schaffhauser, D.; Burg, B.R.; Dittrich, P.S. A microfluidic approach for the formation of conductive nanowires and hollow hybrid structures. Adv. Mater. 2010, 22, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Jie, J.; Wang, G.; Wang, Q.; Chen, Y.; Han, X.; Wang, X.; Hou, J.G. Synthesis and characterization of aligned ZnO nanorods on porous aluminum oxide template. J. Phys. Chem. B 2004, 108, 11976–11980. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, E.P.A.M.; Verheijen, M.A. Synthesis of InP nanotubes. J. Am. Chem. Soc. 2003, 125, 3440–3441. [Google Scholar] [CrossRef] [PubMed]
- Kenis, P.J.A.; Ismagilov, R.F.; Whitesides, G.M. Microfabrication inside capillaries using multiphase laminar flow patterning. Science 1999, 285, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Thangawng, A.L.; Howell, P.B., Jr.; Richards, J.J.; Erickson, J.S.; Ligler, F.S. A simple sheath-flow microfluidic device for micro/nanomanufacturing: Fabrication of hydrodynamically shaped polymer fibers. Lab Chip 2009, 9, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J.; Rubio-Martínez, M.; Hartfelder, U.; Imaz, I.; Maspoch, D.; Dittrich, P.S. Coordination polymer nanofibers generated by microfluidic synthesis. J. Am. Chem. Soc. 2011, 133, 4216–4219. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Luo, X.; Lee, I.; Hu, Y.; Cui, X.T.; Yun, M. Rapid real-time electrical detection of proteins using single conducting polymer nanowire-based microfluidic aptasensor. Biosens. Bioelectron. 2011, 30, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Wang, Y.; Wang, S.; Tseng, H.-R. Integrated microfluidic reactors. Nano Today 2009, 4, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Classen, S.; Berger, J.M.; Quake, S.R. A microfluidic device for kinetic optimization of protein crystallization and in situ structure determination. J. Am. Chem. Soc. 2006, 128, 3142–3143. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Skordalakes, E.; Berger, J.M.; Quake, S.R. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl. Acad. Sci. USA 2002, 99, 16531–16536. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, B.Z.; Puigmartí-Luis, J.; Schaffhauser, D.; Ryll, T.; Schmid, S.; Dittrich, P.S. Confined synthesis and integration of functional materials in sub-nanoliter volumes. ACS Nano 2013, 7, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-J.; Park, J.-Y.; Lee, J.-Y.; Park, H.; Park, Y.-D.; Lee, K.-B.; Whang, C.-M.; Lee, S.-H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Sugaya, S.; Naganuma, Y.; Seki, M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter 2012, 8, 3122–3130. [Google Scholar] [CrossRef]
- Lee, K.H.; Shin, S.J.; Park, Y.; Lee, S.-H. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small 2009, 5, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Laocharoensuk, R.; Palaniappan, K.; Smith, N.A.; Dickerson, R.M.; Werder, D.J.; Baldwin, J.K.; Hollingsworth, J.A. Flow-based solution-liquid-solid nanowire synthesis. Nat. Nanotechnol. 2013, 8, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Parisi, J.; Su, L.; Lei, Y. In situ synthesis of silver nanoparticle decorated vertical nanowalls in a microfluidic device for ultrasensitive in-channel sers sensing. Lab Chip 2013, 13, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Parisi, J.; Liu, Y.; Su, L.; Lei, Y. In situ synthesis of vertical 3-D copper-core/carbon-sheath nanowalls in microfluidic devices. RSC Adv. 2013, 3, 1388–1396. [Google Scholar] [CrossRef]
