Application of Near Infrared Reflectance Spectroscopy for Rapid and Non-Destructive Discrimination of Hulled Barley, Naked Barley, and Wheat Contaminated with Fusarium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hulled Barley, Naked Barley, and Wheat Samples
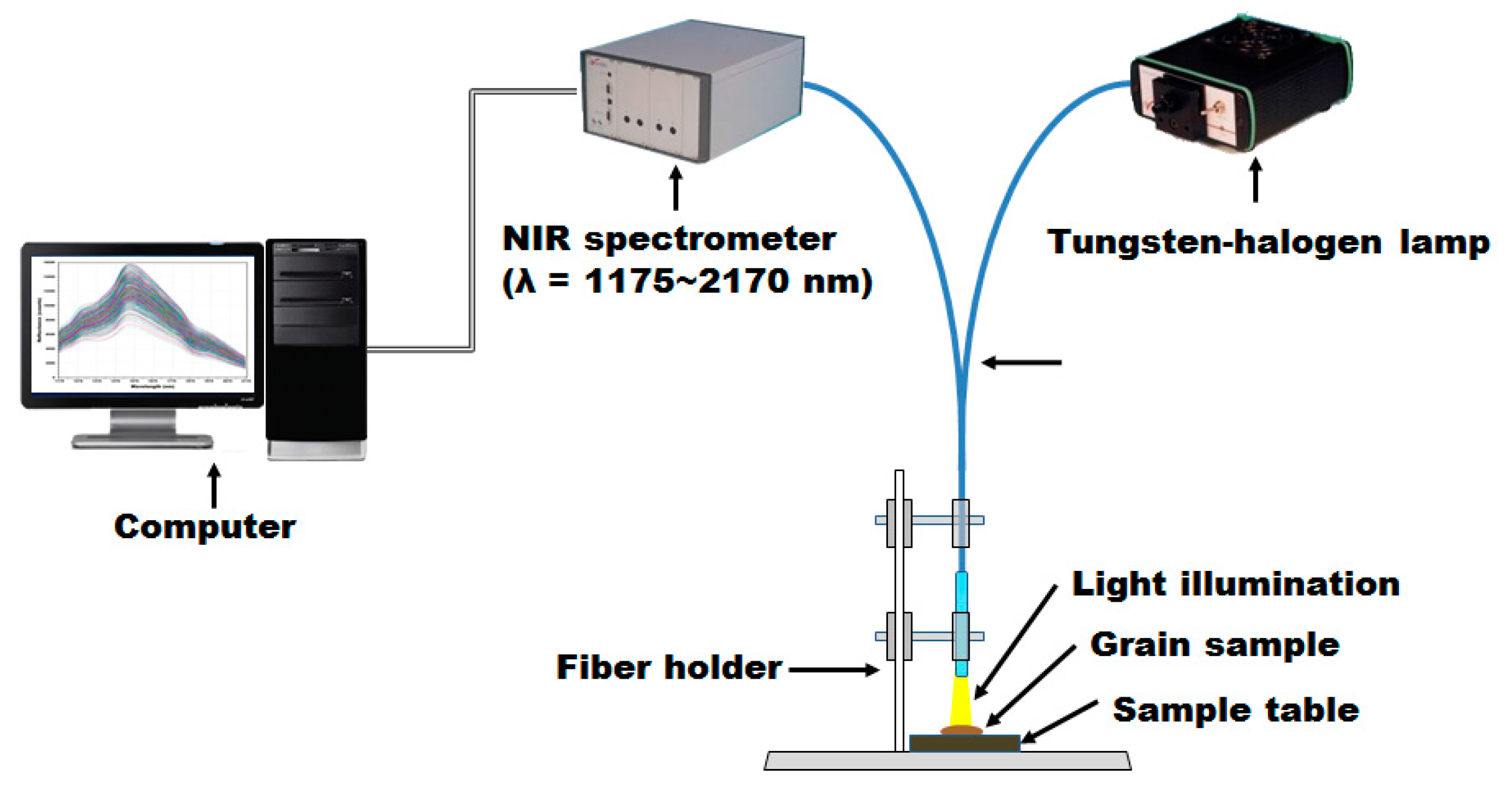
2.2. NIRS Measurement System
2.3. NIR Reflectance Spectrum Acquisition
2.4. Culturing Fusarium
2.5. Development of Fusarium Discrimination Prediction Model
3. Results and Discussion
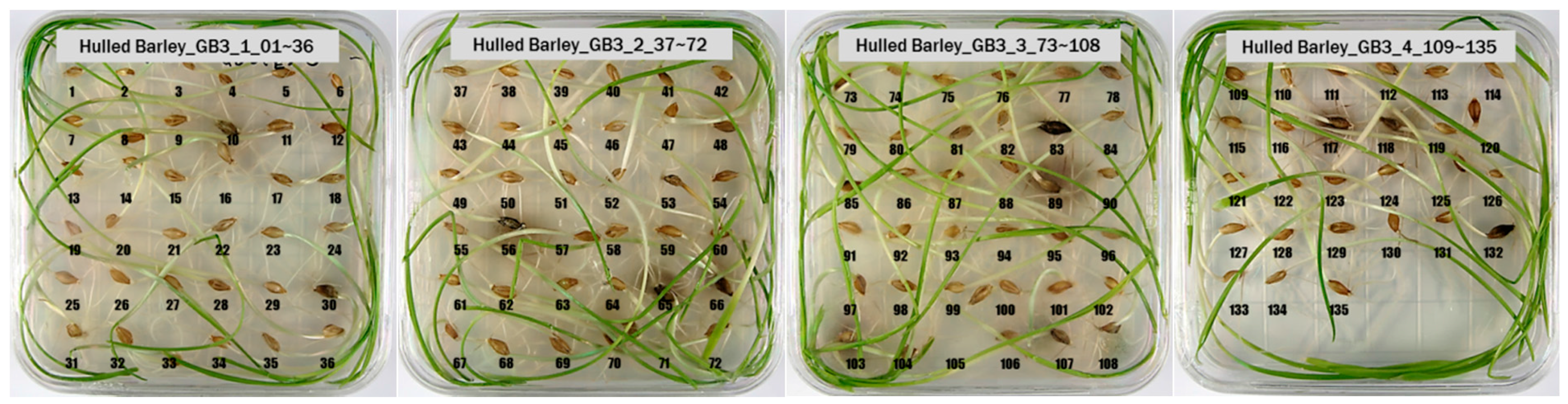
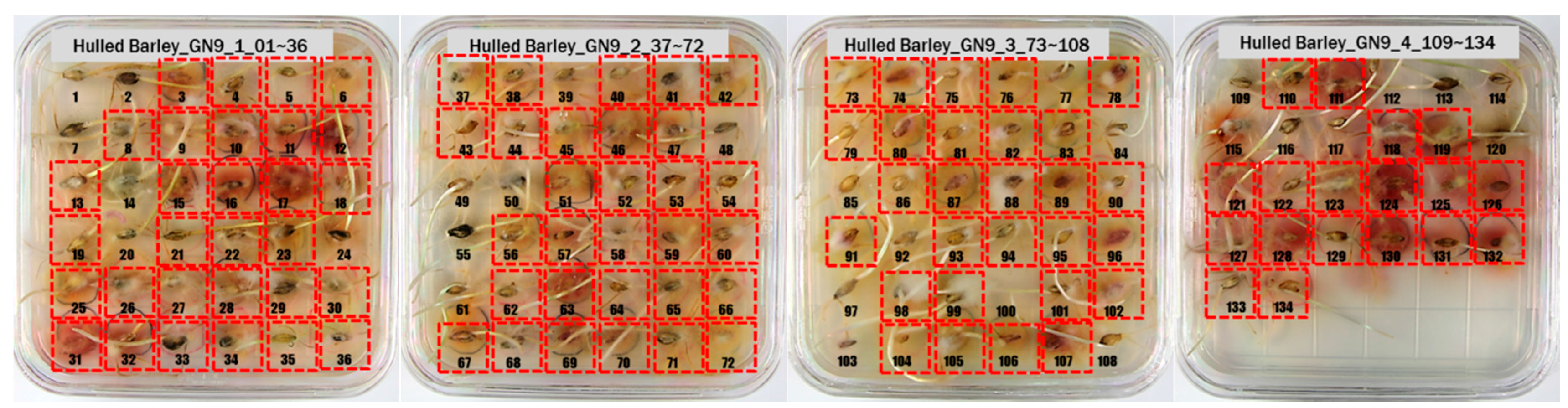
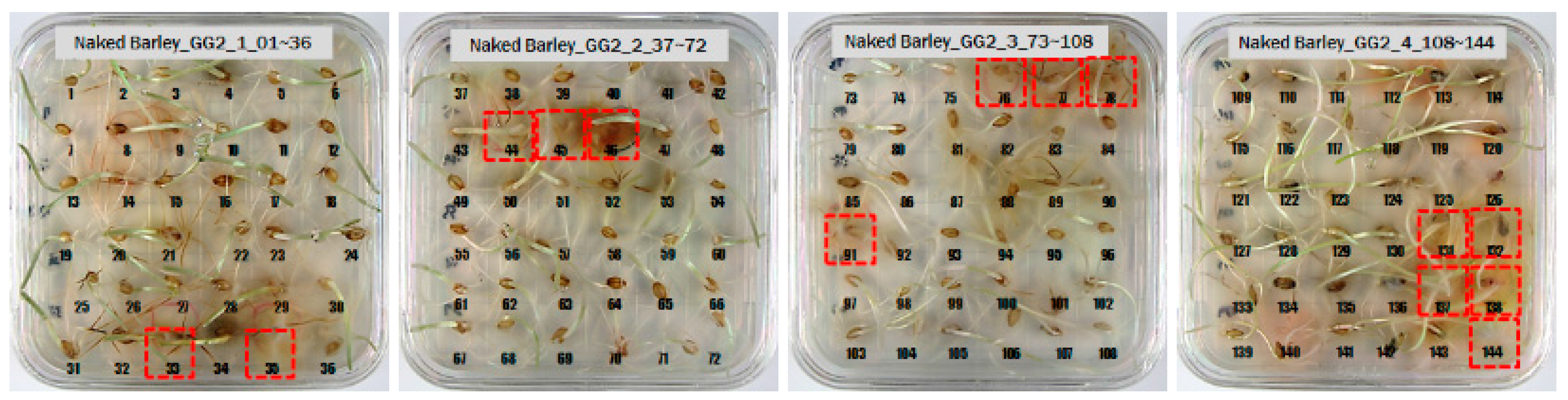
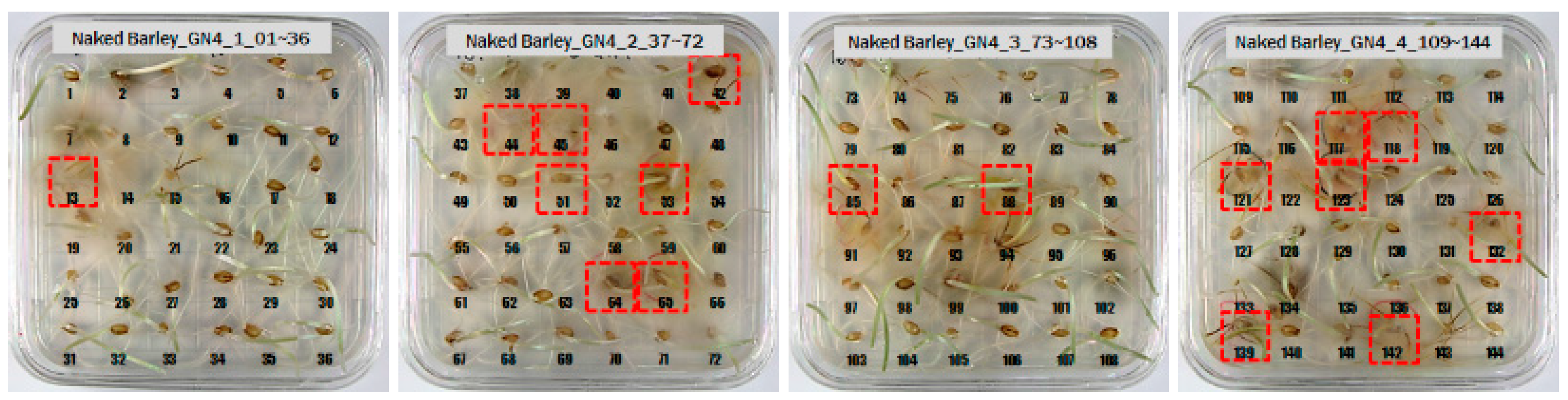
3.1. Culture Results of Hulled Barley, Naked Barley and Wheat
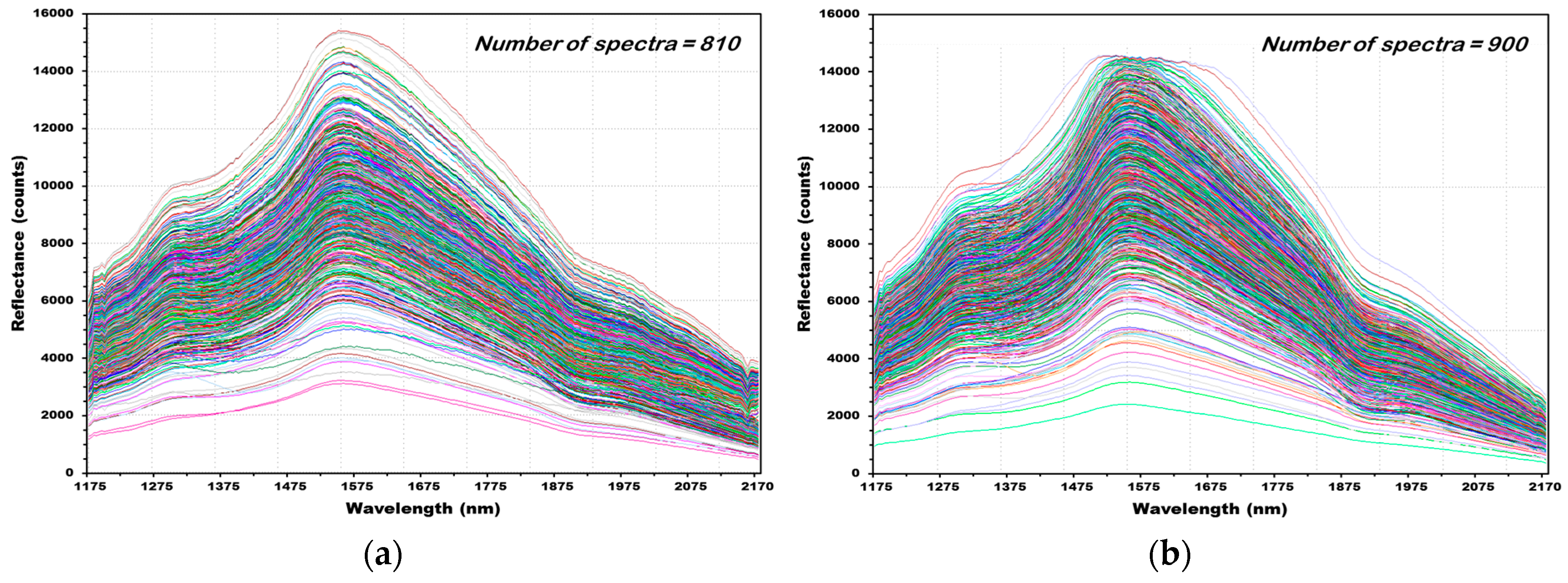
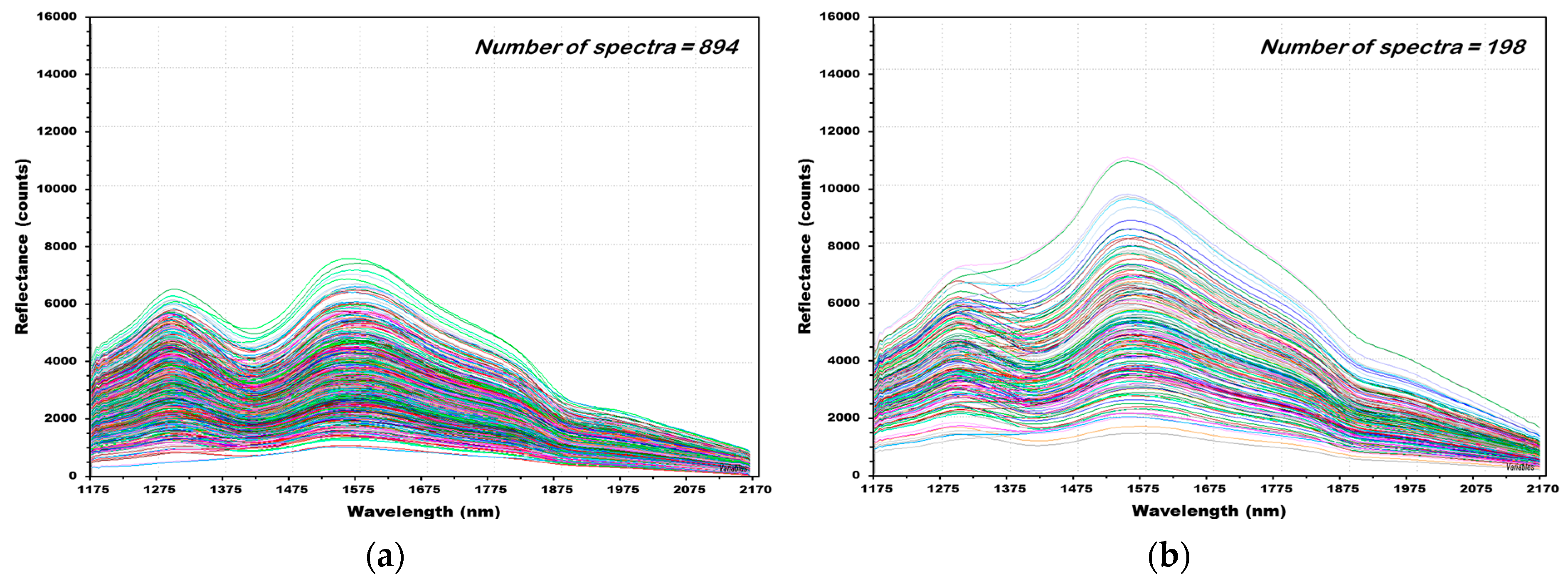
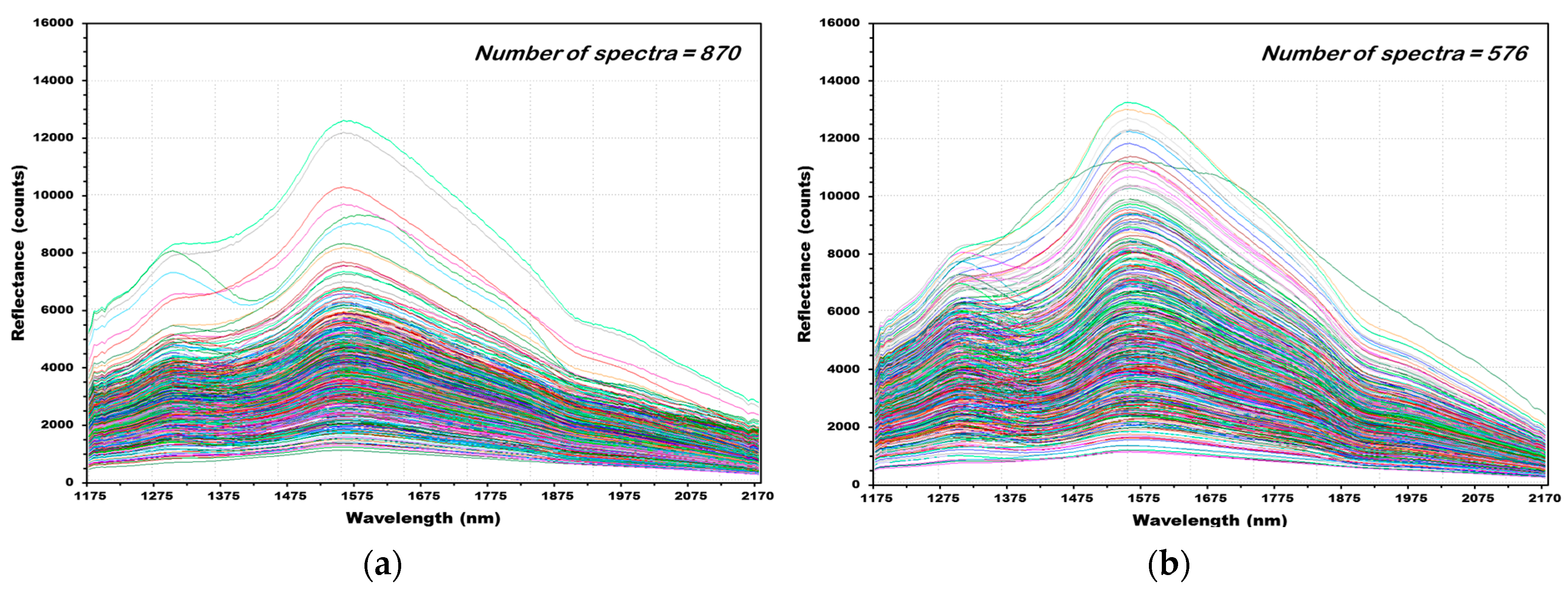
3.2. NIR Reflectance Spectra of Hulled Barley, Naked Barley, and Wheat
3.3. Results of the PLS-DA Prediction Model for Fusarium Discrimination
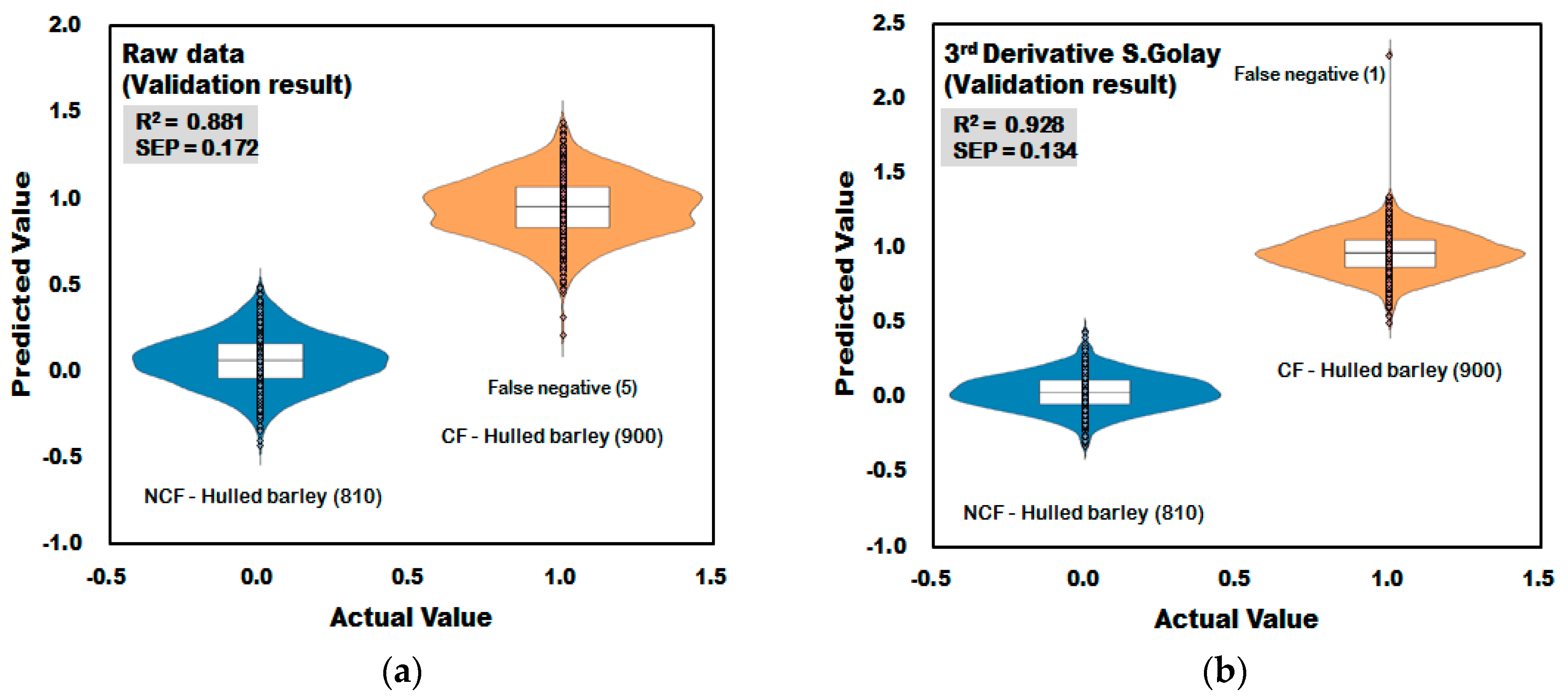
3.3.1. Prediction Results of the PLS-DA Model for Discrimination of Contaminated with Fusarium (CF)-Hulled Barley
3.3.2. Prediction Result of PLS-DA Model for Discrimination of CF-Naked Barley
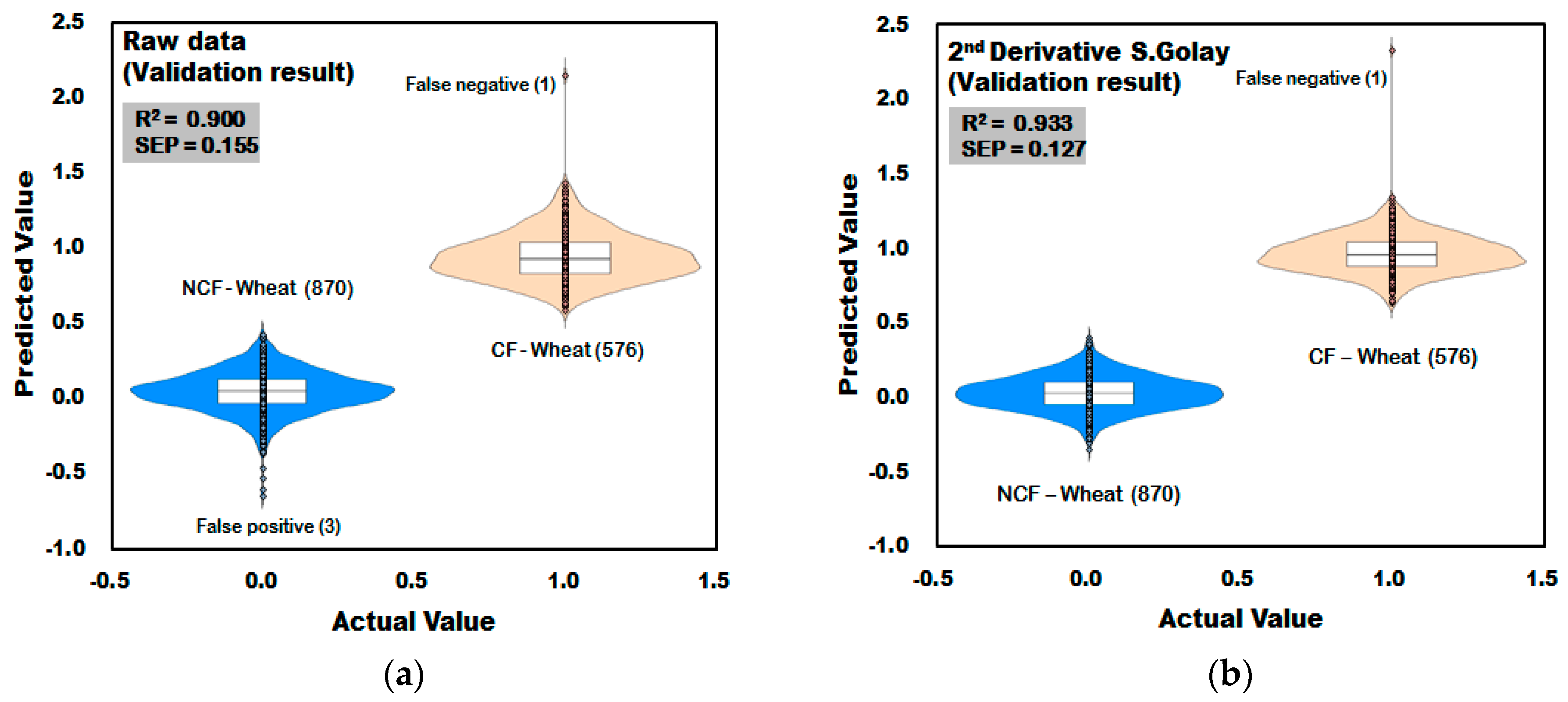
3.3.3. Prediction Result of PLS-DA Model for Discrimination of CF-Wheat
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.H.; Lee, S.K.; Park, H.Y.; Sim, E.Y.; Woo, K.S.; Oh, S.K.; Lee, B.K.; Kim, H.J. Quality characteristics of barley Makgeolli prepared with different barley cultivars and milling recovery. Korean J. Food Preser. 2016, 23, 530–553. [Google Scholar]
