Aptamer-Based Single-Step Assay by the Fluorescence Enhancement on Electroless Plated Nano Au Substrate
Abstract
:1. Introduction
2. Materials and Methods
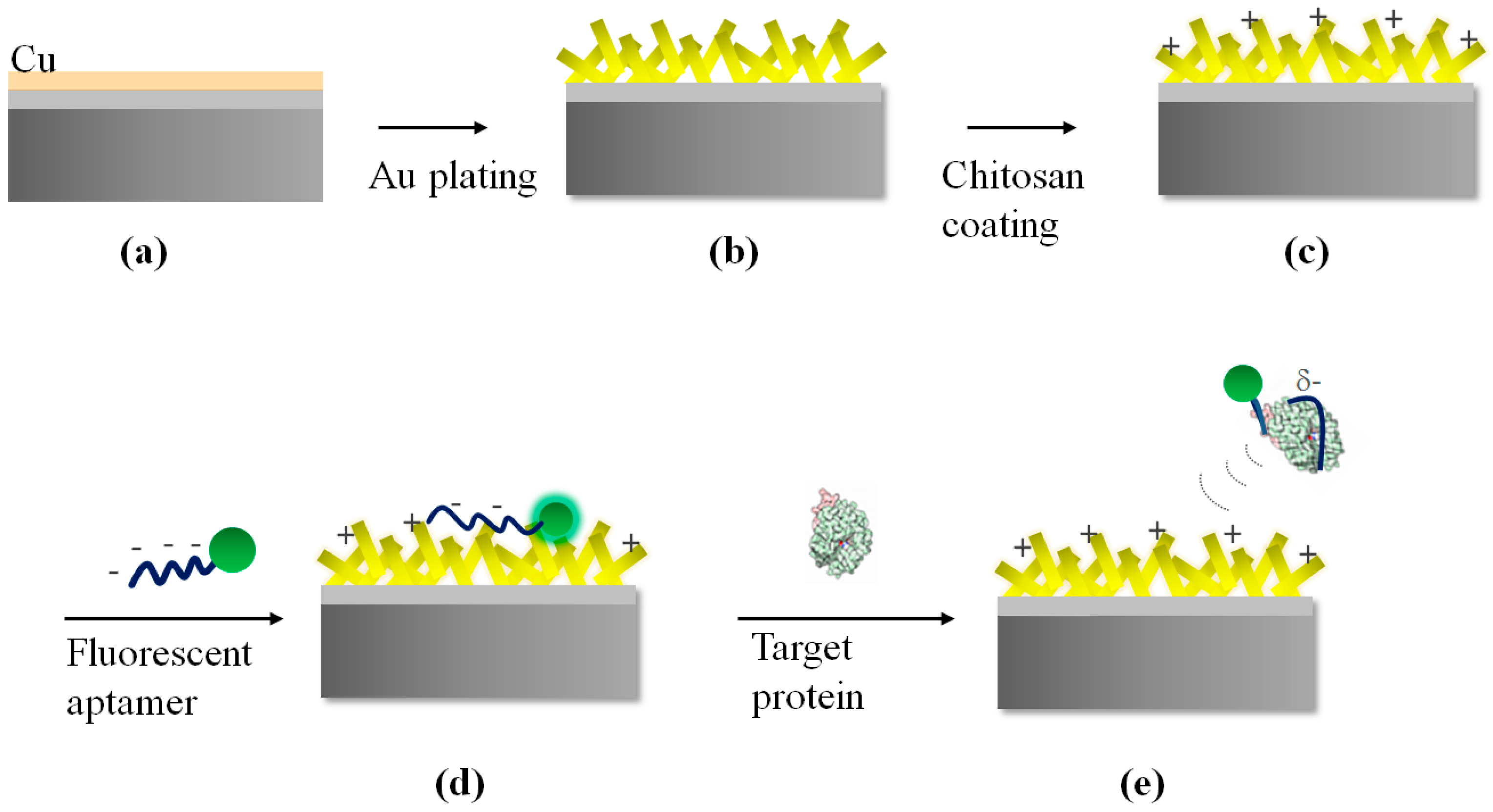
