Highly Integrated MEMS-ASIC Sensing System for Intracorporeal Physiological Condition Monitoring
Abstract
:1. Introduction
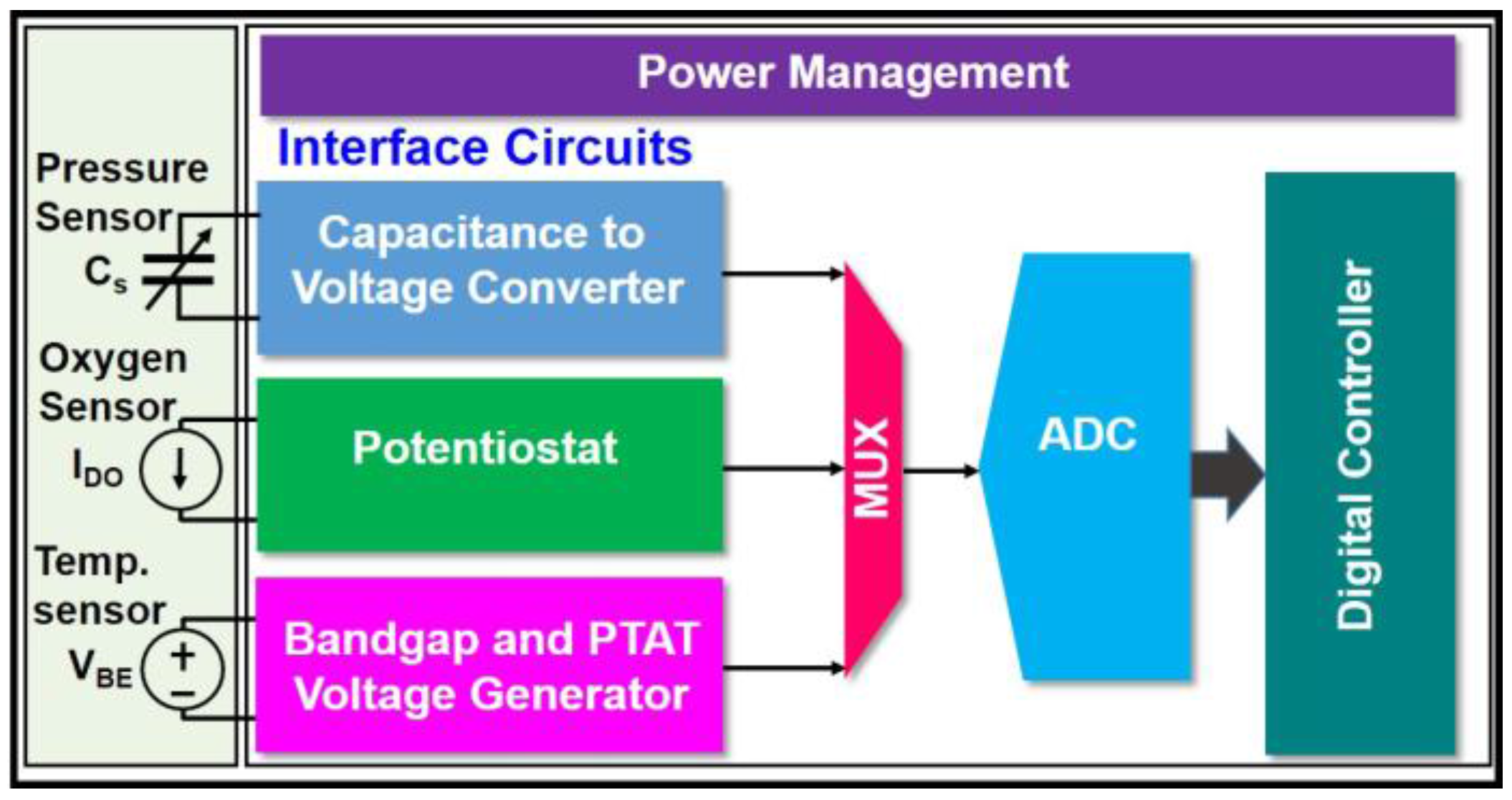
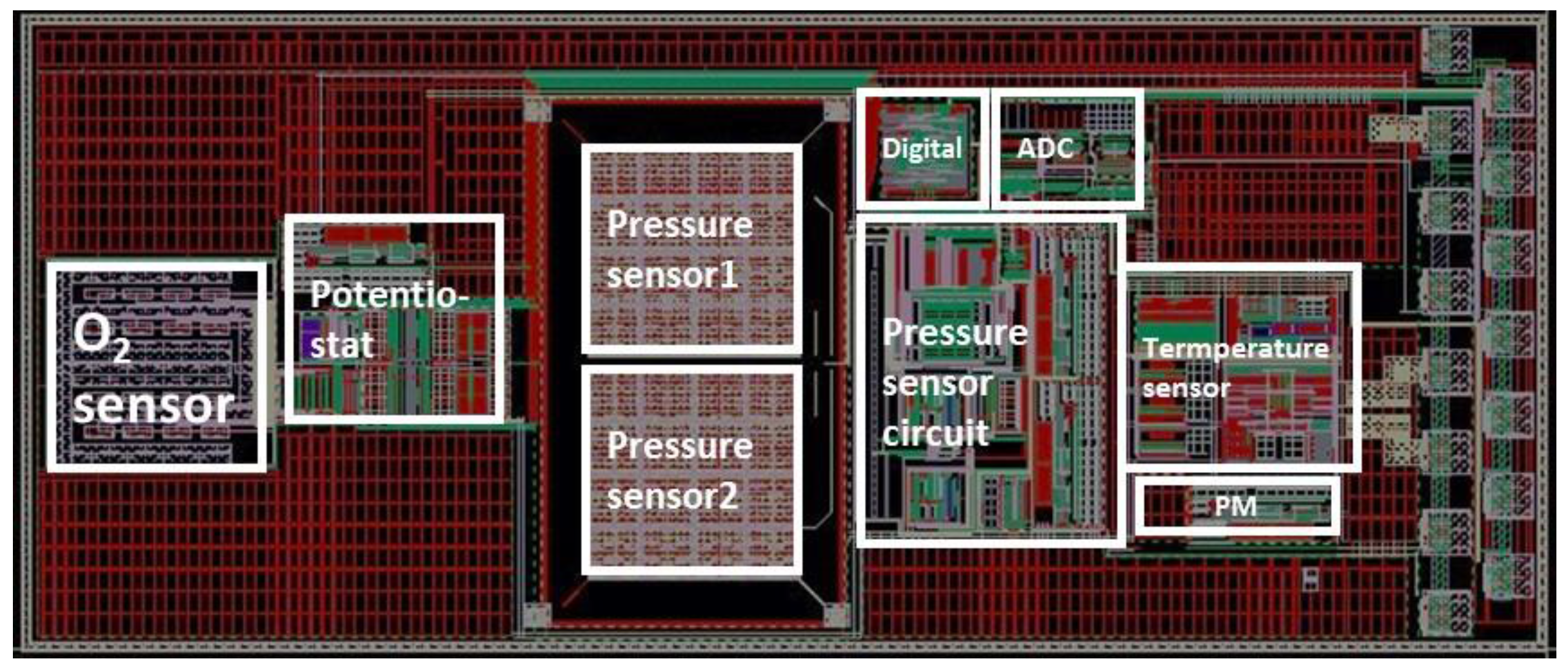
2. Overall View of the Proposed Single-Chip Multi-Sensing System
3. Pressure Sensor Design and Fabrication
3.1. Design and Simulation of the Pressure Sensor
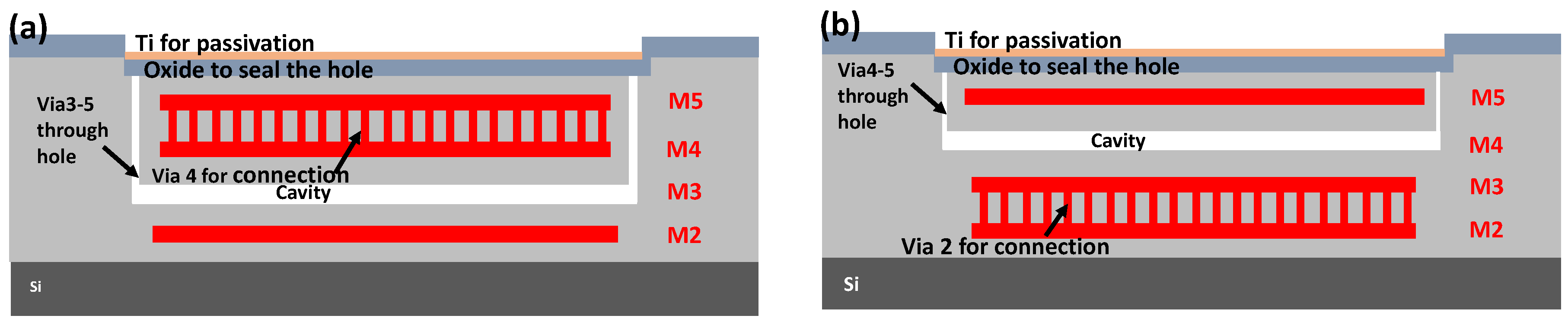
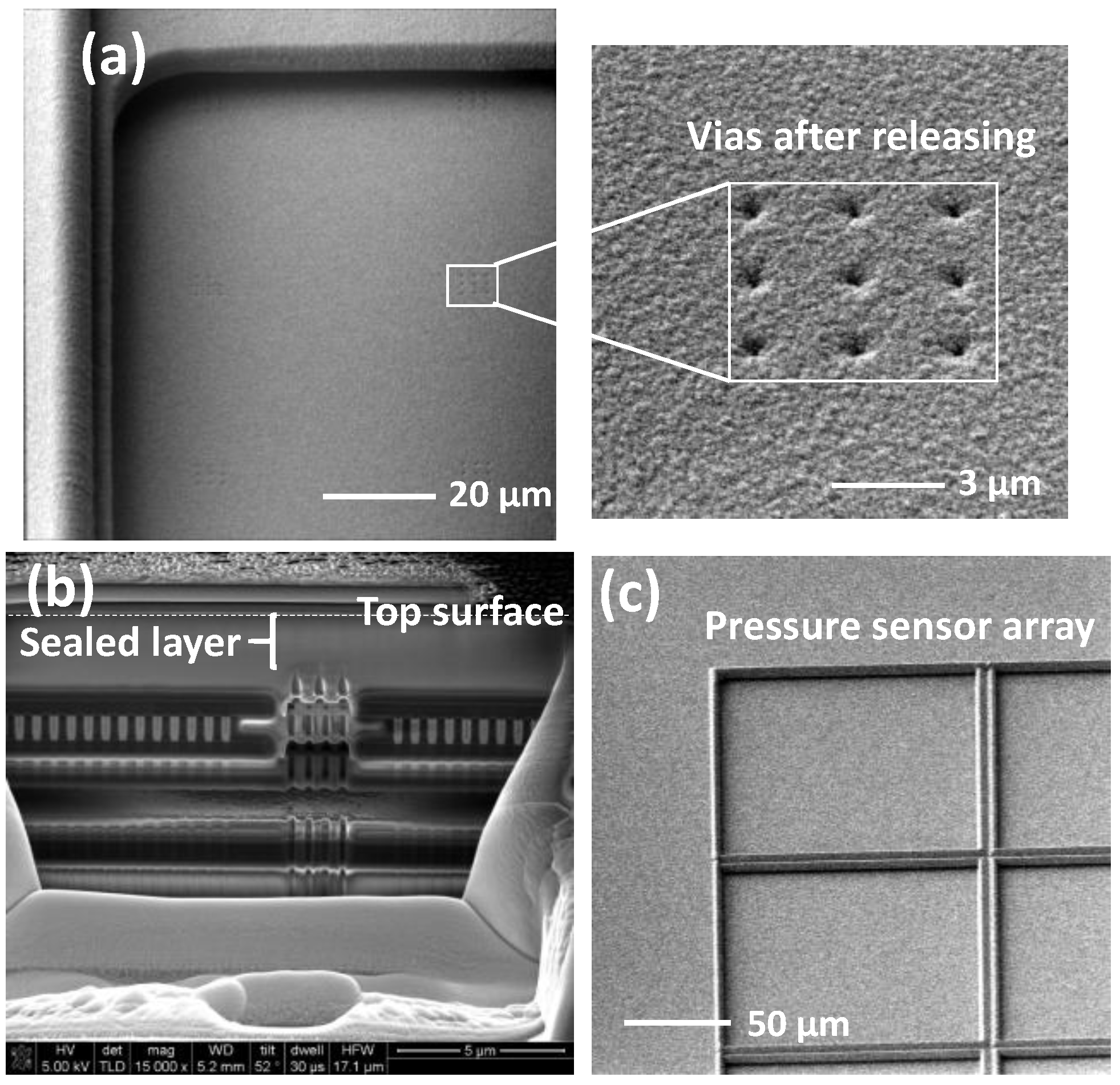
3.2. Fabrication of the Pressure Sensor
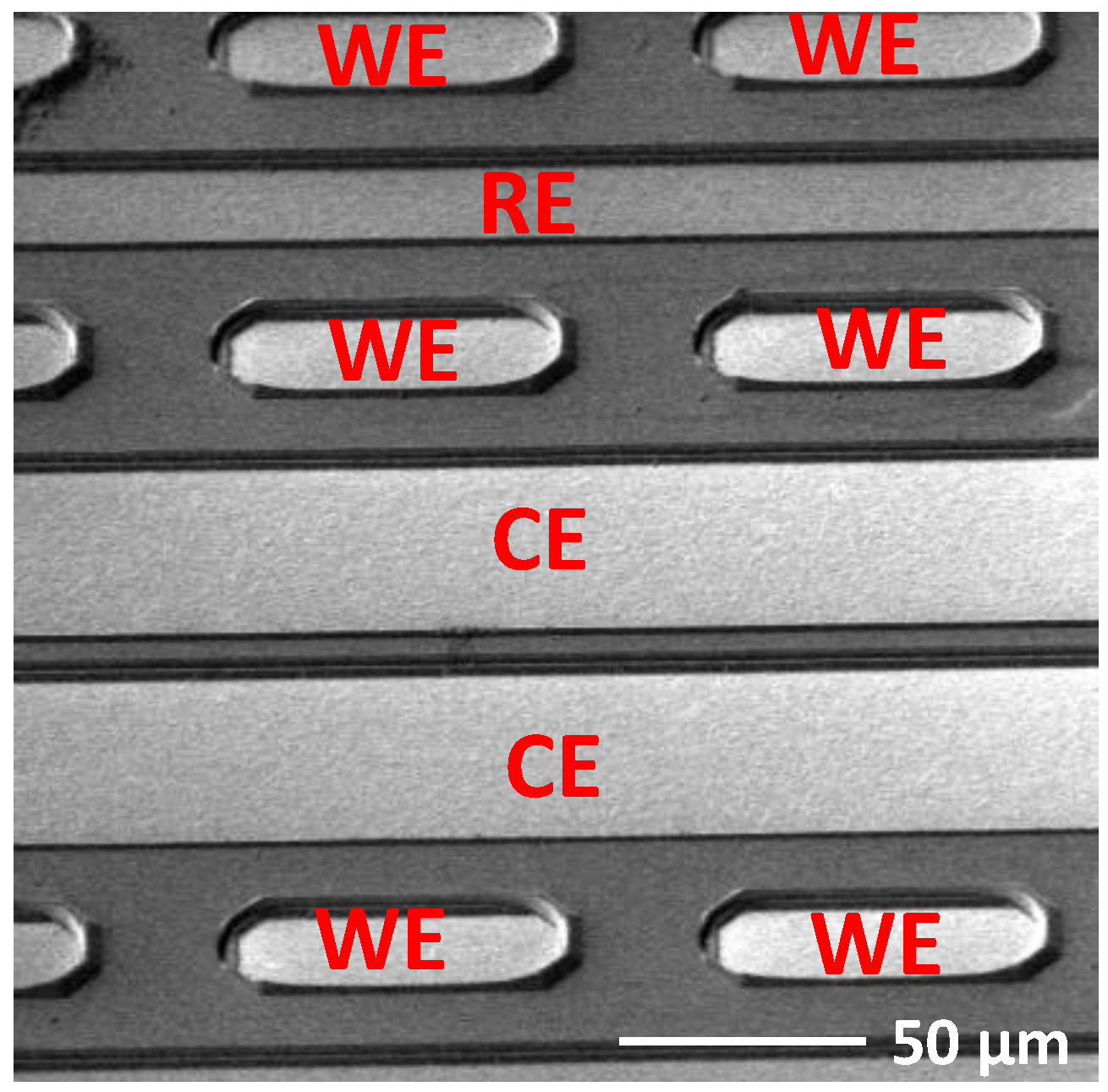
3.3. Oxygen Sensor Design, Fabrication, and Packaging
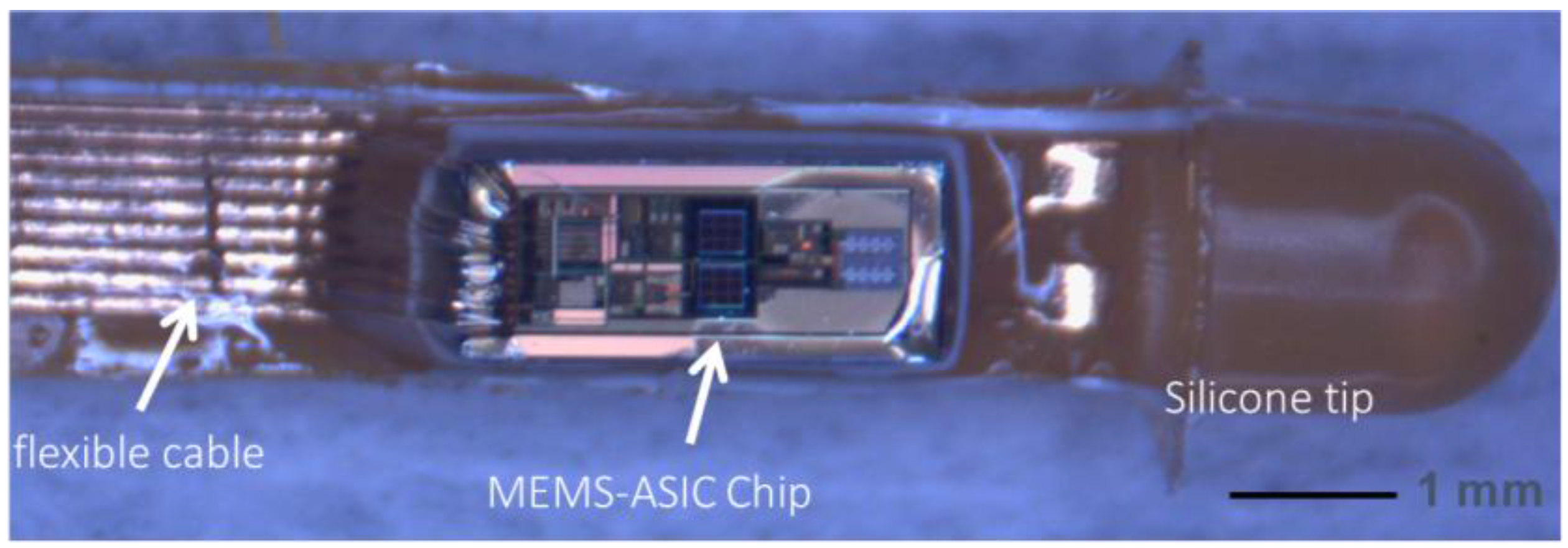
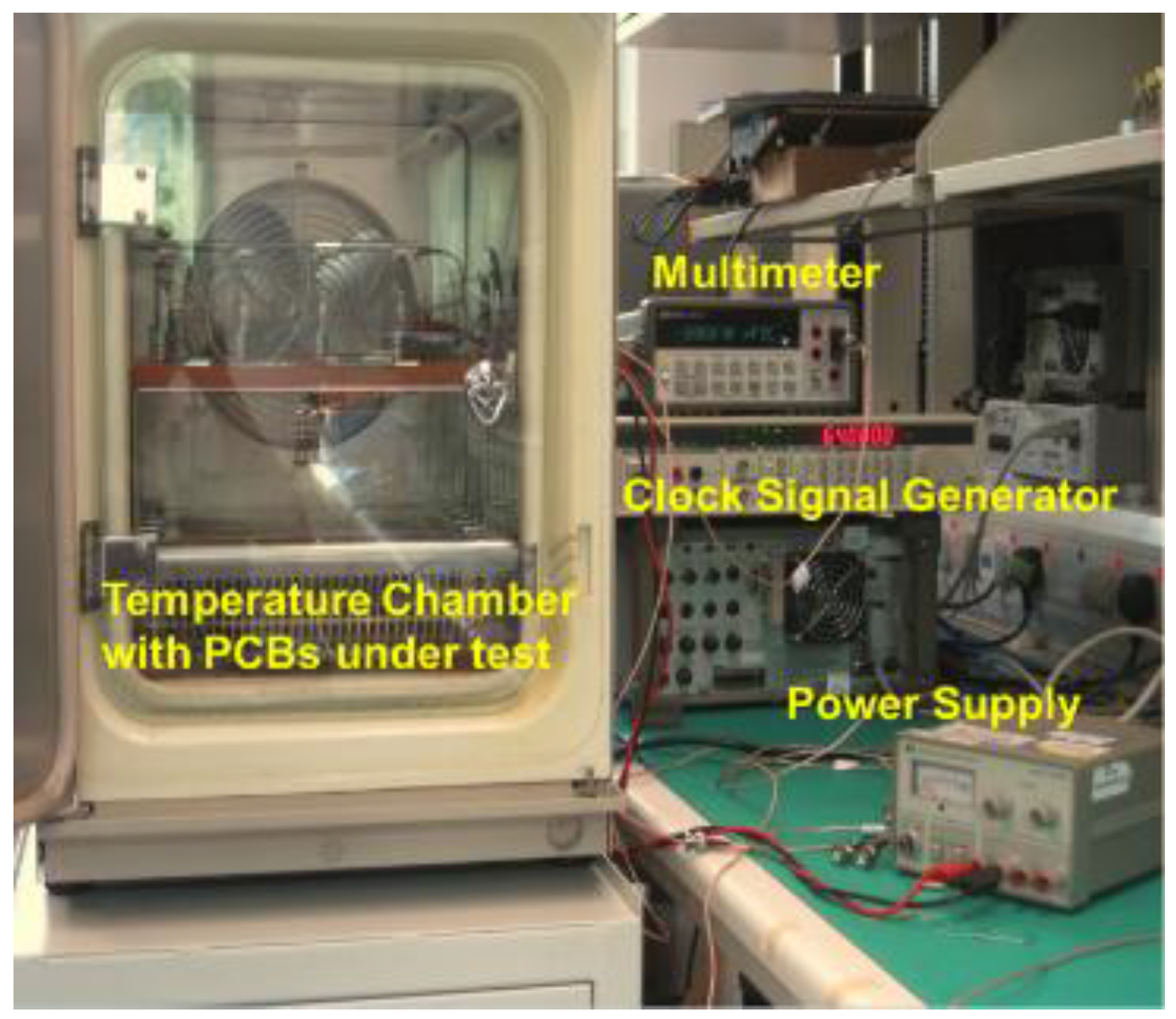
4. Device Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischer, A.C.; Forsberg, F.; Lapisa, M.; Bleiker, S.J.; Stemme, G.; Roxhed, N.; Niklaus, F. Integrating MEMS and ICs. Microsyst. Nanoeng. 2015, 1, 15005. [Google Scholar] [CrossRef]
- Khoshnoud, F.; Silva, C.W. Recent advances in MEMS sensor technology biomedical applications. IEEE Instrum. Meas. Mag. 2012, 15, 8–14. [Google Scholar] [CrossRef]
- Hi, P.; Chung, W. A comprehensive ubiquitous healthcare solution on an Android™ mobile device. Sensors 2011, 11, 6799–6815. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hanson, S.; Blaauw, D.; Sylvester, D. Circuit design advances for wireless sensing applications. Proc. IEEE 2010, 98, 1808–1827. [Google Scholar] [CrossRef]