- Kalra, S.; Jood, S. Effect of dietary barley β-glucan on cholesterol and lipoprotein fractions in rat. J. Cereal Sci. 2000, 31, 141–145. [Google Scholar] [CrossRef]
- Song, E.S.; Park, S.J.; Woo, N.R.A.; Won, M.H.; Cho, J.S.; Kim, J.G.; Kang, M.H. Antioxidant capacity of colored barley extracts by varieties. Korean J. Food Nutr. 2005, 34, 1491–1497. [Google Scholar]
- Kim, Y.S.; Lee, Y.T.; Seong, H.M. Physicochemical properties of starches from waxy and non-waxy hull-less barleys. J. Korean Soc. Agric. Chem. Biotechnol. 1999, 42, 240–245. [Google Scholar]
- Hyun, J.N.; Kweon, S.J.; Park, D.S.; Ko, J.M.; Han, S.I.; Lim, S.G.; Suh, S.J. A new high quality and yielding barley variety “Geungangbori” with lodging resistance. Korean J. Breed. Sci. 2008, 40, 474–478. [Google Scholar]
- Seo, K.H.; Kim, Y.H.; Lee, Y.M.; Ghosh, M.; Park, K.M.; Park, D.H.; Kim, J.S.; Lim, B.O. Evaluation of Anti-Oxidant and Anti-Inflammatory Activities of Ganoderma lucidum Cultured on Hulled Barley. Korean J. Med. Crop Sci. 2017, 25, 29–36. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yi, Y.H. pH, acidity, color, amino acids, reducing sugars, total sugars, and alcohol in puffed millet powder containing millet Takju during fermentation. Korean J. Food Sci. Technol. 2010, 42, 727–732. [Google Scholar]
- Shin, D.S.; Choi, Y.J.; Jeong, S.T.; Sim, E.Y.; Lee, S.K.; Kim, H.J.; Woo, K.S.; Kim, S.J.; Oh, S.K.; Park, H.Y. Quality Characteristics of Mixed Makgeolli with Barley and Wheat. Korean J. Food Nutr. 2016, 29, 565–572. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gellenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Chung, H.S. Cereal scab causing mycotoxicosis in Korea and present status of mycotoxin researches. Korean J. Mycol. 1975, 3, 31–36. [Google Scholar]