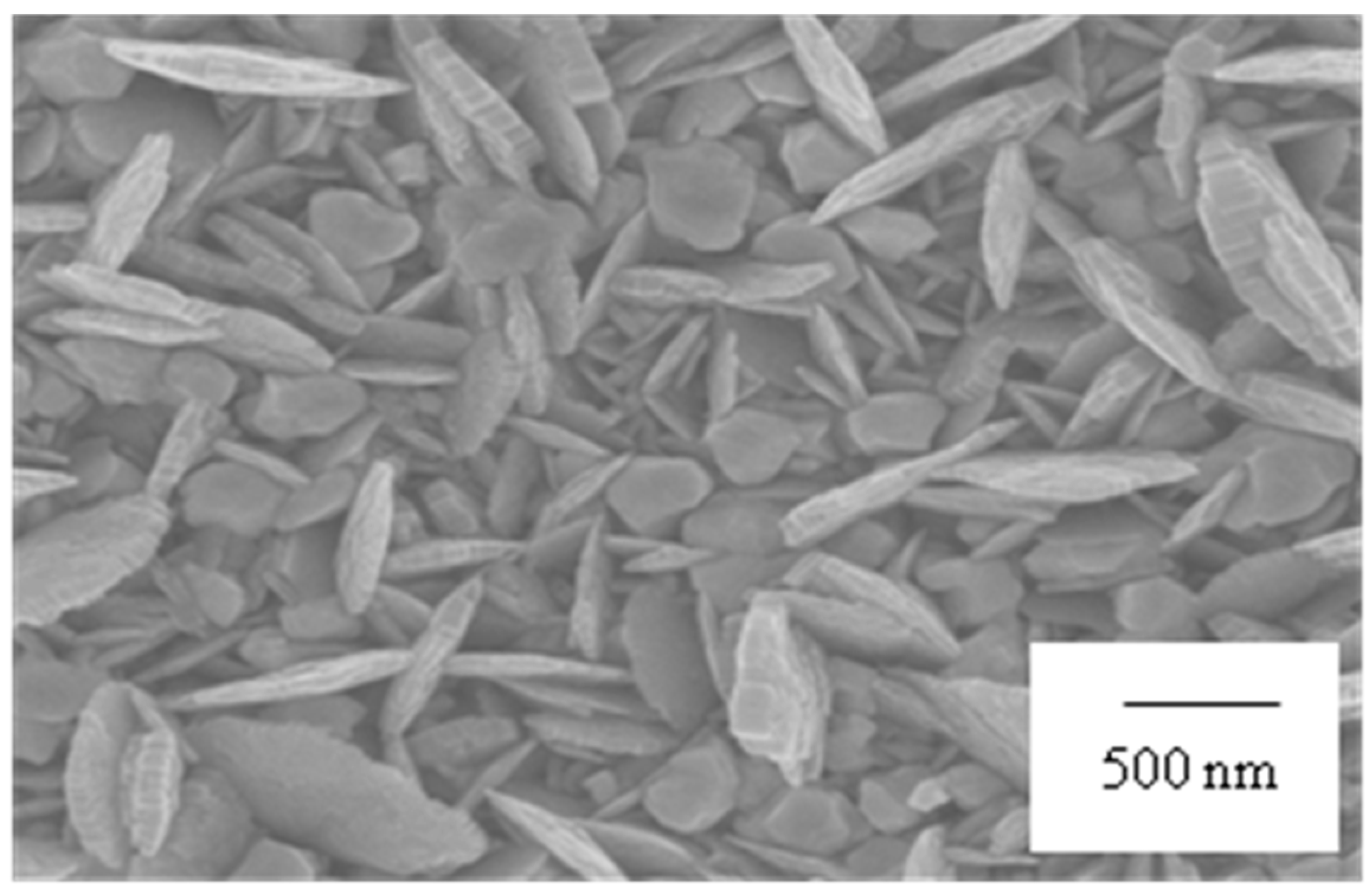
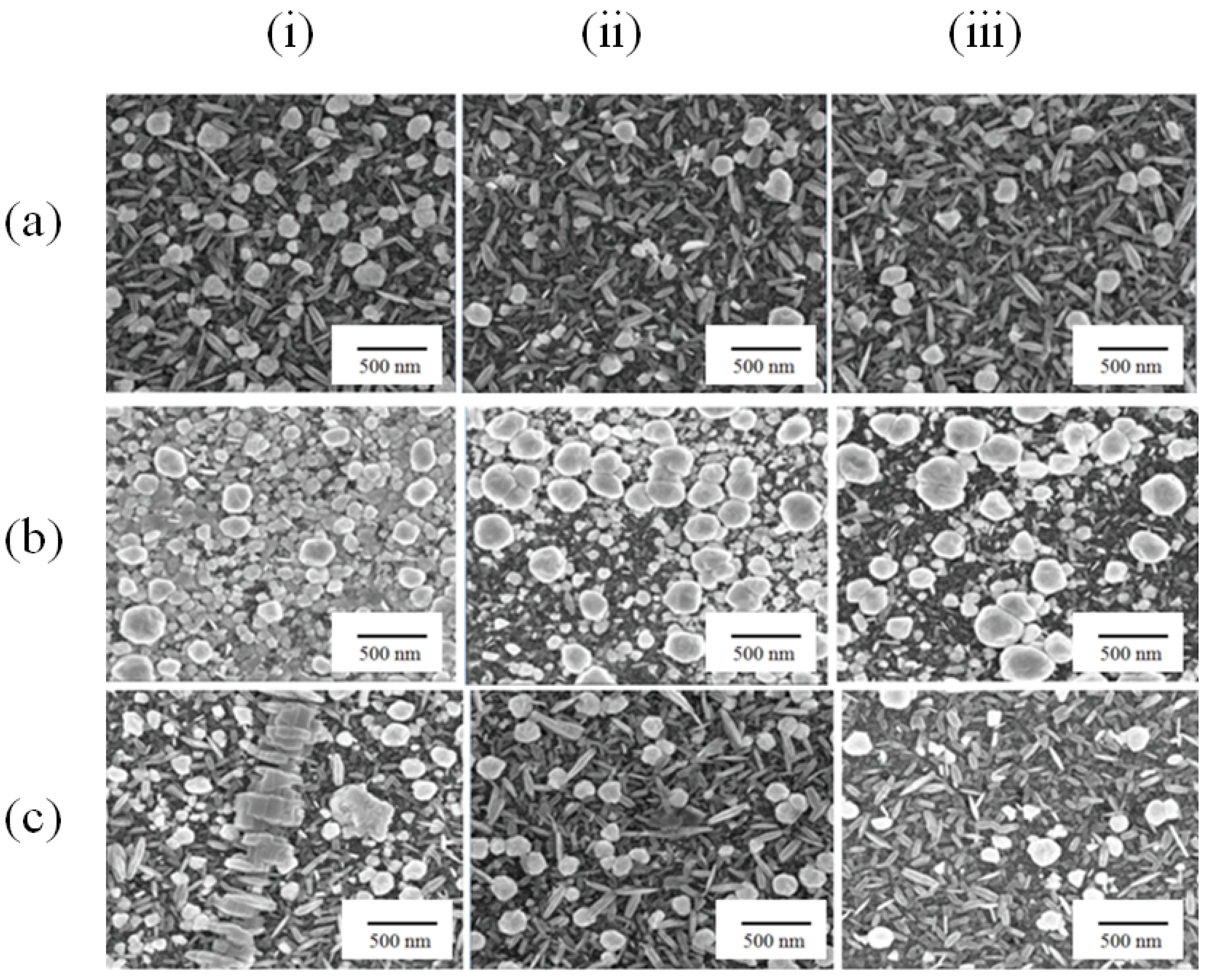
2.1. Fabrication of Substrates with Au Nanostructure
2.2. Chitosan Coating Process
2.3. Immobilization of Aptamers and Optical Signal Measurement
- Thrombin binding aptamer: CAC TGT GGT TGG TGT GGT TGG (21mer)-Cy5 (or FAM);
- Thrombin binding aptamer: GGT TGG TGT GGT TGG (15mer)-Cy5 (or FAM);
- Random DNA sequence: TAG TGA ATG GAT CGG ACA GC (20mer)-Cy5 (or FAM).