- Cho, S.; Xue, N.; Cauller, L.; Rosellini, W.; Lee, J.-B. A SU-8-based fully-integrated biocompatible inductively powered wireless neurostimulator. IEEE/ASME J. Microelectromech. Syst. 2013, 22, 170–176. [Google Scholar] [CrossRef]
- Xue, N.; Chang, S.P.; Lee, J.B. A SU-8-based microfabricated implantable inductively coupled passive RF wireless intraocular pressure sensor. IEEE/ASME J. Microelectromech. Syst. 2012, 21, 1338–1346. [Google Scholar] [CrossRef]
- Chai, K.T.C.; Wang, C.; Tao, J.; Xu, J.; Zhong, L.; Tan, R.S. High performance differential capacitive MEMS sensor readout with relaxation oscillator front-end converter back-end and phase locked loop time-to-digital. In Proceeding of the IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 1528–1531. [Google Scholar]
- Shan, W.; Shi, L.; Yang, J. In-situ timing monitor based adaptive voltage scaling system for wide-voltage-range applications. IEEE Access 2017, 5, 15831–15838. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C. An ultra-low power turning angle based biomedical signal compression engine with adaptive threshold tuning. Sensors 2017, 17, 1809. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.C.; Yih, C.; Su, K.L. Multisensor fusion and integration: Approaches applications and future research directions. IEEE Sens. J. 2002, 2, 107–119. [Google Scholar] [CrossRef]