- Marasas, W.F.O.; Kriek, N.P.J.; Fincham, J.E.; van Rensburg, S.J. Primary liver cancer and oesophageal basal cell hyperplasia in rats caused by Fusarium moniliforme. Int. J. Cancer 1984, 34, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, S.R. Classification of scab- and other mold-damaged wheat kernels by near-infrared reflectance spectroscopy. Trans. ASAE. 2003, 46, 731–738. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Shao, S.; Cai, Z.; Feng, L.; Pan, H.; Wang, Z. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1143, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, H.L.; Warner, R.M.; Prelusky, D.B. Assessment of extraction procedures in the analysis of naturally contaminated grain products for deoxynivalenol (vomitoxin). J. AOAC 1985, 68, 645–649. [Google Scholar]
- Scott, P.M.; Kanhere, S.R.; Tarter, E.J. Determination of nivalenol and deoxynivalenol in cereals by electron-capture gas chromatography. J. AOAC 1986, 69, 889–893. [Google Scholar]
- Tacke, B.K.; Casper, H.H. Determination of deoxynivalenol in wheat, barley, and malt by column cleanup and gas chromatography with electron capture detection. J. AOAC Int. 1996, 79, 472–475. [Google Scholar] [PubMed]
- Scott, P.M.; Lau, P.Y.; Kanhere, S.R. Gas chromatography with electron capture and mass spectrometric detection of deoxynivalenol in wheat and other grains. J. AOAC 1981, 64, 1364–1371. [Google Scholar]