- Joo, J.; Chow, B.Y.; Prakash, M.; Boyden, E.S.; Jacobson, J.M. Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nat. Mater. 2011, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, Z.; Park, I. Direct synthesis and integration of functional nanostructures in microfluidic devices. Lab Chip 2011, 11, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Yi, H.; Hwang, S.; Weitz, D.A.; Lee, C.-S. Microfluidic fabrication of complex-shaped microfibers by liquid template-aided multiphase microflow. Lab Chip 2011, 11, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Li, S.; Lu, Y.; Xu, J.; Luo, G. Controllable preparation of microscale tubes with multiphase co-laminar flow in a double co-axial microdevice. Lab Chip 2009, 9, 3282–3288. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Khademhosseini, A.; Park, Y.; Sun, K.; Lee, S.-H. Microfluidic chip-based fabrication of PLGA microfiber scaffolds for tissue engineering. Langmuir 2008, 24, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Takigami, Y.; Takayama, M.; Kozawa, T.; Hirose, N. Hierarchical supramolecular spinning of nanofibers in a microfluidic channel: Tuning nanostructures at a dynamic interface. Chem. Eur. J. 2012, 18, 13008–13017. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Kozawa, T. Supramolecular polymerization in microfluidic channels: Spatial control over multiple intermolecular interactions. Chem. Eur. J. 2013, 19, 12629–12634. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, S.J.; Kim, C.-B.; Kim, J.K.; Cho, Y.W.; Chung, B.G.; Lee, S.-H. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010, 10, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, K.; Won, S.W.; Cha, C.; Gaharwar, A.K.; Selimović, Š.; Bae, H.; Lee, K.H.; Lee, D.H.; Lee, S.-H.; et al. Microfluidic fabrication of cell adhesive chitosan microtubes. Biomed. Microdevices 2013, 15, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J.; Rubio-Martínez, M.; Imaz, I.; Cvetković, B.Z.; Abad, L.; Pérez del Pino, A.; Maspoch, D.; Amabilino, D.B. Localized, stepwise template growth of functional nanowires from an amino acid-supported framework in a microfluidic chip. ACS Nano 2014, 8, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bunimovich, Y.L.; Sui, G.; Savvas, S.; Wang, J.; Guo, Y.; Heath, J.R.; Tseng, H.-R. Electrochemical fabrication of conducting polymer nanowires in an integrated microfluidic system. Chem. Commun. 2006, 3075–3077. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.H.; Park, H.; Kim, D.-P. Ultrafast and continuous synthesis of unaccommodating inorganic nanomaterials in droplet- and ionic liquid-assisted microfluidic system. J. Am. Chem. Soc. 2011, 133, 14765–14770. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Esser, N.; Dittrich, P.S. Conductive single nanowires formed and analysed on microfluidic devices. J. Mater. Chem. C 2016, 4, 9235–9244. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, G.; Speiser, E.; Esser, N.; Dittrich, P.S. Localized synthesis of conductive copper-tetracyanoquinodimethane nanostructures in ultrasmall microchambers for nanoelectronics. ACS Appl. Mater. Interfaces 2017, 9, 17271–17278. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.K.; Tsai, S.S.H.; Wan, J.; Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D 2013, 46, 114002. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wu, Y.; Jiang, L. The art of aligning one-dimensional (1D) nanostructures. Chem. Soc. Rev. 2012, 41, 7832–7856. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, X.; Wei, Q.; Lieber, C.M. Directed assembly of one-dimensional nanostructures into functional networks. Science 2001, 291, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Niu, C.; Sahi, V.; Chen, J.; Parce, J.W.; Empedocles, S.; Goldman, J.L. High-performance thin-film transistors using semiconductor nanowires and nanoribbons. Nature 2003, 425, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-U.; Meitl, M.A.; Hur, S.-H.; Usrey, M.L.; Strano, M.S.; Kenis, P.J.A.; Rogers, J.A. In situ deposition and patterning of single-walled carbon nanotubes by laminar flow and controlled flocculation in microfluidic channels. Angew. Chem. Int. Ed. 2006, 45, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Messer, B.; Song, J.H.; Yang, P. Microchannel networks for nanowire patterning. J. Am. Chem. Soc. 2000, 122, 10232–10233. [Google Scholar] [CrossRef]