- Depledge, M.H.; Andersen, B.B. A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comp. Biochem. Physiol. Part A Physiol. 1990, 96, 473–477. [Google Scholar] [CrossRef]
- Widdows, J.; Phelps, D.K.; Galloway, W. Measurement of physiological condition of mussels transplanted along a pollution gradient in Narragansett Bay. Mar. Environ. Res. 1981, 4, 181–194. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.M.; Jung, W.; Ahn, C.H.; Shutter, L.A.; Narayan, R.K. A novel lab-on-a-tube for multimodality neuromonitoring of patients with traumatic brain injury (TBI). Lab Chip 2009, 9, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P. Intracranial pressure after the BEST TRIP trial: A call for more monitoring. Curr. Opin. Crit. Care 2004, 20, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Stangel, K.; Kolnsberg, S.; Hammerschmidt, D.; Hosticka, B.; Trieu, H.; Mokwa, W. A programmable intraocular CMOS pressure sensor system implant. IEEE J. Solid-State Circuits 2001, 36, 1094–1100. [Google Scholar] [CrossRef]
- Narayan, R.K.; Michel, M.E.; Ansell, B.; Baethmann, A.; Biegon, A.; Bracken, M.B.; Bullock, M.R.; Choi, S.C.; Clifton, G.L.; Contant, C.F.; et al. Clinical trials in head injury. J. Neurotrauma 2002, 19, 503–557. [Google Scholar] [CrossRef] [PubMed]