- Usleber, E.; Schneider, E.; Märtlbauer, E.; Terplan, G. Two formats of enzyme immunoassay for 15-acetyldeoxynivalenol applied to wheat. J. Agric. Food Chem. 1993, 41, 2019–2023. [Google Scholar] [CrossRef]
- Abramson, D.; Clear, R.M.; Usleber, E.; Gessler, R.; Nowicki, T.W.; Märtlbauer, E. Fusarium species and 8-keto-trichothecene mycotoxins in Manitoba barley. Cereal Chem. 1998, 75, 137–141. [Google Scholar] [CrossRef]
- De Girolamo, A.; Cervellieri, S.; Visconti, A.; Pascale, M. Rapid Analysis of Deoxynivalenol in Durum Wheat by FT-NIR Spectroscopy. Toxins 2014, 6, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.R.; Norris, K.H.; Golumbic, C. Determination of the moisture content of seeds by near-infrared spectrophotometry of their methanol extracts. Cereal Chem. 1962, 39, 94–99. [Google Scholar]
- Wold, H. Path Models with Latent Variables: The NIPALS Approach. In Quantitative Sociology: International Perspectives on Mathematical and Statistical Modeling; Blalock, H.M., Aganbegian, A., Borodkin, F.M., Boudon, R., Capecchi, V., Eds.; Academic Press: New York, NY, USA, 1975; pp. 307–357. [Google Scholar]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-Regression: a Basic Tool of Chemometrics. Chemometr. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Han, I.S.; Kim, M.; Lee, C.H.; Cha, W.; Ham, B.K.; Jeong, J.H.; Lee, H.; Chung, C.B.; Han, C. Application of Partial Least Squares Methods to a Terephthalic Acid Manufacturing Process for Product Quality Control. Korean J. Chem. Eng. 2003, 20, 977–984. [Google Scholar] [CrossRef]
- Han, I.S.; Han, C. Modeling of Multistage Air-Compression Systems in Chemical Processes. Ind. Eng. Chem. Res. 2003, 42, 2209–2218. [Google Scholar] [CrossRef]
