Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes
Abstract
:1. Introduction
2. Experimental Description: Sensor Fabrication and Design
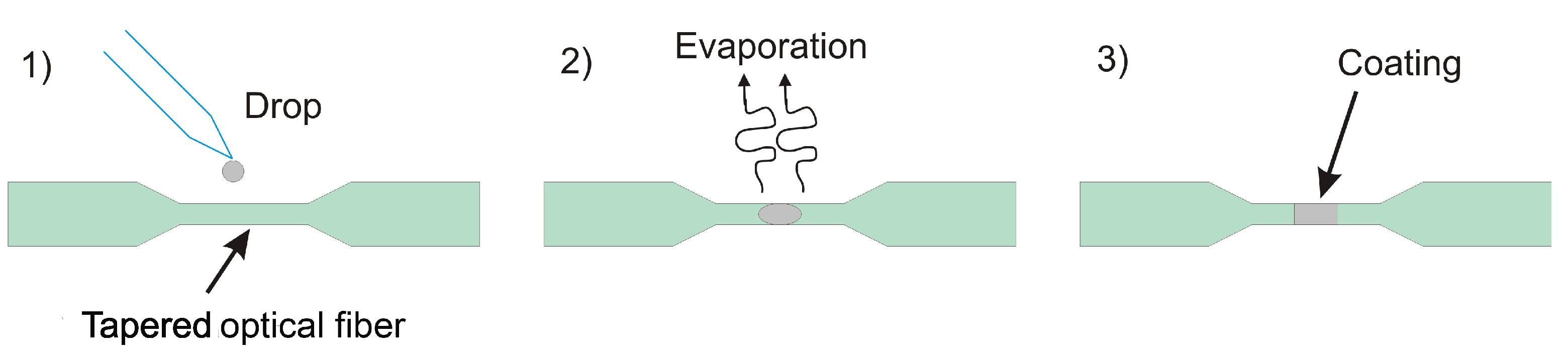
2.1. Fabrication of the Tapered Optical Fiber