- Melgaard, J.; Rijkhoff, N.J.M. Detecting the onset of urinary bladder contractions using an implantable pressure sensor. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Katsios, C.; Ye, C.; Hoad, N.; Piraino, T.; Soth, M.; Cook, D. Intra-abdominal hypertension in the critically ill: Interrater reliability of bladder pressure measurement. J. Crit. Care 2013, 28, 886.e1–886.e6. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Xu, L.; Wald, M.; Coronado, V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006; National Center for Injury Prevention and Control, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Ghajar, J. Traumatic brain injury. Lancet 2000, 356, 923–929. [Google Scholar] [CrossRef]
- Cheatham, M.L.; Safcsak, K.S. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit. Care Med. 2010, 38, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Abdelkalik, M.A.; Elewa, G.M.; Kamaly, A.M.; Elsharnouby, N.M. Incidence and prognostic significance of intra-abdominal pressure in critically ill patients. Ains Shams J. Anesthesil. 2014, 7, 107–113. [Google Scholar]
- Le Roux, P. Intracranial Pressure Monitoring and Management. In Translational Research in Traumatic Brain Injury; Taylor & Francis Group: Oxford, UK, 2016. [Google Scholar]
- Sebastiano, F.; Breems, L.J.; Makinwa, K.A.; Drago, S.; Leenaerts, D.M.; Nauta, B. A 1.2 V 10 μW NPN-based temperature sensor in 65 nm CMOS with an inaccuracy of 0.2 °C from 70 °C to 125 °C. IEEE J. Solid-State Circuits 2010, 45, 2591–2601. [Google Scholar] [CrossRef]