- Han, I.S.; Han, C.; Chung, C.B. Melt Index Modeling with Support Vector Machines, Partial Least Squares, and Artificial Neural Networks. J. Appl. Polym. Sci. 2004, 95, 967–974. [Google Scholar] [CrossRef]
- Lee, K.J.; Hruschka, W.R.; Abbott, J.A.; Noh, S.H.; Park, B.S. Predicting the soluble solids of apples by near infrared spectroscopy(II)-PLS and ANN moddels. J. Korean Soc. Agric. Mach. 1998, 23, 571–582. [Google Scholar]
- Son, J.R.; Lee, K.J.; Kang, S.W.; Yang, G.M.; Seo, Y.W. Development of prediction model for sugar content of strawberry using NIR spectroscopy. Food Eng. Prog. 2009, 13, 297–301. [Google Scholar]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Detection and identification of bacteria in an isolated system with near-infrared spectroscopy and multivariate analysis. J. Agric. Food Chem. 2008, 56, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.G. Development of On-Line Apple (Fuji) Sorting System by the Soluble Solid and Acid Contents Using VIS/NIR Spectroscopy. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2000. [Google Scholar]
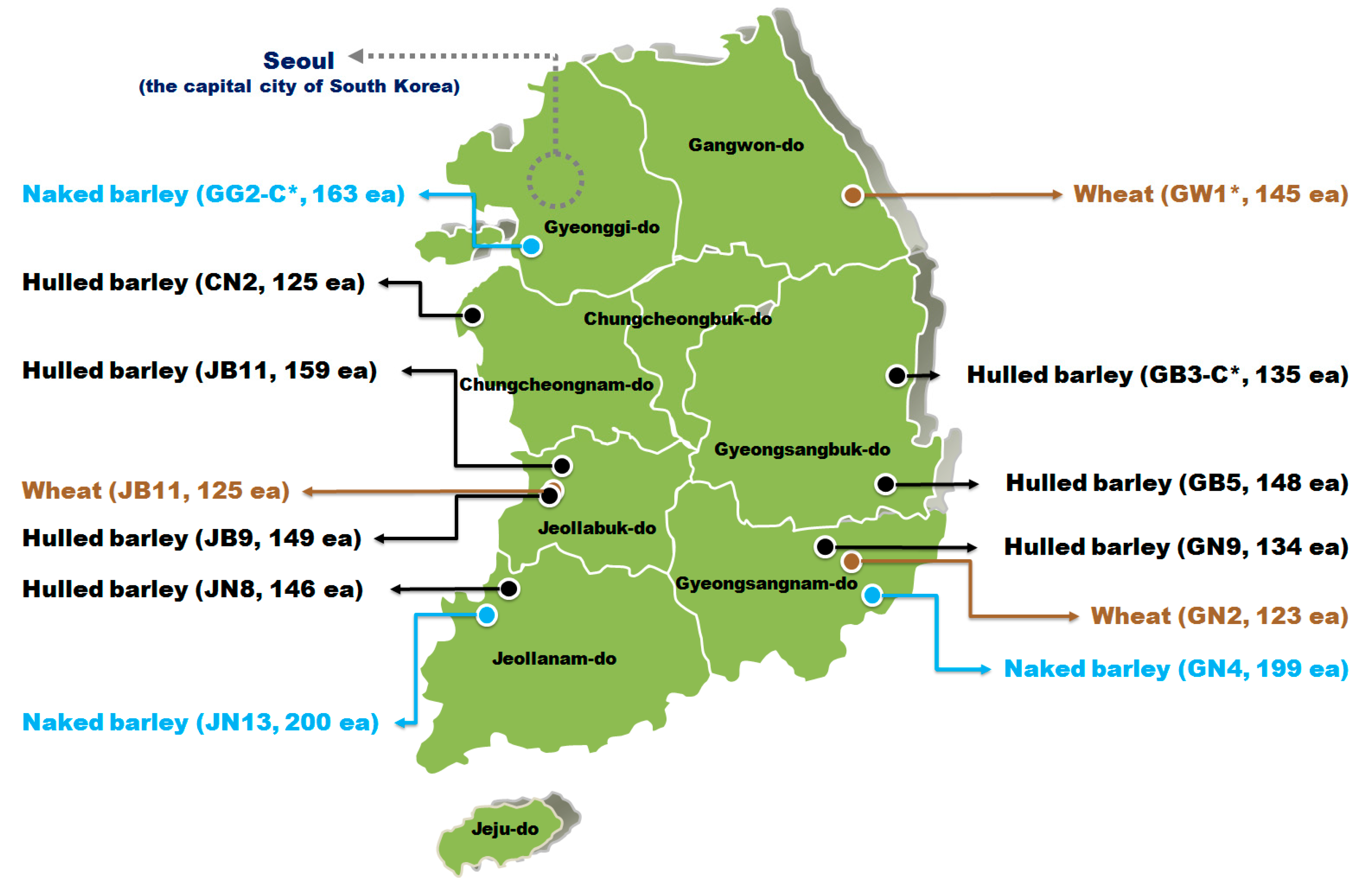




| Grains | Geographic Locations | Group | Quantity | Pictures | |
|---|---|---|---|---|---|
| Hulled barley | Gyeongsangbuk-do (Yeongdeok-gun) | GB3-C * | 135 |  |  |
| Gyeongsangbuk-do (Gyeongju-si) | GB5 | 148 |  |  | |
| Gyeongsangnam-do (Changnyeong-gun) | GN9 | 134 |  |  | |
| Jeollabuk-do (Iksan-si) | JB11 | 159 |  |  | |
| Jeollabuk-do (Gimje-si) | JB9 | 149 |  |  | |
| Jeollanam-do (Yeonggwang-gun) | JN8 | 146 |  |  | |
| Chungcheongnam-do (Taean-gun) | CN2 | 125 |  |  | |