3. Results and Discussion
3.1. Fabrication of Gold Nanostructure Substrate for Aptasensor
3.2. Positive Layer Polymer
3.3. Enhancement of Fluorescence on Au Substrate
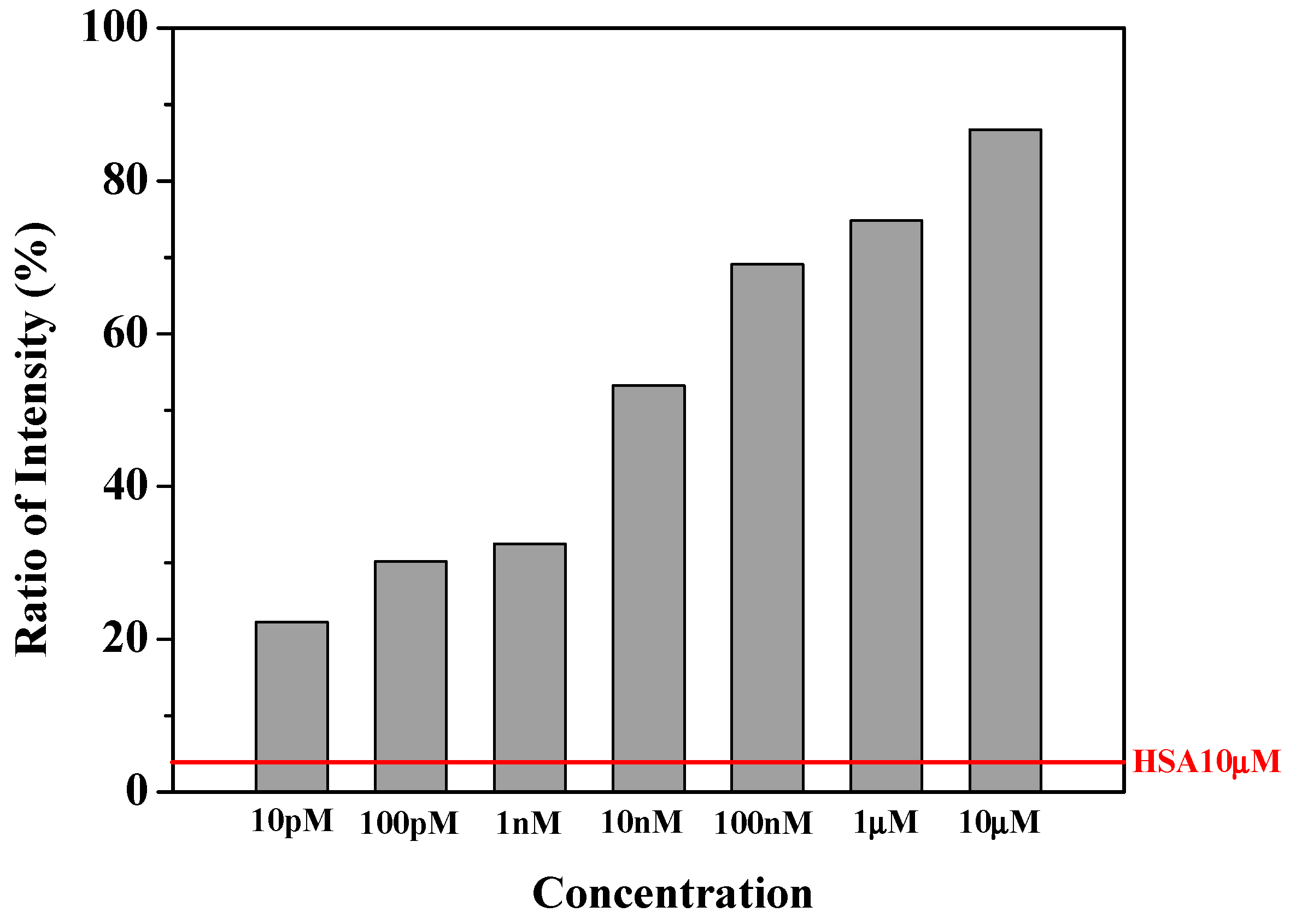
3.4. Injection of Thrombin and Quantitative Measurement of Fluorescence
3.4.1. In Situ Fluorescence upon Thrombin Injection
3.4.2. Effect of the Length of Aptamers
3.4.3. Thrombin Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, Y.C.; Jin, R.; Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Raman dye-labeled nanoparticle probes for proteins. J. Am. Chem. Soc. 2003, 125, 14676–14677. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Nanopraticle-Based Bio-Bar Codes for the Ultrasenstitive Detection of Proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Cai, L.J.; Cuo, J.; Wang, C.X.; Ruan, W.D.; Han, W.Y.; Xu, W.Q.; Zhao, B.; Ozaki, Y. Fluorescein Isothiocyanate Linked Immunoabsorbent Assay Based on Surface-Enhanced Resonance Raman Scattering. Anal. Chem. 2008, 80, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In Vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- De-los-Santos-Álvarez, N.; Lobo-Castañón, M.A.J.; Miranda-Orderes, A.J.; Tuñón-Blanco, P. Aptamers as recognition elements for label-free analytical devices. TrAC Trends Anal. Chem. 2008, 27, 437–446. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. Adaptive Recognition by Nucleic Acid Aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Lin, Y.; Abrams, B.; Jayasena, S.D. Staining of cell surface human CD4 with 2’-F-pyrimidine-containing RNA aptamers for flow cytometry. Nucleic Acids Res. 1998, 26, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khadem-hosseini, A.; Tran, T.-N.T.; La Van, D.A.; Langer, R. Nanoparticle-Aptamer Bioconjugates—A New Approach for Targeting Prostate Cancer Cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Moon-McDermott, L.; Roming, T.S. An enzyme-linked oligonucleotide assay. Nat. Biotechnol. 1996, 14, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, D.D.; Jameson, E.E.; Perlette, J.; Malik, A.; Kennedy, R.T. Effect of buffer, electric field, and separation time on detection of aptamer-ligand complexes for affinity probe capillary electrophoresis. Electrophoresis 2003, 24, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Ehrentreich-Förster, E.; Orgel, D.; Krause-Griep, A.; Cech, B.; Erdmann, V.; Bier, F.; Scheller, F.; Rimmele, M. Biosensor-based on-site explosives detection using aptamers as recognition elements. Anal. Bioanal. Chem. 2008, 391, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Marion, C.; Chang, Y.F.; Gould, T.; Lynott, C.K.; Parma, D.; Schemidt, P.G.; Warren, S. Tenascin-C aptamers are generated using tumor cells and purified protein. J. Biol. Chem. 2001, 276, 48644–48654. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Inuyama, H.; Saito, T.; Takagi, J.; Saito, Y. Factor X-dependent, thrombin-generating activities on a neuroblastoma cell and their disappearance upon differentiation. J. Cell. Physol. 1997, 173, 406–414. [Google Scholar] [CrossRef]
- Shuman, M.A.; Majerus, P.W. The measurement of thrombin in clotting blood by radioimmunoassay. J. Clin. Investig. 1976, 58, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Bichler, J.; Heit, J.A.; Owen, W.G. Detection of thrombin in human blood by ex vivo hirudin. Thromb. Res. 1996, 84, 289–294. [Google Scholar] [CrossRef]
- Bang, G.S.; Cho, S.; Kim, B. A novel electrochemical detection method for aptamer biosensors. Biosens. Bioelectron. 2005, 21, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Hwang, S.; Kwak, J.; Park, S.; Lee, S.S.; Lee, K.C. An electrochemical impedance biosensor with aptamer-modified pyrolized carbon electrode for label-free protein detection. Sens. Actuators B Chem. 2008, 129, 372–379. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. SERS detection of thrombin by protein recognition using functionalized gold nanoparticles. Nanomed. Nanotechnol. Bol. Med. 2007, 3, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, C.; Qian, M.X.; Zhao, X.S. Aptamer biosensor for protein detection based on guanine-quenching. Sens. Actuators B Chem. 2008, 129, 211–217. [Google Scholar] [CrossRef]
- Cho, H.; Yeh, E.C.; Sinha, R.; Laurennce, T.A.; Bearinger, J.P.; Lee, L.P. Single-step nanoplasmonic VEGF165 aptasensor for early cancer diagnosis. ACS Nano 2012, 6, 7607–7614. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Baker, B.R.; Hogu, S.W.; Pagba, C.V.; Laurence, T.A.; Lane, S.M.; Lee, L.P.; Tok, T.B.-H. Aptamer-Based SERRS Sensor for Thrombin Detection. Nano Lett. 2008, 8, 4386–4390. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Hong, J.-Y.; Han, A.R.; Lee, J.S.; Lee, J.S.; Oh, J.H.; Jang, J. Flexible FET-Type VEGF Aptasensor Based on Nitrogen-Doped Graphene converted from Conducting Polymer. ACS Nano 2012, 6, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, Y.; Cao, Z.; Tan, W.; Chang, H. Aptamer-Modified Gold Nanoparticles for Colorimetric Determination of Platelet-Derived Growth Factors and Their Receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Jennings, T.L.; Singh, M.P.; Strouse, G.F. Fluorescent lifetime quenching near d = 1.5 nm gold nanoparticles: Probing NSET validity. J. Am. Chem. Soc. 2006, 128, 5462–5467. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Hong, M.-Y.; Lee, D.; Nam, S.-H.; Yoon, H.C.; Kim, H.-S. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Radiative decay engineering: biophysical and biomedical applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Shen, Y.; Auria, S.D.; Malicka, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.M.; Wokaun, A.; Heritage, J.P.; Bergman, J.G.; Liao, P.F.; Olson, D.H. Enhanced two-photon fluorescence of molecules adsorbed on silver particle films. Phys. Rev. B 1981, 24, 4906–4909. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, S. Enhancement of Dye Fluorescence by Gold Nanoparticles: Analysis of Particle Size Dependence. Jpn. J. Appl. Phys. 2005, 44, 6833–6837. [Google Scholar] [CrossRef]
- Oroval, M.; Climent, E.; Coll, C.; Eritja, R.; Avino, A.; Marcos, M.D.; Sancenon, F.; Manez, R.M.; Amoros, P. An aptamer-gated silica mesoporous material for thrombin detection. Chem. Commun. 2013, 49, 5480–5482. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.J.; Kim, I.T.; Ahn, S.-H.; Kim, Z.H.; Cho, S.-H.; Kim, S.K. Electroless Deposition of SERS active Au nanostructures on variety of metallic substrates. Biochip. J. 2013, 7, 375–385. [Google Scholar] [CrossRef]
- Park, W.; Kim, M.J.; Choe, Y.; Kim, S.K.; Woo, K. Highly photoluminescent superparamagnetic silica composites for on-site biosensors. J. Mater. Chem. B 2014, 2, 1938–1944. [Google Scholar] [CrossRef]



| Chitosan | In/Ib FAM (Q.Y: 90%) | In/Ib Cy3 (Q.Y: 10%) |
|---|---|---|
| With “M.W. = 5000“ | 2.64 ± 0.08 | 2.98 ± 0.90 |
| With “M.W. = 1000“ | 2.58 ± 0.09 | 7.62 ± 0.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nambi Krishnan, J.; Park, S.-H.; Kim, S.K. Aptamer-Based Single-Step Assay by the Fluorescence Enhancement on Electroless Plated Nano Au Substrate. Sensors 2017, 17, 2044. https://doi.org/10.3390/s17092044
Nambi Krishnan J, Park S-H, Kim SK. Aptamer-Based Single-Step Assay by the Fluorescence Enhancement on Electroless Plated Nano Au Substrate. Sensors. 2017; 17(9):2044. https://doi.org/10.3390/s17092044
Chicago/Turabian StyleNambi Krishnan, Jegatha, Sang-Hwi Park, and Sang Kyung Kim. 2017. "Aptamer-Based Single-Step Assay by the Fluorescence Enhancement on Electroless Plated Nano Au Substrate" Sensors 17, no. 9: 2044. https://doi.org/10.3390/s17092044