- Lou, L.; Zhang, S.; Park, W.-T.; Tsai, J.M.; Kwong, D.-L.; Lee, C. Optimization of NEMS pressure sensors with a multilayered diaphragm using silicon nanowires as piezoresistive sensing elements. J. Micromech. Microeng. 2012, 22, 1–15. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Kyoung Yoo, Y.; Kang, S.; Kim, J.; Hoon Lee, J. Enhanced sensitivity of piezoelectric pressure sensor with microstructured polydimethylsiloxane layer. Appl. Phys. Lett. 2014, 104, 123701. [Google Scholar] [CrossRef]
- Cong, P.; Ko, W.H.; Young, D.J. Wireless batteryless implantable blood pressure monitoring microsystem for small laboratory animals. IEEE Sens. J. 2010, 10, 243–254. [Google Scholar] [CrossRef]
- Martin, C.S.; Dadamos, T.R.; Teixeira, M.F. Development of an electrochemical sensor for determination of dissolved oxygen by nickel-salen polymeric film modified electrode. Sens. Actuators B Chem. 2012, 175, 111–117. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Abruna, H.D.; Spector, J.A.; Olbricht, W.L. Micromachined dissolved oxygen sensor based on solid polymer electrolyte. Sens. Actuators B Chem. 2011, 153, 145–151. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices—Part 5: In Tests for In Vitro Cytotoxicity. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=36406 (accessed on 1 June 2009).