| Naked barley | Gyeonggi-do (Hwaseong-si) | GG2-C * | 163 |  |  |
| Jeollanam-do (Hampyeong-gun) | JN13 | 200 |  |  | |
| Gyeongsangnam-do (Gimhae-si) | GN4 | 199 |  |  | |
| Wheat | Gangwon-do (Donghae-si) | GW1-C * | 145 |  |  |
| Jeollabuk-do (Gimje-si) | JB11 | 125 |  |  | |
| Gyeongsangnam-do (Milyang-si) | GN2 | 123 |  |  | |
| Types of Grain | (a) Primary Culture Results (1) NCF + 2) CF = Sum) | (b) Secondary Culture Results (NCF + CF = Sum) | |
|---|---|---|---|
| Hulled barley | GB3-C * | 135 + 0 = 135 | 135 + 0 = 135 |
| GB5 | 147 + 1 = 148 | 147 + 1 = 148 | |
| GN9 | 39 + 95 = 134 | 28 + 106 = 134 | |
| JB11 | 158 + 1 = 159 | 159 + 0 = 159 | |
| JB9 | 107 + 42 = 149 | 123 + 26 = 149 | |
| JN8 | 117 + 29 = 146 | 131 + 15 = 146 | |
| CN2 | 114 + 11 = 125 | 123 + 2 = 125 | |
| Naked barley | GG2-C * | 163 + 0 = 163 | 149 + 14 = 163 |
| JN13 | 166 + 34 = 200 | 188 + 12 = 200 | |
| GN4 | 172 + 27 = 199 | 178 + 21 = 199 | |
| Wheat | GW1-C * | 145 + 0 = 145 | 145 + 0 = 145 |
| JB11 | 25 + 100 = 125 | 92 + 33 = 125 | |
| GN2 | 73 + 50 = 123 | 60 + 63 = 123 | |
| Pretreatment | 1) PC | Performances of Calibration | Performances of Validation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2) Rc2 | 3) SEC | 4) CCR (%) | 5) Rv2 | 6) SEP | CCR (%) | ||||
| NCF | CF | NCF | CF | ||||||
| Hulled barley | |||||||||
| Non-pretreatment | 16 | 0.888 | 0.167 | 100 | 99.56 | 0.881 | 0.172 | 100 | 99.44 |
| 1st order Derivative | 11 | 0.918 | 0.143 | 100 | 100 | 0.911 | 0.149 | 100 | 99.56 |
| 2nd order Derivative | 10 | 0.950 | 0.112 | 100 | 99.78 | 0.946 | 0.116 | 100 | 99.78 |