- Mahajan, N.; Fang, J. Two-dimensional ordered arrays of aligned lipid tubules on substrates with microfluidic networks. Langmuir 2005, 21, 3153–3157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Weimer, W.A. Room-temperature assembly of directional carbon nanotube strings. J. Am. Chem. Soc. 2002, 124, 758–759. [Google Scholar] [CrossRef] [PubMed]
- Joon, S.S.; Yeo-Heung, Y.; Michael, J.R.; Jaephil, D.; Vesselin, S.; Mark, J.S.; Chong, H.A. The precise self-assembly of individual carbon nanotubes using magnetic capturing and fluidic alignment. Nanotechnology 2009, 20, 325607–325614. [Google Scholar] [CrossRef]
- Mathai, P.P.; Carmichael, P.T.; Shapiro, B.A.; Liddle, J.A. Simultaneous positioning and orientation of single nano-wires using flow control. RSC Adv. 2013, 3, 2677–2682. [Google Scholar] [CrossRef]
- Chang, C.-C.F.; Fu, L.-M.; Yang, R.-J. Active mixer. In Encyclopedia of Microfluidics and Nanofluidics, 1st ed.; Li, D., Ed.; Springer: New York, NY, USA, 2008; pp. 33–35. ISBN 978-0-387-48998-8. [Google Scholar]
- Kuhn, P.; Puigmarti-Luis, J.; Imaz, I.; Maspoch, D.; Dittrich, P.S. Controlling the length and location of in situ formed nanowires by means of microfluidic tools. Lab Chip 2011, 11, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-grown zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.J.; Oh, D.; Lee, S.W.; Goto, H.; Buckmaster, R.; Yasukawa, T.; Matsue, T.; Hong, S.-K.; Chul, H.; et al. Control of the ZnO nanowires nucleation site using microfluidic channels. J. Phys. Chem. B 2006, 110, 3856–3859. [Google Scholar] [CrossRef] [PubMed]
- Gang, A.; Haustein, N.; Baraban, L.; Weber, W.; Mikolajick, T.; Thiele, J.; Cuniberti, G. Microfluidic alignment and trapping of 1d nanostructures—A simple fabrication route for single-nanowire field effect transistors. RSC Adv. 2015, 5, 94702–94706. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Q.; Wang, H.; Li, Y. Continuous high-throughput phosphopeptide enrichment using microfluidic channels modified with aligned ZnO/TiO2 nanorod arrays. Biomed. Microdevices 2011, 13, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Rahong, S.; Yasui, T.; Yanagida, T.; Nagashima, K.; Kanai, M.; Meng, G.; He, Y.; Zhuge, F.; Kaji, N.; Kawai, T.; et al. Three-dimensional nanowire structures for ultra-fast separation of DNA, protein and RNA molecules. Sci. Rep. 2015, 5, 10584. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, T.J.; Ko, S.; Yeo, H.; Kim, S.; Noh, T.; Song, S.; Sung, M.M.; Lee, H. Hierarchical and multifunctional three-dimensional network of carbon nanotubes for microfluidic applications. Adv. Mater. 2012, 24, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Berenschot, E.J.W.; Burouni, N.; Schurink, B.; van Honschoten, J.W.; Sanders, R.G.P.; Truckenmuller, R.; Jansen, H.V.; Elwenspoek, M.C.; van Apeldoorn, A.A.; Tas, N.R. 3D nanofabrication of fluidic components by corner lithography. Small 2012, 8, 3823–3831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, J.W.; Kim, D.P.; Shin, J.H.; Park, I. Nanowire-integrated microfluidic devices for facile and reagent-free mechanical cell lysis. Lab Chip 2012, 12, 2914–2921. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Jiang, Z.; Wei, X. Emerging microfluidic devices for cell lysis: A review. Lab Chip 2014, 14, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Shahini, M.; Yeow, J.T.W. Carbon nanotubes for voltage reduction and throughput enhancement of electrical cell lysis on a lab-on-a-chip. Nanotechnology 2011, 22, 325705. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.-I.; Lee, J.S.; Park, C.B.; Kim, D.-P. A microfluidic system incorporated with peptide/Pd nanowires for heterogeneous catalytic reactions. Lab Chip 2011, 11, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, X.; Hu, J.; Lieber, C.M. Doping and electrical transport in silicon nanowires. J. Phys. Chem. B 2000, 104, 5213–5216. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.S.; Ivanisevic, A. Molecular analysis of blood with micro-/nanoscale field-effect-transistor biosensors. Small 2011, 7, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Krivitsky, V.; Hsiung, L.-C.; Lichtenstein, A.; Brudnik, B.; Kantaev, R.; Elnathan, R.; Pevzner, A.; Khatchtourints, A.; Patolsky, F. Si nanowires forest-based on-chip biomolecular filtering, separation and preconcentration devices: Nanowires do it all. Nano Lett. 2012, 12, 4748–4756. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Gao, A.; Dai, P.; Mao, H.; Zuo, X.; Fan, C.; Wang, Y.; Li, T. Ultrasensitive detection of dual cancer biomarkers with integrated cmos-compatible nanowire arrays. Anal. Chem. 2015, 87, 11203–11208. [Google Scholar] [CrossRef] [PubMed]
- Schütt, J.; Ibarlucea, B.; Illing, R.; Zörgiebel, F.; Pregl, S.; Nozaki, D.; Weber, W.M.; Mikolajick, T.; Baraban, L.; Cuniberti, G. Compact nanowire sensors probe microdroplets. Nano Lett. 2016, 16, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.U.; Chen, C.; Lin, K.-H.; Fang, Y.; Lieber, C.M. Label-free detection of small-molecule—Protein interactions by using nanowire nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 3208–3212. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with cmos-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M.; Zhang, G.-J. Label-free electrical detection of cardiac biomarker with complementary metal-oxide semiconductor-compatible silicon nanowire sensor arrays. Anal. Chem. 2009, 81, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.-I.; Lieber, C.M. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2003, 4, 51–54. [Google Scholar] [CrossRef]
- De, A.; van Nieuwkasteele, J.; Carlen, E.T.; van den Berg, A. Integrated label-free silicon nanowire sensor arrays for (bio)chemical analysis. Analyst 2013, 138, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.; Cardenas, P.P.; Otón, F.; Martinez, J.; Mas-Torrent, M.; Garcia, F.; Alonso, J.C.; Rovira, C.; Garcia, R. Detection of the early stage of recombinational DNA repair by silicon nanowire transistors. Nano Lett. 2012, 12, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Wu, C.-S.; Chuang, C.-K.; Pang, S.-T.; Pan, T.-M.; Yang, Y.-S.; Ko, F.-H. Real-time and label-free detection of the prostate-specific antigen in human serum by a polycrystalline silicon nanowire field-effect transistor biosensor. Anal. Chem. 2013, 85, 7912–7918. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Li, C.A.; Seong, G.H. Microfluidic chips for immunoassays. Annu. Rev. Anal. Chem. 2013, 6, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Lee, S.; Hong, J.; Jang, E.; Lee, T.; Koh, W.-G. Mutiscale substrates based on hydrogel-incorporated silicon nanowires for protein patterning and microarray-based immunoassays. Biosens. Bioelectron. 2013, 45, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Kuan, D.-H.; Wang, I.S.; Lin, J.-R.; Yang, C.-H.; Huang, C.-H.; Lin, Y.-H.; Lin, C.-T.; Huang, N.-T. A microfluidic device integrating dual cmos polysilicon nanowire sensors for on-chip whole blood processing and simultaneous detection of multiple analytes. Lab Chip 2016, 16, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Lee, C.H.; Zheng, X. Probing flow velocity with silicon nanowire sensors. Nano Lett. 2009, 9, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, D.; Gao, X.P.A.; Alexander, J.I.D. Sensing and energy harvesting of fluidic flow by InAs nanowires. Nano Lett. 2013, 13, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.A.; Maqableh, M.M.; Delahunt, M.J.; Tondra, M.; Flatau, A.B.; Shield, C.K.; Stadler, B.J.H. Fabrication of bioinspired inorganic nanocilia sensors. IEEE Trans. Magn. 2013, 49, 191–196. [Google Scholar] [CrossRef]
- Shirale, D.J.; Bangar, M.A.; Chen, W.; Myung, N.V.; Mulchandani, A. Effect of aspect ratio (length:Diameter) on a single polypyrrole nanowire FET device. J. Phys. Chem. C 2010, 114, 13375–13380. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Leu, P.W.; McIntyre, P.C. Vertical germanium nanowire arrays in microfluidic channels for charged molecule detection. J. Electrochem. Soc. 2009, 156, K11–K16. [Google Scholar] [CrossRef]
