A Fully Integrated Paper-Microfluidic Electrochemical Device for Simultaneous Analysis of Physiologic Blood Ions
Abstract
:1. Introduction
2. Materials and Methods
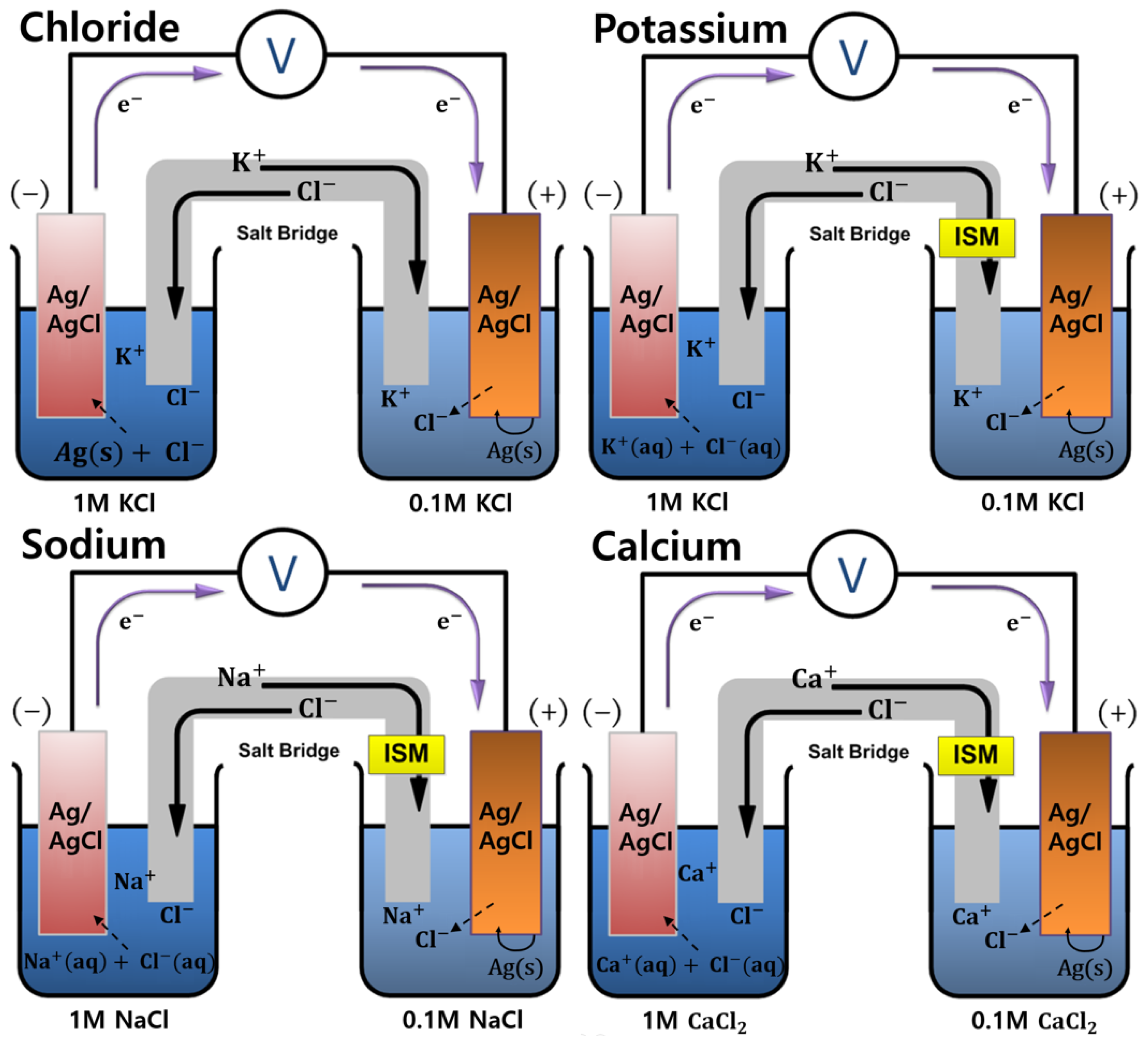
2.1. Conceptual Theories
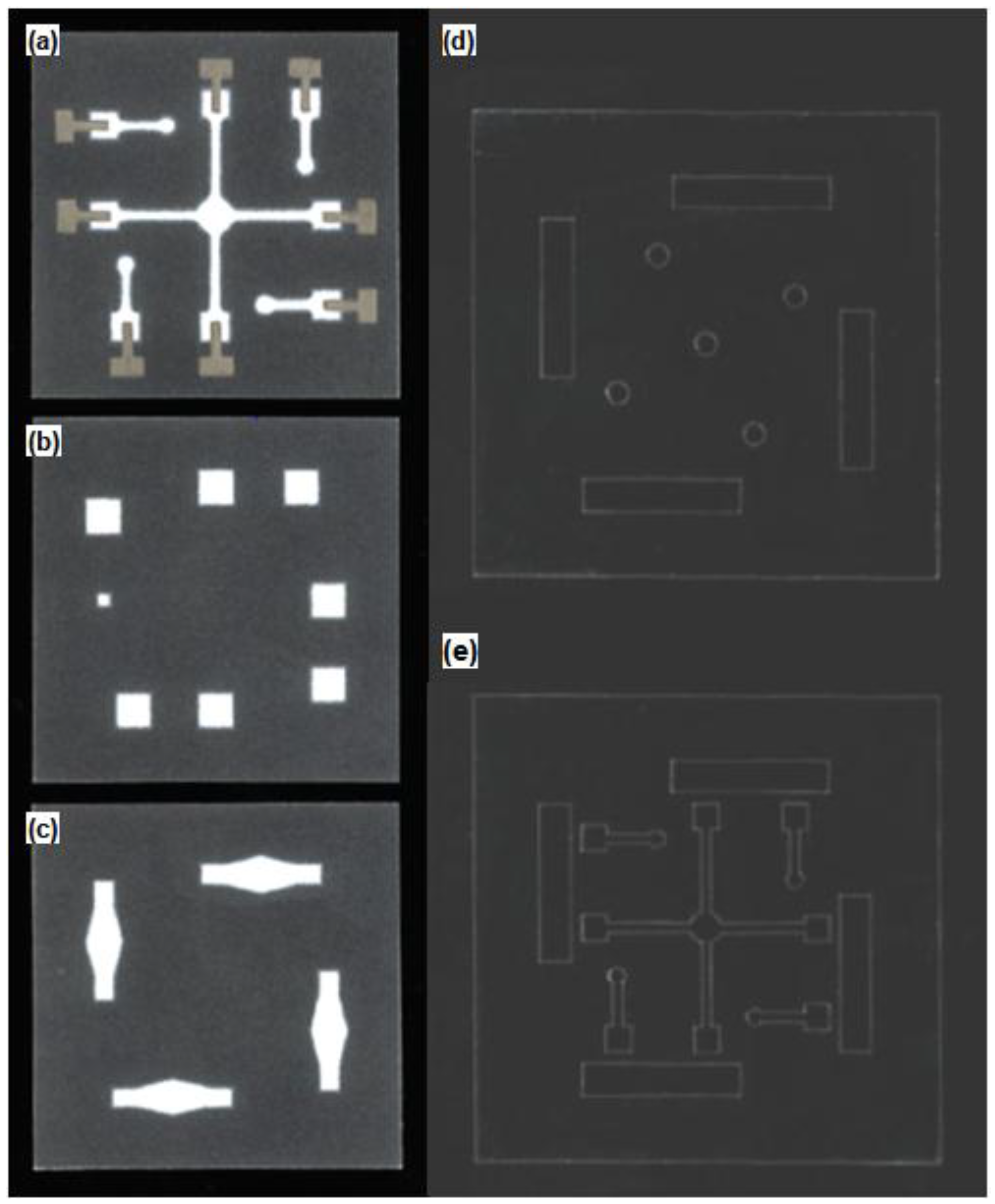
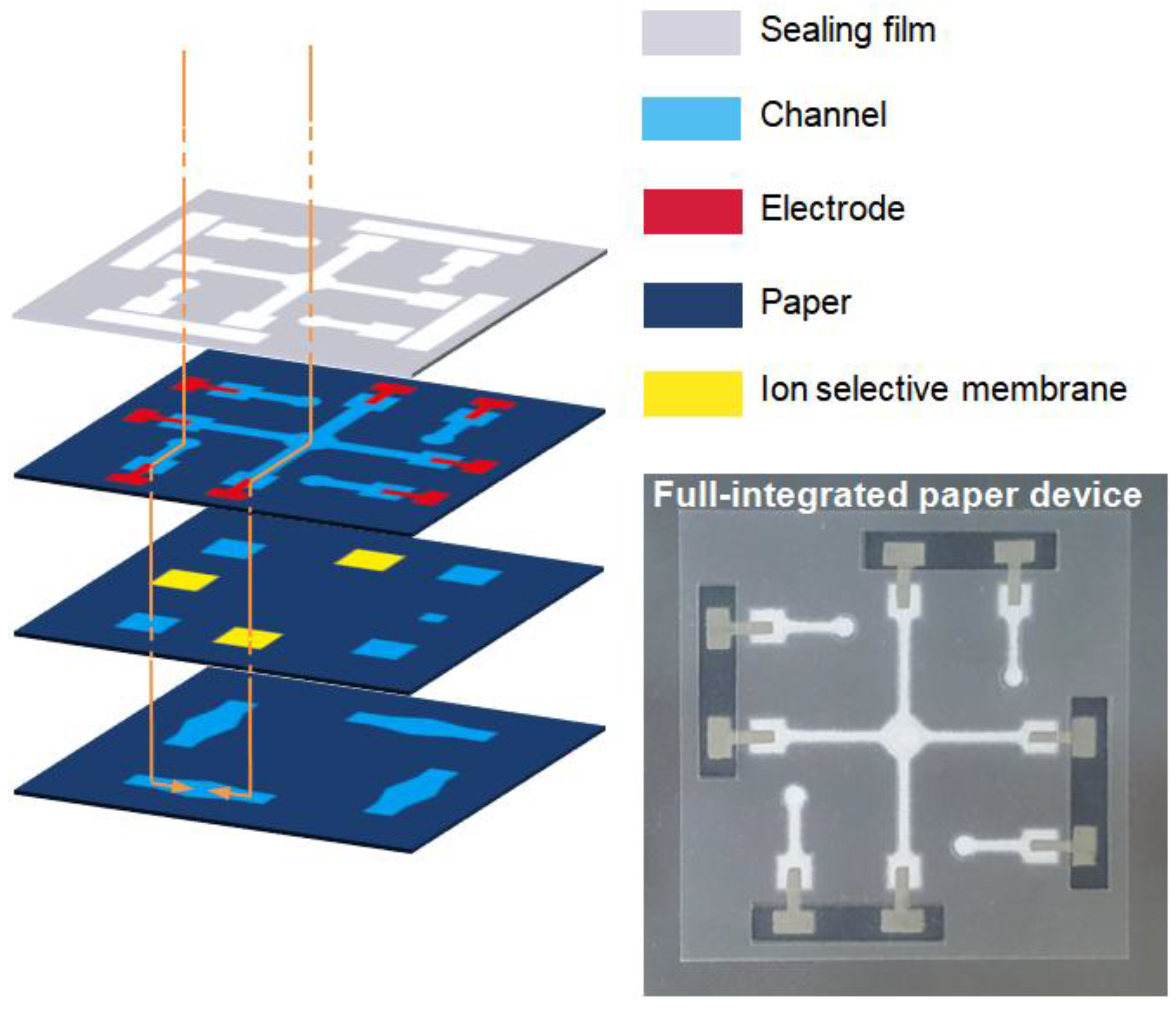
2.2. Fabrication of the Paper Microfluidic Components
3. Results
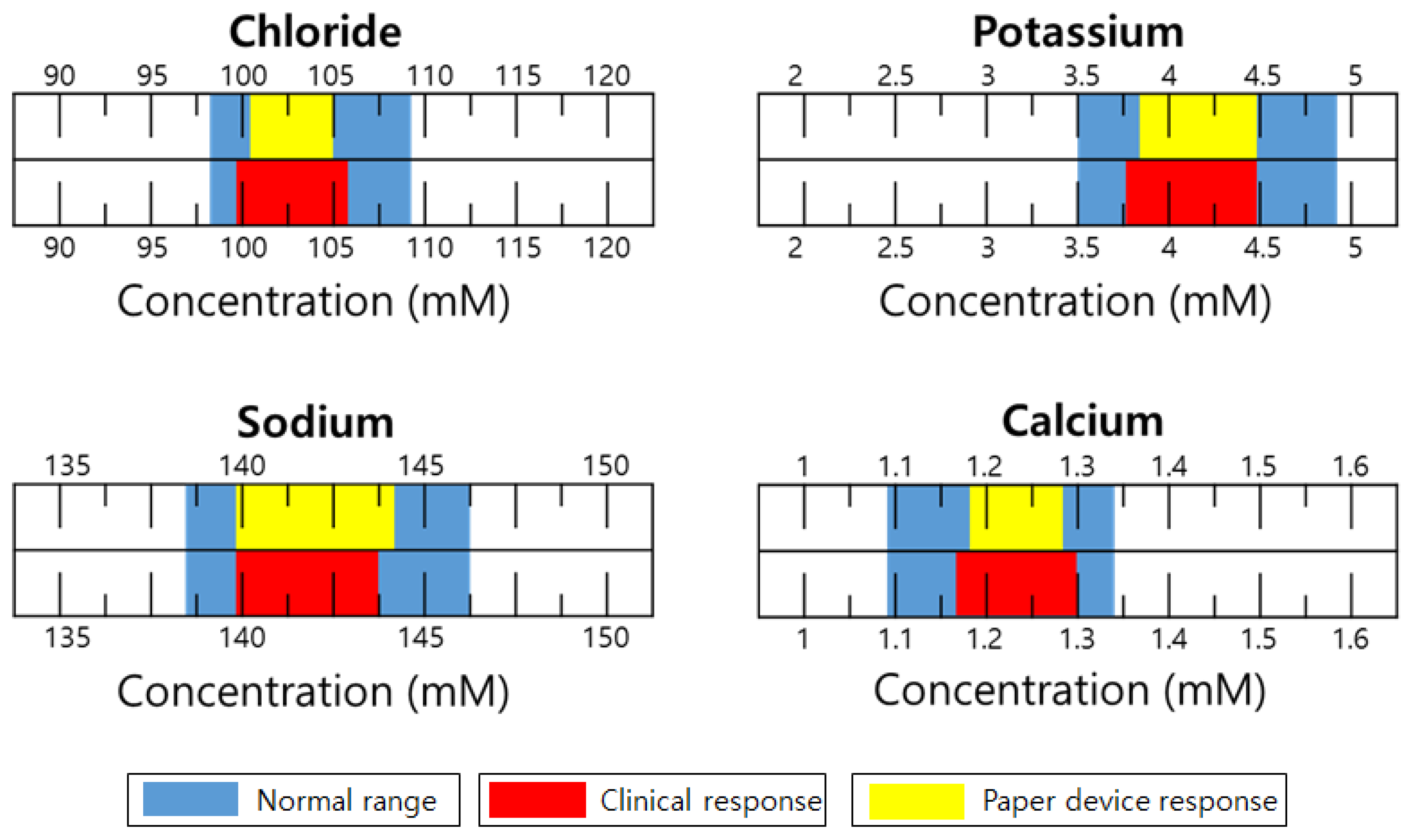
3.1. Structural Optimization of Paper Microfluidic Systems
3.2. Shape of the Salt-Bridged Mixing Channel
3.3. Heating and Drying Processes for Wax Diffusion
3.4. Oxygen Plasma-Based Surface Hydrophilization
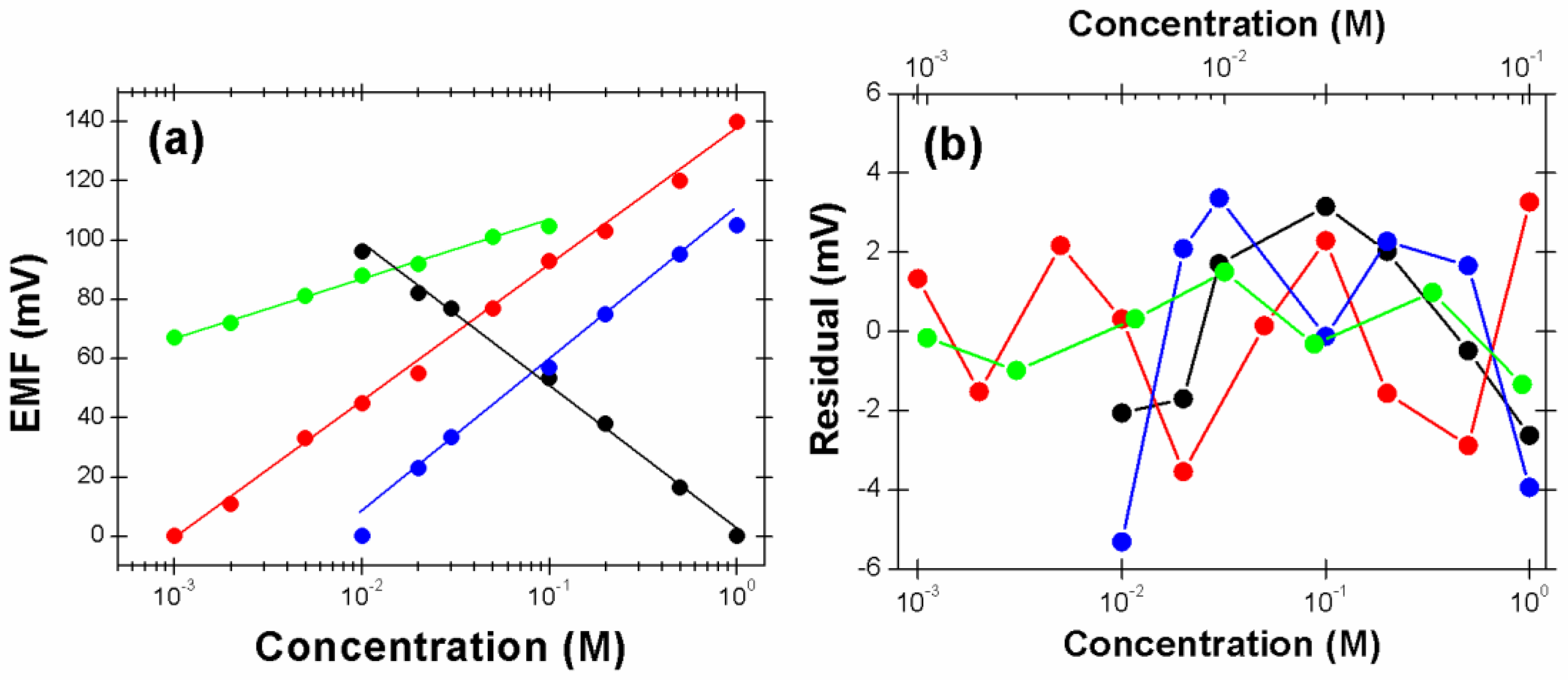
3.5. Performance Characterization of Paper-Microfluidic Devices by Electrochemical Potentiometry
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guery, B.; Poissy, J.; el Mansouf, L.; Sejourne, C.; Ettahar, N.; Lemaire, X.; Vuotto, F.; Goffard, A.; Behillil, S.; Enouf, V.; et