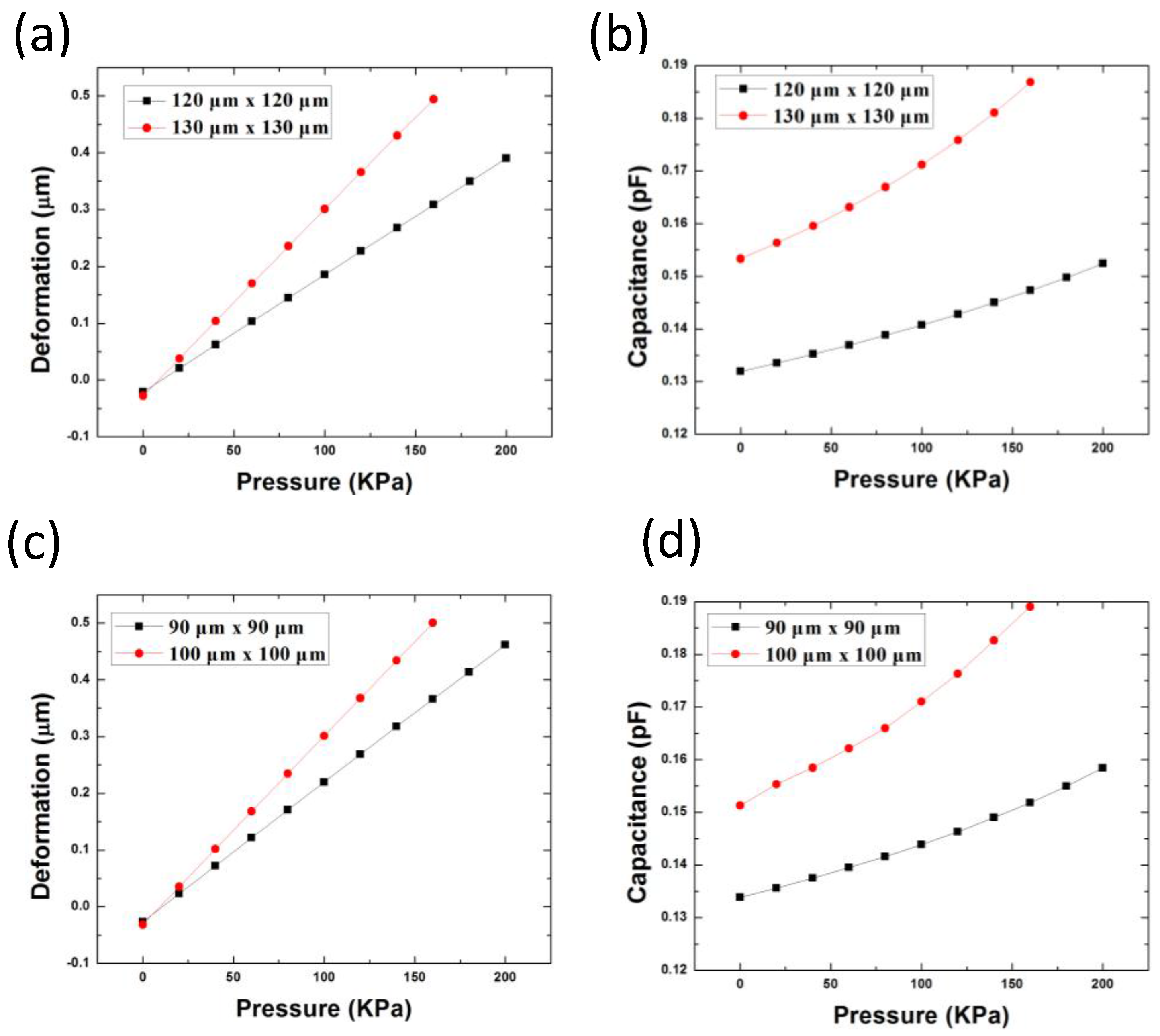



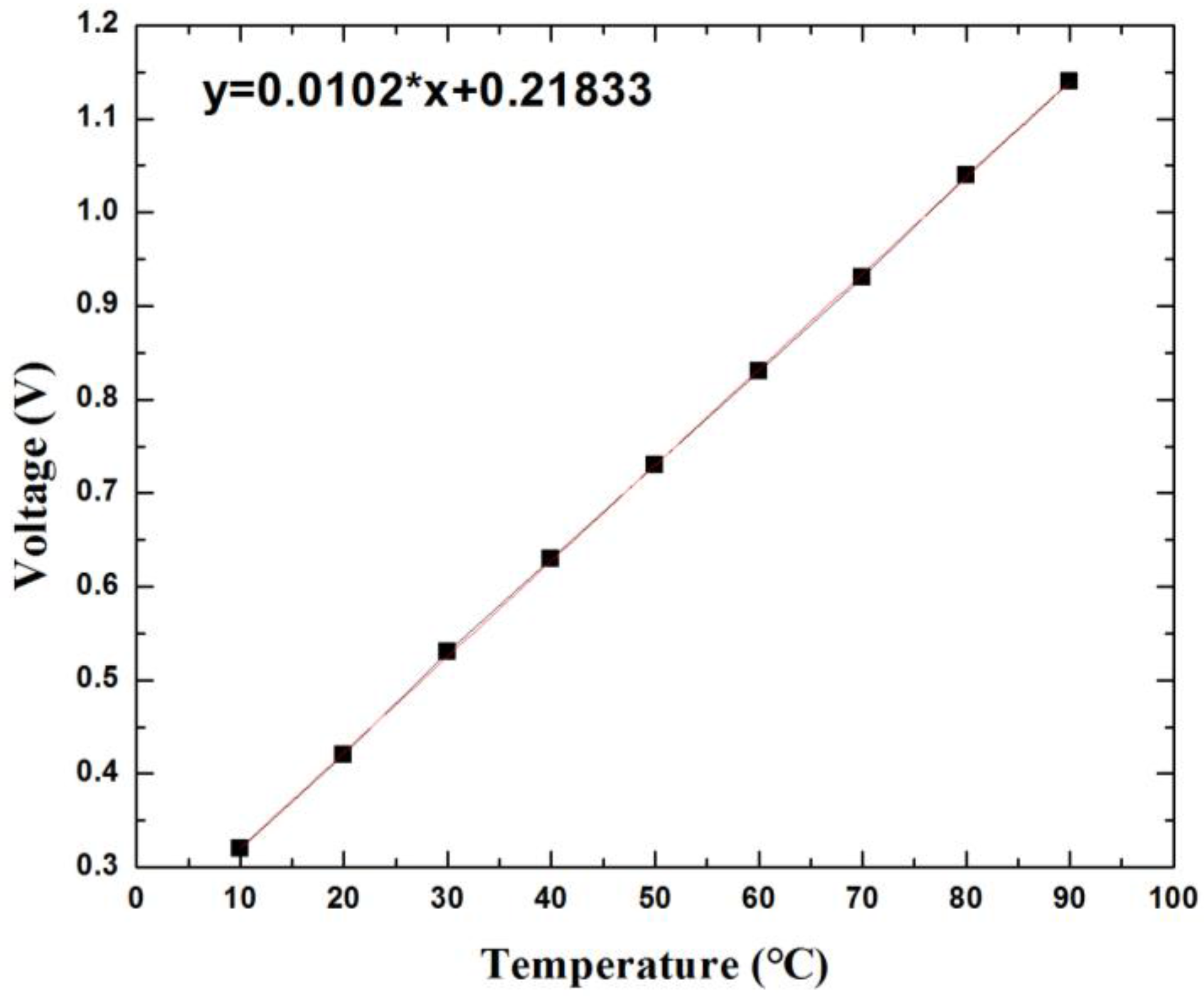
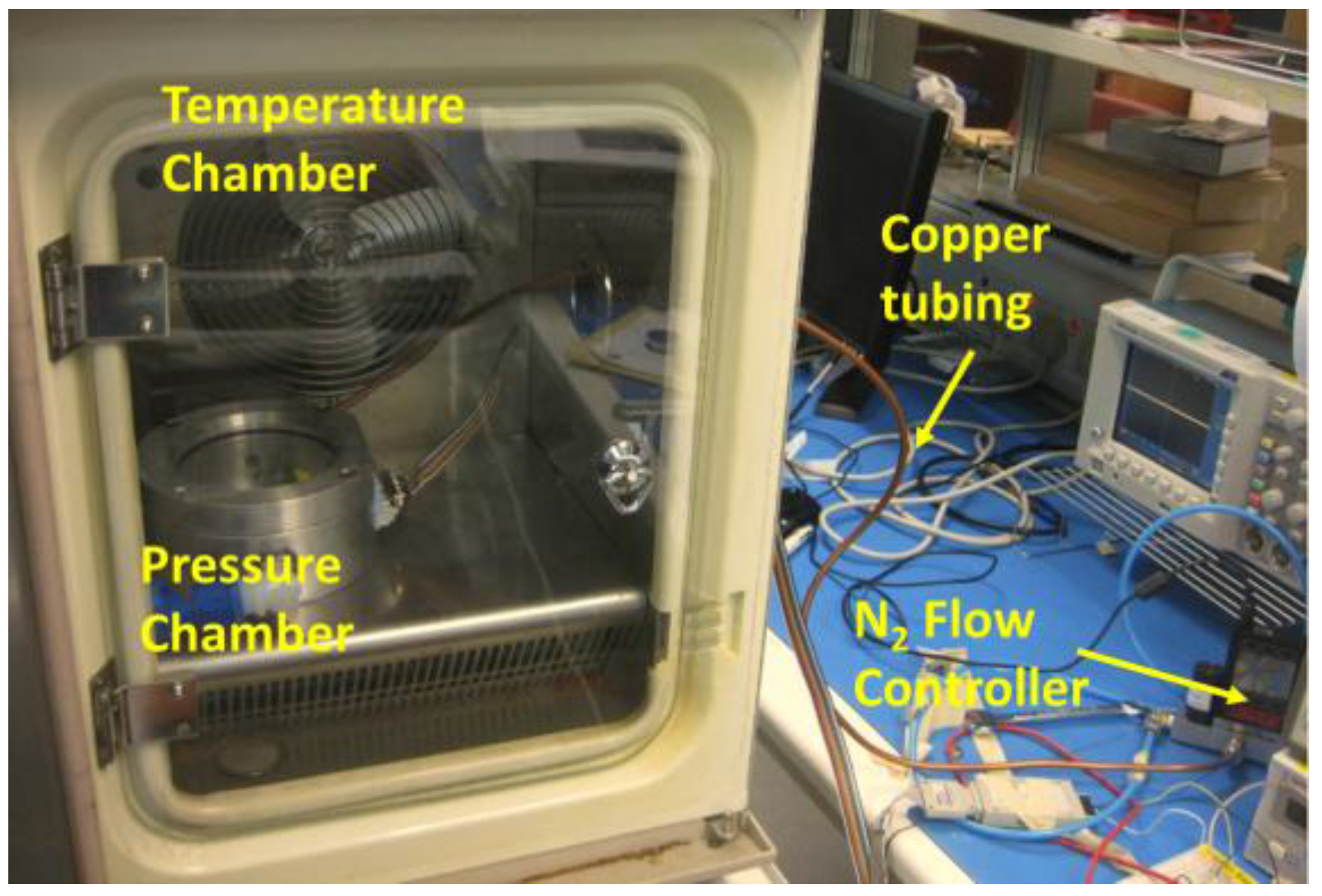
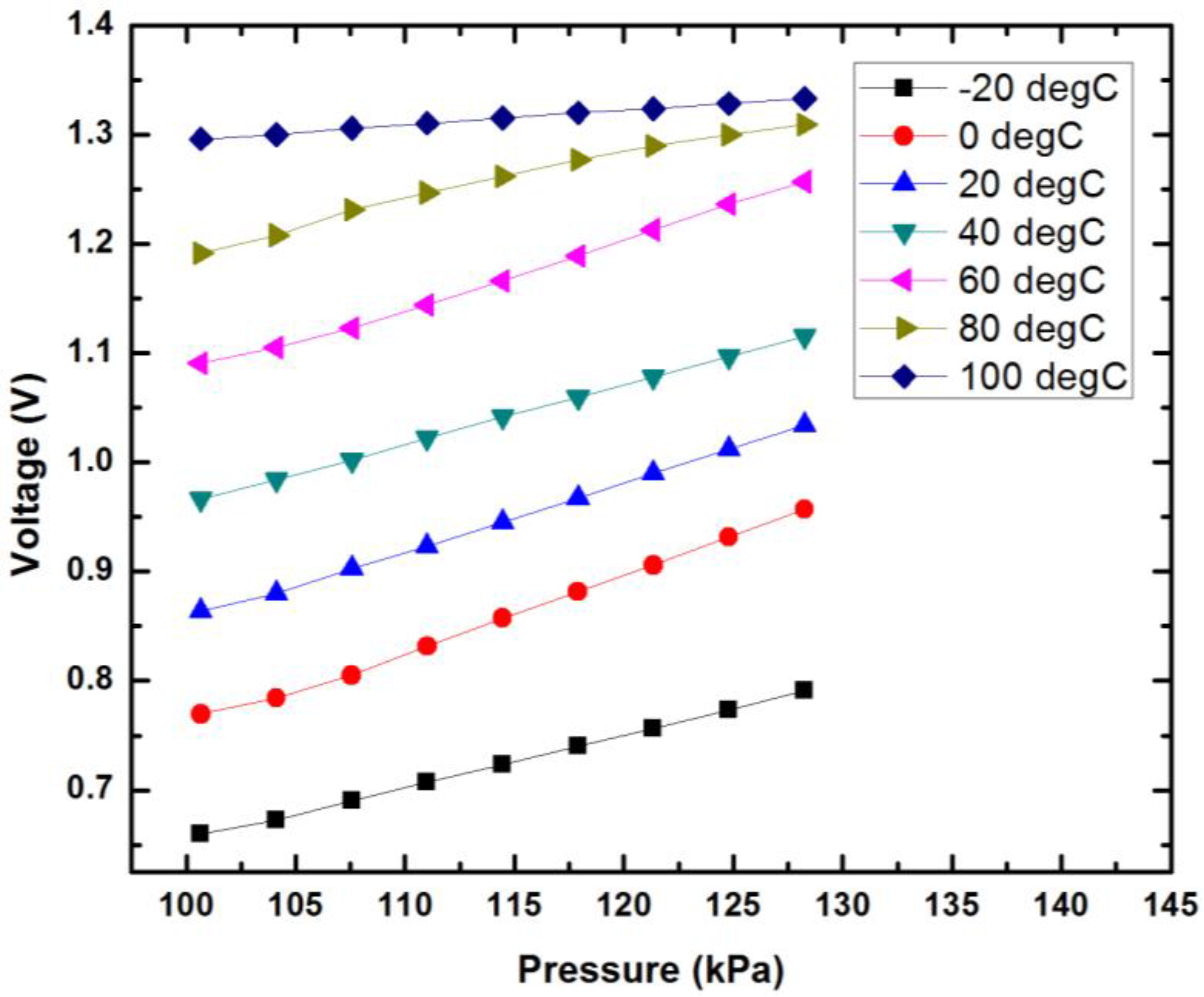

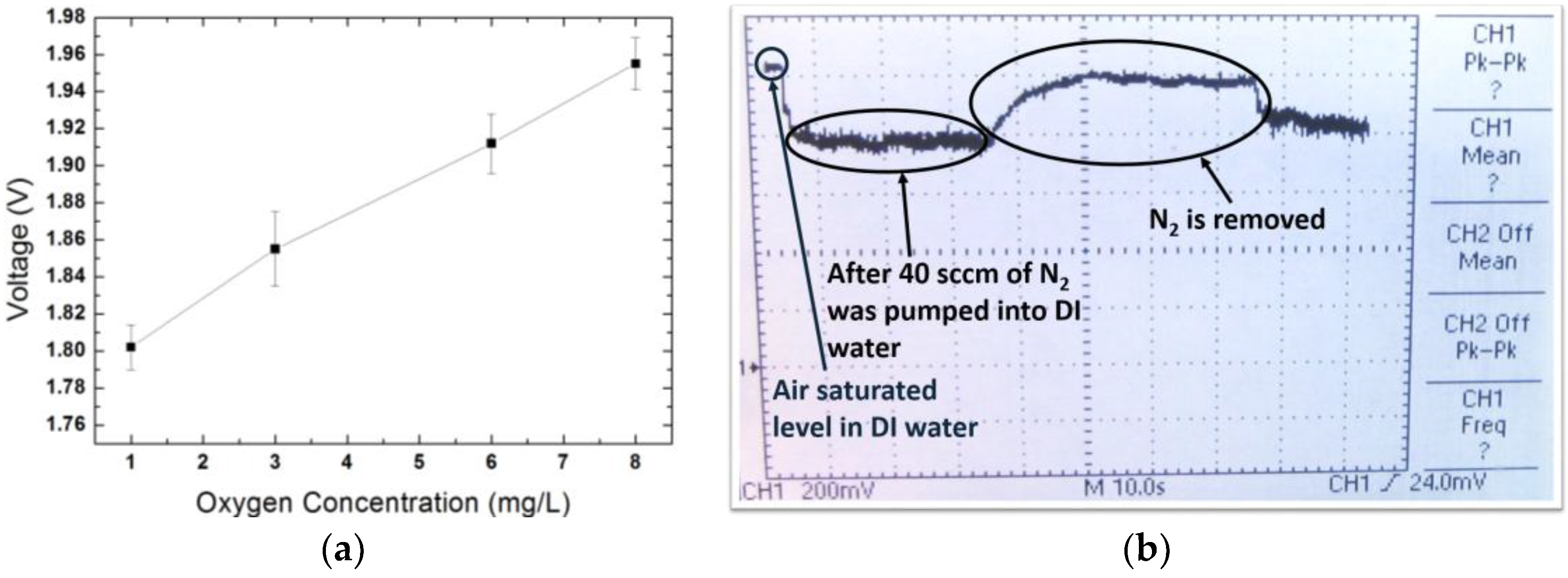

| Sensor Dimension (µm) | Gap between Two Metals (µm) | Thickness of Pressure Membrane (µm) | |
|---|---|---|---|
| Design 1 | 120 × 120 130 × 130 | 2.34 (0.54 µm cavity) | 5 |
| Design 2 | 90 × 90 100 × 100 | 2.34 (0.54 µm cavity) | 3.54 |
| Physiological Monitoring Requirement | Measured System Linear Sensing Range | Measured System Voltage Output Variation after 30 Days | Measured System Sensing Accuracy | |
|---|---|---|---|---|
| Temperature | 35–42 °C | 10–90 °C | 0.3% | ±0.2 °C |