- Xing, Y.; Wyss, A.; Esser, N.; Dittrich, P.S. Label-free biosensors based on in situ formed and functionalized microwires in microfluidic devices. Analyst 2015, 140, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Sirbuly, D.J.; Tao, A.; Law, M.; Fan, R.; Yang, P. Multifunctional nanowire evanescent wave optical sensors. Adv. Mater. 2007, 19, 61–66. [Google Scholar] [CrossRef]
- Tey, J.N.; Wijaya, I.P.M.; Wei, J.; Rodriguez, I.; Mhaisalkar, S.G. Nanotubes-/nanowires-based, microfluidic-integrated transistors for detecting biomolecules. Microfluid. Nanofluid. 2010, 9, 1185–1214. [Google Scholar] [CrossRef]
- Crevillén, A.G.; Ávila, M.; Pumera, M.; González, M.C.; Escarpa, A. Food analysis on microfluidic devices using ultrasensitive carbon nanotubes detectors. Anal. Chem. 2007, 79, 7408–7415. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Rani, C.; Vijay Anand, A.; Ho, J.-A.A. Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosens. Bioelectron. 2009, 24, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Larrimore, L.; Nad, S.; Zhou, X.; Abruña, H.; McEuen, P.L. Probing electrostatic potentials in solution with carbon nanotube transistors. Nano Lett. 2006, 6, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Heller, I.; Kong, J.; Heering, H.A.; Williams, K.A.; Lemay, S.G.; Dekker, C. Individual single-walled carbon nanotubes as nanoelectrodes for electrochemistry. Nano Lett. 2005, 5, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Chatrathi, M.P.; Musameh, M. Capillary electrophoresis microchip with a carbon nanotube-modified electrochemical detector. Anal. Chem. 2004, 76, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Merkoçi, A.; Alegret, S. Carbon nanotube detectors for microchip CE: Comparative study of single-wall and multiwall carbon nanotube, and graphite powder films on glassy carbon, gold, and platinum electrode surfaces. Electrophoresis 2007, 28, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Crevillen, A.G.; Pumera, M.; Gonzalez, M.C.; Escarpa, A. Towards lab-on-a-chip approaches in real analytical domains based on microfluidic chips/electrochemical multi-walled carbon nanotube platforms. Lab Chip 2009, 9, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Elsnab, J.; Gehrke, C.; Li, J.; Gale, B.K. Microfluidic integrated multi-walled carbon nanotube (MWCNT) sensor for electrochemical nucleic acid concentration measurement. Sens. Actuators B Chem. 2013, 185, 370–376. [Google Scholar] [CrossRef]
- Panini, N.V.; Messina, G.A.; Salinas, E.; Fernández, H.; Raba, J. Integrated microfluidic systems with an immunosensor modified with carbon nanotubes for detection of prostate specific antigen (PSA) in human serum samples. Biosens. Bioelectron. 2008, 23, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Mahendra Wijaya, I.P.; Nie, T.J.; Gandhi, S.; Boro, R.; Palaniappan, A.; Hau, G.W.; Rodriguez, I.; Suri, C.R.; Mhaisalkar, S.G. Femtomolar detection of 2,4-dichlorophenoxyacetic acid herbicides via competitive immunoassays using microfluidic based carbon nanotube liquid gated transistor. Lab Chip 2010, 10, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Bourlon, B.; Wong, J.; Miko, C.; Forro, L.; Bockrath, M. A nanoscale probe for fluidic and ionic transport. Nat. Nanotechnol. 2007, 2, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Rocha-Santos, T.A.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Advances in point-of-care technologies with biosensors based on carbon nanotubes. Trends Anal. Chem. 2013, 45, 24–36. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kim, S.N.; Papadimitrakopoulos, F.; Rusling, J.F. Protein immunosensor using single-wall carbon nanotube forests with electrochemical detection of enzyme labels. Mol. BioSyst. 