High-Performance Wireless Ammonia Gas Sensors Based on Reduced Graphene Oxide and Nano-Silver Ink Hybrid Material Loaded on a Patch Antenna
Abstract
:1. Introduction
2. Synthesis and Characterization
2.1. Synthesis of Reduction Graphene Oxide
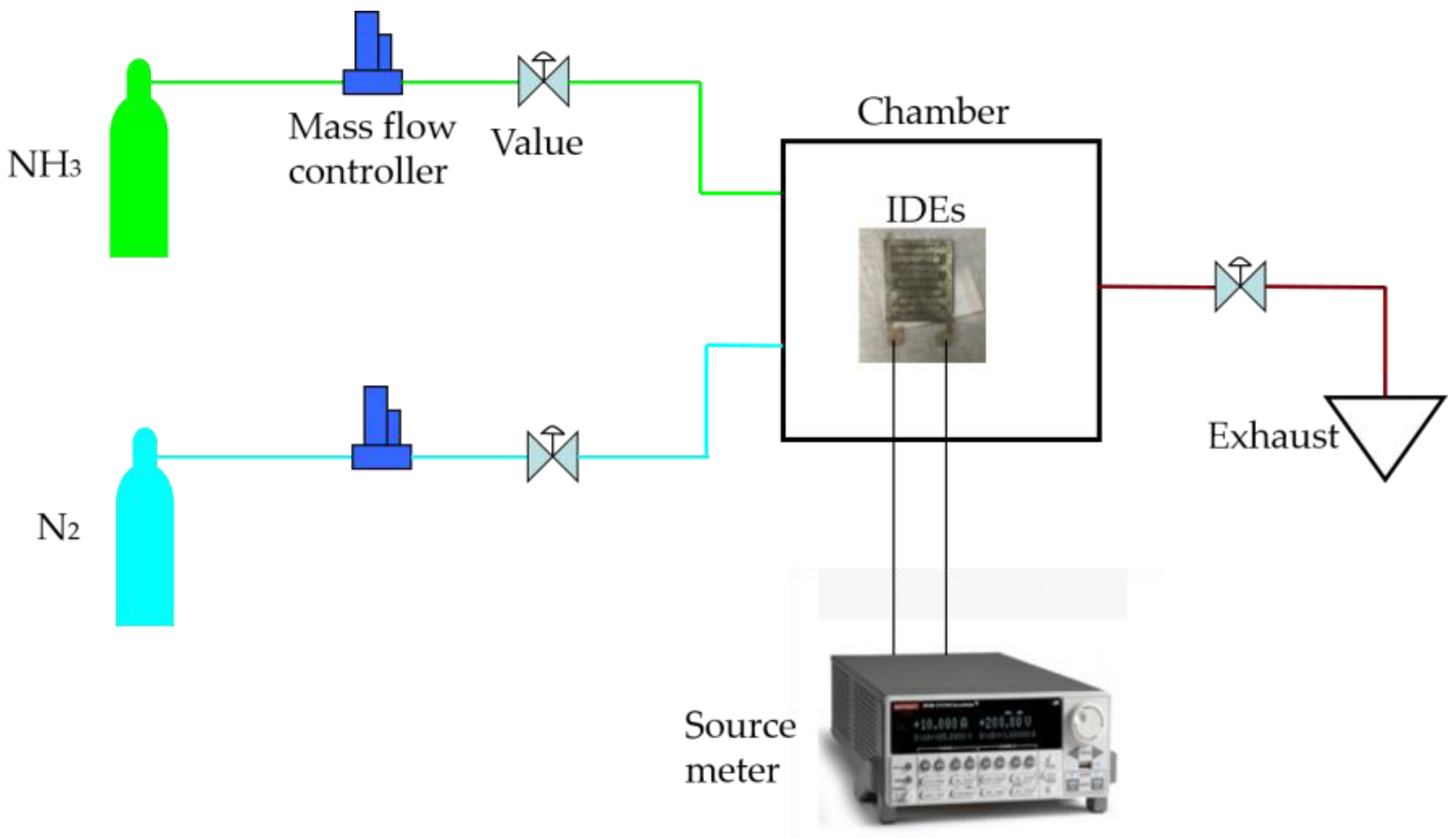
2.2. Fabrication of Sensor Devices with rGO/Ag-ink
2.3. Characterization of rGO/Ag-ink Hybrid Material
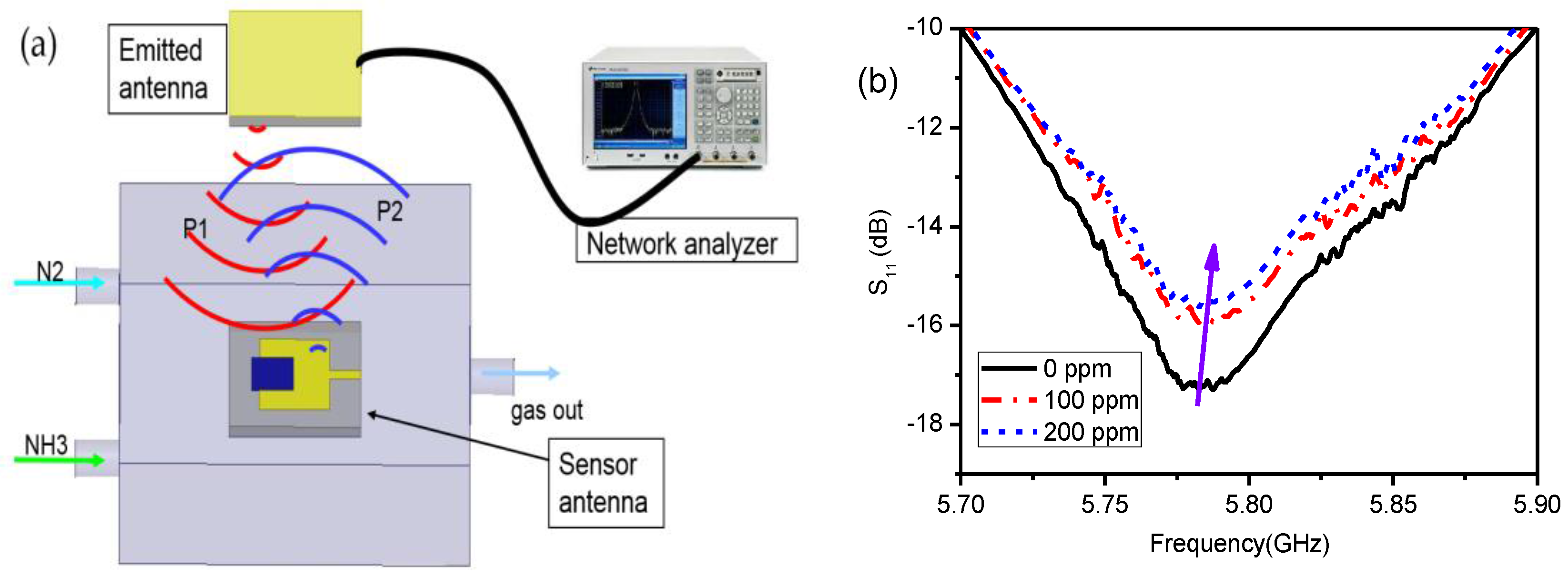
3. Experimental Results and Discussions
3.1. The Sensitivity of rGO/Ag-ink on IDEs
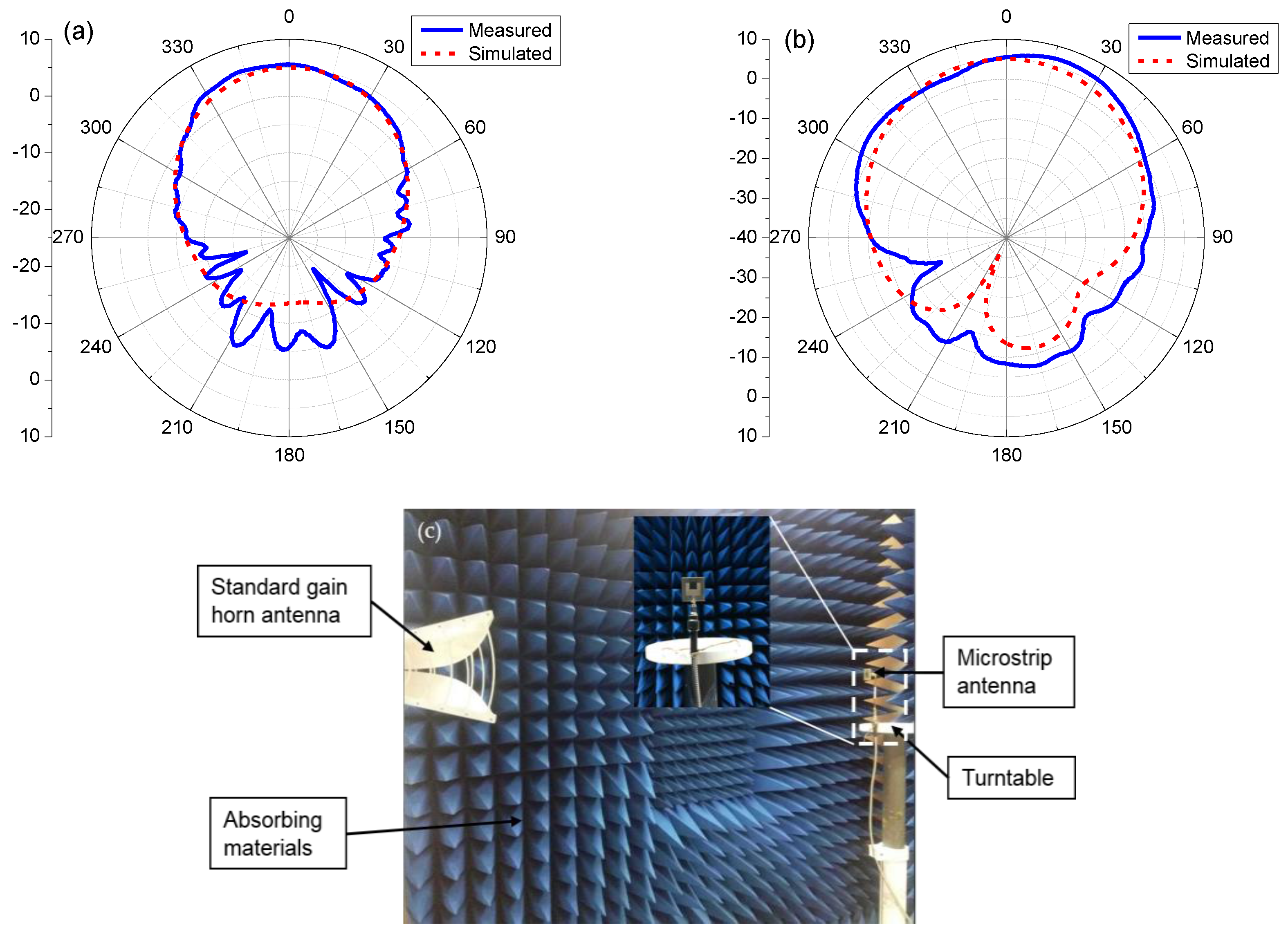
3.2. The Reflection of the Patch Antenna with rGO/Ag-ink
3.3. Wireless Sensing Using Patch Antennas with rGO/Ag-ink
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications: A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Armelao, L.; Mari, C.M.; Polizzi, S.; Ruffo, R.; Scottiand, R.; Morazzoni, F. Macroporous WO3 thin films active in NH3 sensing: Role of the hosted Cr isolated centers and Pt nanoclusters. J. Am. Chem. Soc. 2011, 133, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lou, Z.; Fei, T.; Zhang, T. Templating synthesis of ZnO hollow nanospheres loaded with Au nanoparticles and their enhanced gas sensing properties. J. Mater. Chem. 2012, 22, 4767–4771. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave gas sensing with a microstrip interDigital capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B Chem. 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Elouali, S.; Bloor, L.G.; Binions, R.; Parkin, I.P.; Carmalt, C.J.; Darr, J.A. Gas sensing with nano-indium oxides (In2O3) prepared via continuous hydrothermal flow synthesis. Langmuir Acs J. Surf. Colloids 2012, 28, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shaker, G.; Naishadham, K.; Song, K. Carbon-nanotube loaded antenna-based ammonia gas sensor. IEEE Trans. Microwave Theor. Tech. 2011, 59, 2665–2673. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC, Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloys Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuators B Chem. 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Xie, T.; Su, Y.; Yuan, Z. A facile method to develop novel TiO2/rGO layered film sensor for detecting ammonia at room temperature. Mater. Lett. 2016, 165, 127–130. [Google Scholar] [CrossRef]
- Su, P.G.; Yang, L.Y. NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens. Actuators Chem. 2016, 223, 202–208. [Google Scholar] [CrossRef]
- Cui, S.; Mao, S.; Wen, Z.; Chang, J.; Zhang, Y.; Chen, J. Controllable synthesis of silver nanoparticle-decorated reduced graphene oxide hybrids for ammonia detection. Analyst 2013, 138, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Cui, S.; Lu, G.; Yu, K.; Wen, Z.; Chen, J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009–11013. [Google Scholar] [CrossRef]
- Na, K.; Ma, H.; Park, J.; Yeo, J.; Park, J.U. Graphene-based wireless environmental gas sensor on PET substrate. IEEE Sens. J. 2016, 16, 5003–5009. [Google Scholar] [CrossRef]
- Ali, M.A.; Cheng, M.C.; Chen, C.M.; Wu, C.T.M. Microwave gas sensor based on graphene-loaded substrate integrated waveguide cavity resonator. In Proceedings of the 2016 IEEE MTT-S International, San Francisco, CA, USA, 22–27 May 2016. [Google Scholar]
- Jun, J.; Oh, J.; Shin, D.H.; Kim, S.G.; Lee, J.S. Wireless, room temperature volatile organic compound sensor based on polypyrrole nanoparticle immobilized ultrahigh frequency radio frequency identification tag. Acs Appl. Mater. Interfaces 2016, 8, 33139–33147. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhao, Y.; Sun, J.; Tian, Y.; Luo, R.; Li, D.; Chen, A. Ultrasensitive room temperature NH3 sensor based on a graphene-polyaniline hybrid loaded on PET thin film. Chem. Commun. 2015, 51, 7524–7527. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Yuan, Z.; Zheng, W.; Ye, Z.; Liu, C.; Du, X. ZnO nanoparticles/reduced graphene oxide bilayer thin films for improved NH3-sensing performances at room temperature. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, P.V.; Rao, K.V.S. Theory and measurement of backscattering from RFID tags. IEEE Antennas Propag. Mag. 2006, 48, 212–218. [Google Scholar] [CrossRef]
- Fuschini, F.; Piersanti, C.; Paolazzi, F.; G Falciasecca, G. Analytical approach to the backscattering from UHF RFID transponder. IEEE Antennas Wirel. Propag. Lett. 2008, 7, 33–35. [Google Scholar] [CrossRef]
- Virtanen, J.; Yang, F.; Ukkonen, L.; Elsherbeni, A.Z.; Babar, A.A.; Sydanheimo, S. Dual port temperature sensor tag for passive UHF RFID systems. Chem. Rev. 2014, 34, 154–169. [Google Scholar] [CrossRef]
- Vyas, R.; Lakafosis, V.; Lee, H.; Shaker, G.; Li, Y.; Orecchini, G.; Traille, A.; Tentzeris, M.M.; Roselli, M. Inkjet printed, self-powered, wireless sensors for environmental, gas, and authentication-based sensing. IEEE Sens. J. 2011, 11, 3139–3152. [Google Scholar] [CrossRef]
- Wang, W.; Lee, K.; Kim, T.; Park, I.; Yang, S. A novel wireless, passive CO2 sensor incorporating a surface acoustic wave reflective delay line. Smart Mater. Struct. 2007, 16, 1382–1389. [Google Scholar] [CrossRef]
- Wang, W.; Lim, C.; Lee, K.K.; Yang, S.S. Wireless surface acoustic wave chemical sensor for simultaneous measurement of CO and humidity. J. Micro/Nanolithogr. MEMS MOEMS 2009, 8, 1717–1721. [Google Scholar]
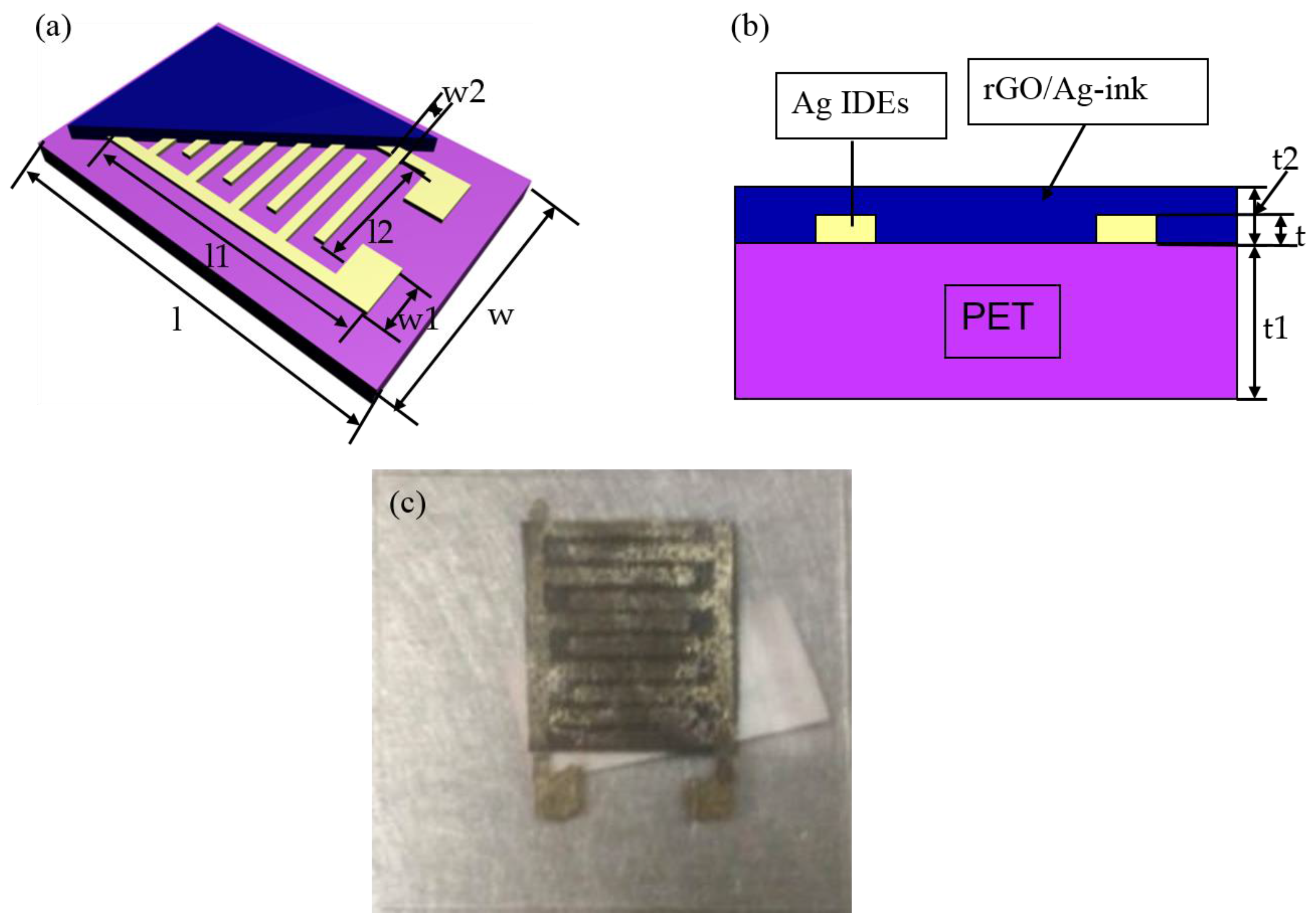
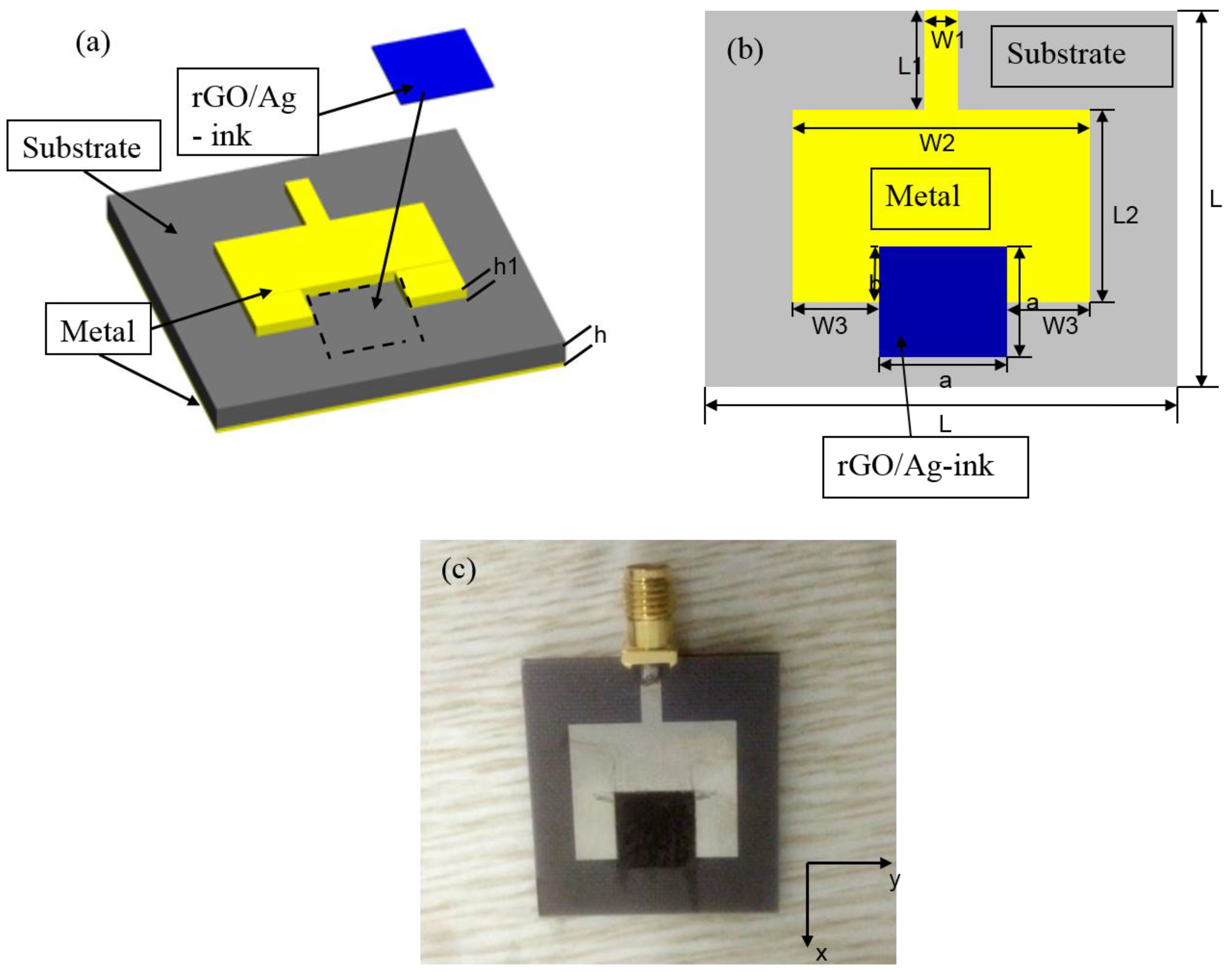



| Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) |
|---|---|---|---|---|---|---|---|---|---|
| l | 30 | l1 | 15 | l2 | 12 | t | 0.0005 | t2 | 0.0015 |
| w | 20 | w1 | 5 | w2 | 0.5 | t1 | 0.25 |
| Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) | Parameters | Values (mm) |
|---|---|---|---|---|---|---|---|---|---|
| L | 30 | L2 | 13.8 | W2 | 20 | a | 9.5 | h | 1.9 |
| L1 | 8 | W1 | 2.5 | W3 | 5.25 | b | 6.75 | h1 | 0.017 |
| Sensor Type | Gain Level | Integration Level | Identification Range | Operation Bandwidth | Reference |