| Pressure (relative value) | 0–60 mmHg (0–8 kPa) | 0–187 mmHg (0–25 kPa) | 0.5% | ±1 mmHg |
| Oxygen partial pressure (Oxygen concentration) | 22–100 mmHg (1–4.47 mg/L) | 22–178 mmHg (1–8 mg/L) | 5% | ±1 mmHg |
| [24] | [27] | [30] | This Work | |
|---|---|---|---|---|
| Process | 0.16 μm CMOS | 1.5 μm CMOS | MEMS | 0.18 μm CMOS |
| Sensor type | Temperature sensor | Pressure sensor | Oxygen sensor | Pressure, temperature and oxygen multi-sensor |
| Sensing area | N. A. | 0.2 mm2 | 0.43 mm2 | 0.6 mm2 (three sensors) |
| Sensitivity | N. A. | 0.7 fF/mmHg 58.6 mV/kPa | 633 pA/mmHg | 10.2 mV/°C (Temperature) 5.58 mV/kPa (Pressure) 20 mV·L/mg (Oxgen) |
| Biocompatible packaging size | N. A. | 6.4 mm (Diameter) 4 mm (Height) | 1.1 mm × 1.5 mm (no IC) | 3.65 mm × 1.65 mm × 0.72 mm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, N.; Wang, C.; Liu, C.; Sun, J. Highly Integrated MEMS-ASIC Sensing System for Intracorporeal Physiological Condition Monitoring. Sensors 2018, 18, 107. https://doi.org/10.3390/s18010107
Xue N, Wang C, Liu C, Sun J. Highly Integrated MEMS-ASIC Sensing System for Intracorporeal Physiological Condition Monitoring. Sensors. 2018; 18(1):107. https://doi.org/10.3390/s18010107
Chicago/Turabian StyleXue, Ning, Chao Wang, Cunxiu Liu, and Jianhai Sun. 2018. "Highly Integrated MEMS-ASIC Sensing System for Intracorporeal Physiological Condition Monitoring" Sensors 18, no. 1: 107. https://doi.org/10.3390/s18010107