| 3rd order Derivative | 9 | 0.934 | 0.128 | 100 | 99.89 | 0.928 | 0.134 | 100 | 99.89 |
| Mean 7) Nor | 13 | 0.863 | 0.185 | 98.52 | 99.00 | 0.855 | 0.190 | 98.52 | 98.89 |
| Maximum Nor | 13 | 0.855 | 0.190 | 98.52 | 99.00 | 0.846 | 0.196 | 98.15 | 98.78 |
| Range Nor | 14 | 0.869 | 0.181 | 98.89 | 99.11 | 0.860 | 0.187 | 98.77 | 99.00 |
| 8) MSC | 12 | 0.835 | 0.203 | 98.02 | 99.11 | 0.826 | 0.208 | 97.90 | 98.78 |
| Baseline | 15 | 0.875 | 0.177 | 100 | 99.44 | 0.868 | 0.182 | 99.88 | 99.22 |
| 9) SNV | 13 | 0.836 | 0.202 | 97.90 | 99.11 | 0.827 | 0.208 | 97.90 | 98.78 |
| Naked barley | |||||||||
| Non-pretreatment | 13 | 0.868 | 0.140 | 99.89 | 98.99 | 0.859 | 0.145 | 99.89 | 98.48 |
| 1st order Derivative | 10 | 0.939 | 0.095 | 100 | 100 | 0.932 | 0.101 | 100 | 100 |
| 2nd order Derivative | 7 | 0.944 | 0.091 | 100 | 100 | 0.941 | 0.093 | 100 | 100 |
| 3rd order Derivative | 7 | 0.950 | 0.086 | 100 | 100 | 0.948 | 0.088 | 100 | 100 |
| Mean Nor | 12 | 0.810 | 0.168 | 99.22 | 98.99 | 0.800 | 0.174 | 99.22 | 98.99 |
| Maximum Nor | 13 | 0.810 | 0.170 | 99.22 | 98.99 | 0.792 | 0.176 | 99.22 | 98.99 |
| Range Nor | 13 | 0.807 | 0.169 | 99.22 | 98.99 | 0.792 | 0.176 | 99.22 | 98.99 |
| MSC | 11 | 0.777 | 0.182 | 99.33 | 98.99 | 0.758 | 0.190 | 98.99 | 98.48 |
| Baseline | 13 | 0.865 | 0.142 | 99.89 | 98.99 | 0.855 | 0.147 | 99.89 | 98.48 |
| SNV | 12 | 0.781 | 0.181 | 99.33 | 98.48 | 0.763 | 0.188 | 99.11 | 98.48 |
| Wheat | |||||||||
| Non-pretreatment | 12 | 0.908 | 0.149 | 99.66 | 100 | 0.900 | 0.155 | 99.66 | 99.83 |
| First-order Derivative | 8 | 0.923 | 0.136 | 100 | 100 | 0.909 | 0.147 | 100 | 99.83 |