|---|---|---|---|---|---|
| Patch Antenna | high | easy | long | narrow | This work |
| Coil Antenna | low | hard | short | wide | [14] |
| SIW Slot Antenna | low | easy | short | wide | [15] |
| Dipole Antenna | average | hard | average | wide | [16] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zhang, X.; Huang, B.; Zhao, Y.; Cheng, C.; Chen, H. High-Performance Wireless Ammonia Gas Sensors Based on Reduced Graphene Oxide and Nano-Silver Ink Hybrid Material Loaded on a Patch Antenna. Sensors 2017, 17, 2070. https://doi.org/10.3390/s17092070
Wu B, Zhang X, Huang B, Zhao Y, Cheng C, Chen H. High-Performance Wireless Ammonia Gas Sensors Based on Reduced Graphene Oxide and Nano-Silver Ink Hybrid Material Loaded on a Patch Antenna. Sensors. 2017; 17(9):2070. https://doi.org/10.3390/s17092070
Chicago/Turabian StyleWu, Bian, Xingfei Zhang, Beiju Huang, Yutong Zhao, Chuantong Cheng, and Hongda Chen. 2017. "High-Performance Wireless Ammonia Gas Sensors Based on Reduced Graphene Oxide and Nano-Silver Ink Hybrid Material Loaded on a Patch Antenna" Sensors 17, no. 9: 2070. https://doi.org/10.3390/s17092070