2005, 1, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Wong Shi Kam, N.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Schadler, L.S.; Siegel, R.W.; Zhang, X.; Zhang, H.; Terrones, M. Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J. Mater. Chem. 2004, 14, 37–39. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Sritongkham, P.; Karuwan, C.; Phokharatkul, D.; Maturos, T.; Tuantranont, A. Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor. Biosens. Bioelectron. 2010, 26, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Baek, J.; Kim, H.; Lee, K.; Lee, S. Integration of enzyme immobilized single-walled carbon nanotubes mass into the microfluidic platform and its application for the glucose-detection. Sens. Actuators A Phys. 2006, 128, 7–13. [Google Scholar] [CrossRef]
- Campbell, J.K.; Sun, L.; Crooks, R.M. Electrochemistry using single carbon nanotubes. J. Am. Chem. Soc. 1999, 121, 3779–3780. [Google Scholar] [CrossRef]
- Ghosh, S.; Sood, A.K.; Kumar, N. Carbon nanotube flow sensors. Science 2003, 299, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Ghosh, S. Direct generation of a voltage and current by gas flow over carbon nanotubes and semiconductors. Phys. Rev. Lett. 2004, 93, 086601. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Choo, J. Recent advances in surface-enhanced Raman scattering detection technology for microfluidic chips. Electrophoresis 2008, 29, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.S.; Erickson, D. Aptamer based surface enhanced Raman scattering detection of vasopressin using multilayer nanotube arrays. Biosens. Bioelectron. 2010, 25, 1240–1243. [Google Scholar] [CrossRef] [PubMed]



| Microfluidic Method | Class of Material | Composition | Synthetic Approach | Morphology | Ref. |
|---|---|---|---|---|---|
| Continuous flow (Coaxial flow or Hydrodynamic flow focussing) | Inorganic | Alginate | Ionic crosslinking | Microfiber | [23,24,25] |
| Cadmium selenide; Zinc selenide | Solution-liquid-solid growth | Nanowire | [26] | ||
| Copper-carbon | Hydrothermal growth | Nanowire | [27,28] | ||
| Silver | Self-assembly | Microwire | [11] | ||
| Zinic oxide (ZnO) | Hydrothermal, ZnO seed | Nanowire | [29,30] | ||
| Organic | 4-Hydroxybutyl acrylate; Poly(ethylene glycol) diacrylate | Photo-polymerization | Microfiber/microtube | [6,31] | |
| Polyacrylonitrile; Polysulfone; Polystyrene; Poly(methylmethacrylate) | Solvent-extraction hardening | Nano-/microfiber | [12,32] | ||
| Poly(lactic-co-glycolic acid) | Solvent-exchange solidification | Microfiber | [33] | ||
| Bio-organic | B-1,3-glucan; Guanosine 5′-monophosphate | Self-assembly | Nanofiber | [34,35] | |
| Chitosan | Ionic crosslinking | Microfiber/microtube | [36,37] | ||
| Metal-organic | Copper-aspartate; Silver-cysteine; Zinic-4,4′-bipyridine | Self-assembly | Nanofiber | [14] | |
| Gold-tetrathiafulvalene (Au-TTF) | Self-assembly | Nano-/microwire | [7] | ||
| Silver-tetracyanoquinodimethane (Ag-TCNQ) | Self-assembly | Nanowire | [38] | ||
| Valve-based | Organic | Polyaniline/polypyrrole | Electro-polymerization | Nanowire | [39] |
| Metal-organic | Au-TTF/Ag-TCNQ | Self-assembly | Microwire | [22] | |
| Copper-tetracyanoquinodimethane | Self-assembly | Nano-/microwire | [22] | ||
| Microdroplet | Inorganic | γ-AlOOH/β-FeOOH | Ion liquid template | Nanofiber/nanorod | [40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Dittrich, P.S. One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration towards Functional Sensing Devices. Sensors 2018, 18, 134. https://doi.org/10.3390/s18010134
Xing Y, Dittrich PS. One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration towards Functional Sensing Devices. Sensors. 2018; 18(1):134. https://doi.org/10.3390/s18010134
Chicago/Turabian StyleXing, Yanlong, and Petra S. Dittrich. 2018. "One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration towards Functional Sensing Devices" Sensors 18, no. 1: 134. https://doi.org/10.3390/s18010134