| Second-order Derivative | 7 | 0.941 | 0.119 | 100 | 100 | 0.933 | 0.127 | 100 | 99.83 |
| Third-order Derivative | 5 | 0.916 | 0.141 | 100 | 99.83 | 0.911 | 0.146 | 100 | 99.83 |
| Mean Nor | 9 | 0.939 | 0.121 | 99.89 | 99.83 | 0.934 | 0.126 | 99.89 | 99.83 |
| Maximum Nor | 10 | 0.933 | 0.127 | 99.89 | 99.83 | 0.928 | 0.132 | 99.89 | 99.83 |
| Range Nor | 10 | 0.928 | 0.131 | 99.54 | 100 | 0.921 | 0.138 | 99.54 | 99.83 |
| MSC | 9 | 0.912 | 0.145 | 99.54 | 100 | 0.906 | 0.150 | 99.54 | 99.65 |
| Baseline | 13 | 0.909 | 0.147 | 99.89 | 100 | 0.901 | 0.154 | 99.89 | 99.65 |
| SNV | 10 | 0.913 | 0.144 | 99.54 | 100 | 0.908 | 0.149 | 99.54 | 99.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Kim, G.; Mo, C.; Oh, K.; Kim, G.; Ham, H.; Kim, S.; Kim, M.S. Application of Near Infrared Reflectance Spectroscopy for Rapid and Non-Destructive Discrimination of Hulled Barley, Naked Barley, and Wheat Contaminated with Fusarium. Sensors 2018, 18, 113. https://doi.org/10.3390/s18010113
Lim J, Kim G, Mo C, Oh K, Kim G, Ham H, Kim S, Kim MS. Application of Near Infrared Reflectance Spectroscopy for Rapid and Non-Destructive Discrimination of Hulled Barley, Naked Barley, and Wheat Contaminated with Fusarium. Sensors. 2018; 18(1):113. https://doi.org/10.3390/s18010113
Chicago/Turabian StyleLim, Jongguk, Giyoung Kim, Changyeun Mo, Kyoungmin Oh, Geonseob Kim, Hyeonheui Ham, Seongmin Kim, and Moon S. Kim. 2018. "Application of Near Infrared Reflectance Spectroscopy for Rapid and Non-Destructive Discrimination of Hulled Barley, Naked Barley, and Wheat Contaminated with Fusarium" Sensors 18, no. 1: 113. https://doi.org/10.3390/s18010113