al. Clinical features and viral diagnosis of two cases of infection with middle east respiratory syndrome coronavirus: A report of nosocomial transmission. Lancet 2013, 381, 2265–2272. [Google Scholar] [CrossRef]
- Jaax, N.K.; Davis, K.J.; Geisbert, T.J.; Vogel, P.; Jaax, G.P.; Topper, M.; Jahrling, P.B. Lethal experimental infection of rhesus monkeys with ebola-zaire (mayinga) virus by the oral and conjunctival route of exposure. Arch. Pathol. Lab. Med. 1996, 120, 140–155. [Google Scholar] [PubMed]
- Jeong, S.G.; Kim, J.M.; Nam, J.O.; Song, Y.S.; Lee, C.S. Paper-based analytical device for quantitative urinalysis. Int. Neurourol. J. 2013, 17, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Jayawardane, B.M.; McKelvie, I.D.; Kolev, S.D. Development of a gas-diffusion microfluidic paper-based analytical device (mu pad) for the determination of ammonia in wastewater samples. Anal. Chem. 2015, 87, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, H.; Rong, L.Y.; Chen, X.Q. A gas-diffusion microfluidic paper-based analytical device (mu pad) coupled with portable surface-enhanced raman scattering (sers): Facile determination of sulphite in wines. Analyst 2016, 141, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Martinez, A.W.; Gong, J.L.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based elisa. Angew. Chem. Int. Ed. 2010, 49, 4771–4774. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, M.; Jasinski, A.; Jasinska, M.; Drucis, K.; Ekman, M.; Szarmach, A.; Suchodolski, R.; Pomecko, R.; Bochenska, M. Simultaneous determination of Na+, K+, Ca2+, Mg2+ and Cl− in unstimulated and stimulated human saliva using all solid state multisensor platform. Electroanal 2017, 29, 2232–2238. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.J.; Zou, X.U.; Hamedi, M.M.; Hu, J.B.; Parolo, C.; Maxwell, E.J.; Buhlmann, P.; Whitesides, G.M. Paper-based potentiometric ion sensing. Anal. Chem. 2014, 86, 9548–9553. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; p. xxi, 833. [Google Scholar]
- Bakker, E.; Buhlmann, P.; Pretsch, E. Polymer membrane ion-selective electrodes—What are the limits. Electroanal 1999, 11, 915–933. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic paper-based analytical devices (mu pads) and micro total analysis systems (mu tas): Development, applications and future trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.L.; Ramsey, S.; Kauffman, P.; Lutz, B.; Yager, P. Transport in two-dimensional paper networks. Microfluid. Nanofluid. 2011, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kwon, E.J.; Kang, J.Y.; Skalak, M.; Anglin, E.J.; Mann, A.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Porous silicon-graphene oxide core-shell nanoparticles for targeted delivery of sirna to the injured brain. Nanoscale Horiz. 2016, 1, 407–414. [Google Scholar] [CrossRef]
- Rhee, Y.H.; Lee, C.J.; Ko, M.J.; Jin, J.H.; Min, N.K. Enhancing the efficiency of electron conduction in spray-coated anode of photoelectrochemical cell using oxygenated multi-walled carbon nanotubes. J. Phys. Chem. C 2015, 119, 9085–9091. [Google Scholar] [CrossRef]
- Rhee, Y.H.; Ahn, D.J.; Ko, M.J.; Jin, H.Y.; Jin, J.H.; Min, N.K. Enhanced electrocatalytic activity of plasma functionalized multiwalled carbon nanotube-entrapped poly(3,4-ethylendioxythiophene): Poly(styrene sulfonate) photocathode. Electrochim. Acta 2014, 146, 68–72. [Google Scholar] [CrossRef]
- Jin, J.H.; Min, N.K.; Hong, S.I. Poly(3-methylthiophene)-based porous silicon substrates as a urea-sensitive electrode. Appl. Surf. Sci. 2006, 252, 7397–7406. [Google Scholar] [CrossRef]
- Tietz, N.W.; Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry, 2nd ed.; Saunders: Philadelphia, PA, USA, 1994. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.-H.; Kim, J.H.; Lee, S.K.; Choi, S.J.; Park, C.W.; Min, N.K. A Fully Integrated Paper-Microfluidic Electrochemical Device for Simultaneous Analysis of Physiologic Blood Ions. Sensors 2018, 18, 104. https://doi.org/10.3390/s18010104
Jin J-H, Kim JH, Lee SK, Choi SJ, Park CW, Min NK. A Fully Integrated Paper-Microfluidic Electrochemical Device for Simultaneous Analysis of Physiologic Blood Ions. Sensors. 2018; 18(1):104. https://doi.org/10.3390/s18010104
Chicago/Turabian StyleJin, Joon-Hyung, Joon Hyub Kim, Sang Ki Lee, Sam Jin Choi, Chan Won Park, and Nam Ki Min. 2018. "A Fully Integrated Paper-Microfluidic Electrochemical Device for Simultaneous Analysis of Physiologic Blood Ions" Sensors 18, no. 1: 104. https://doi.org/10.3390/s18010104