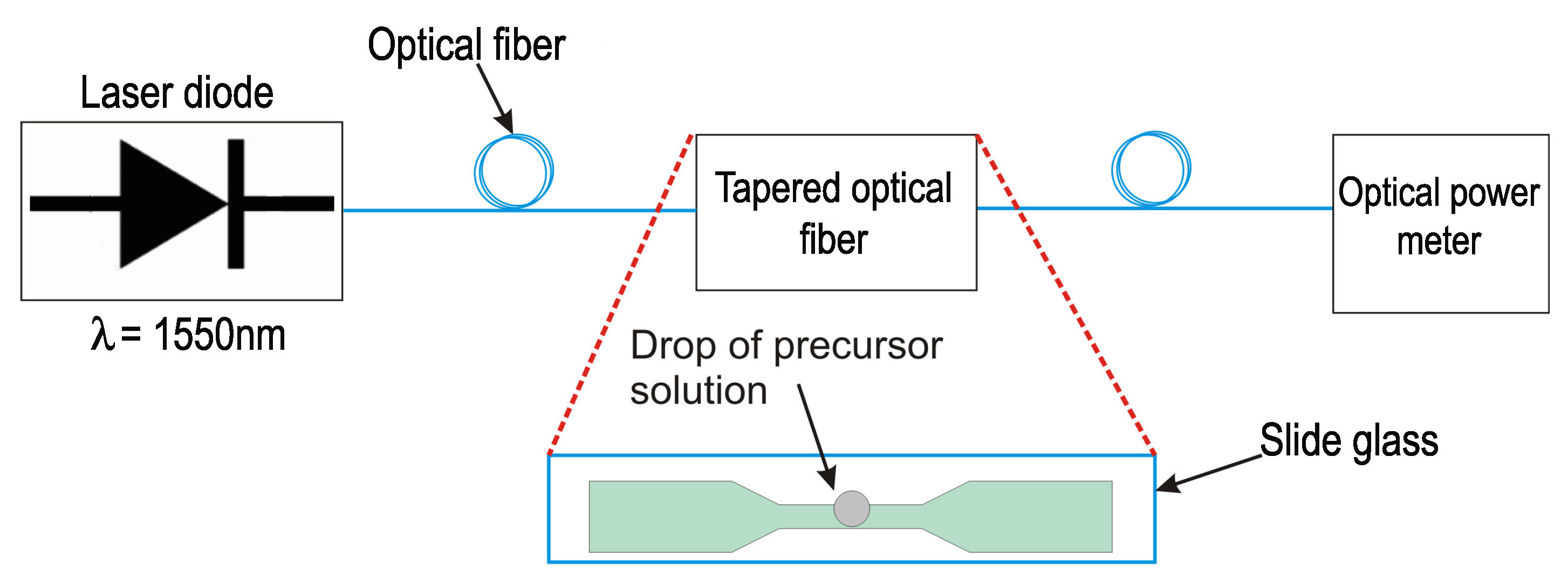
2.2. Functionalizing of Tapered Optical Fiber with Pd NPs
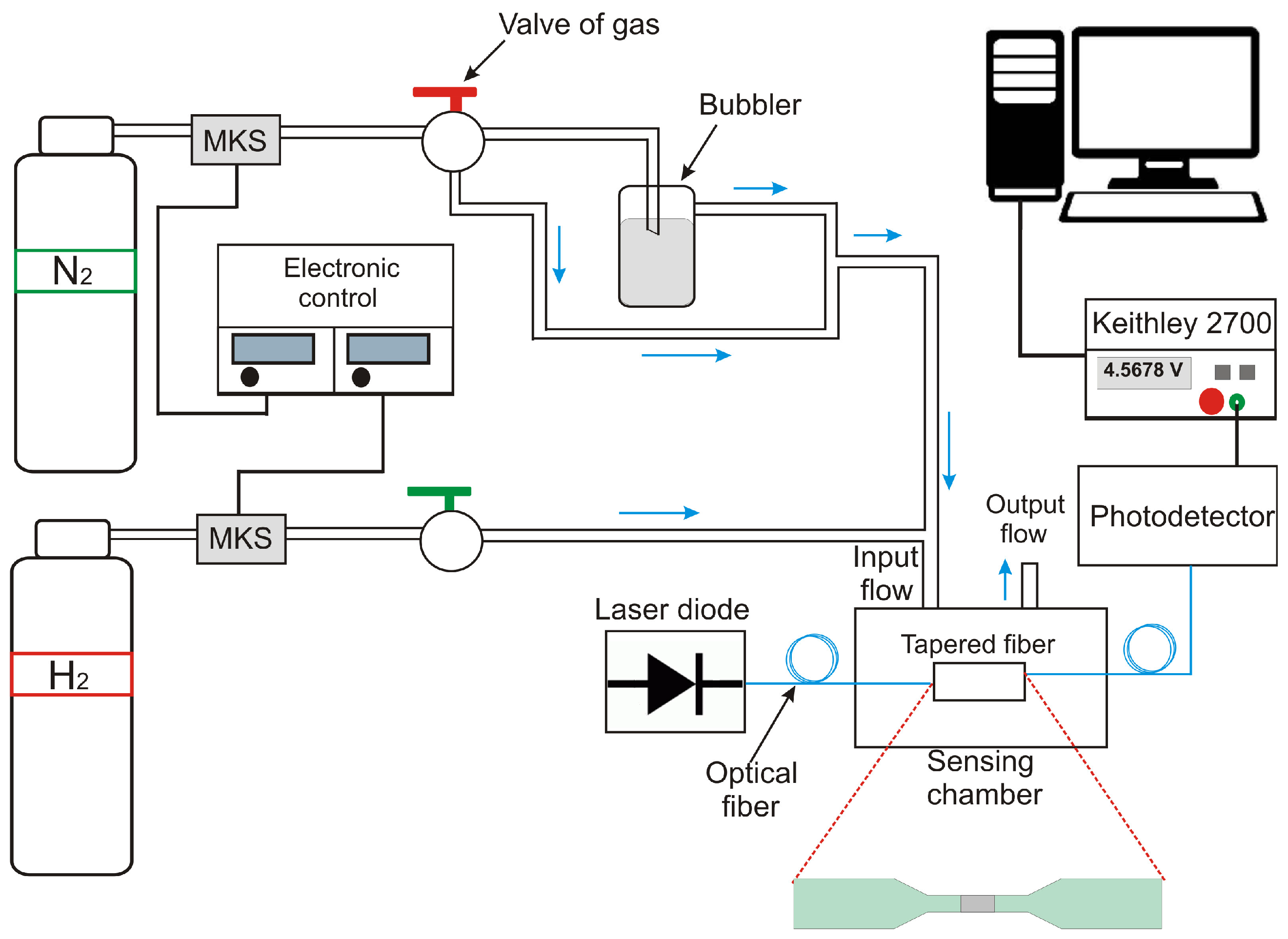
2.3. Characterization of the Optical Sensor
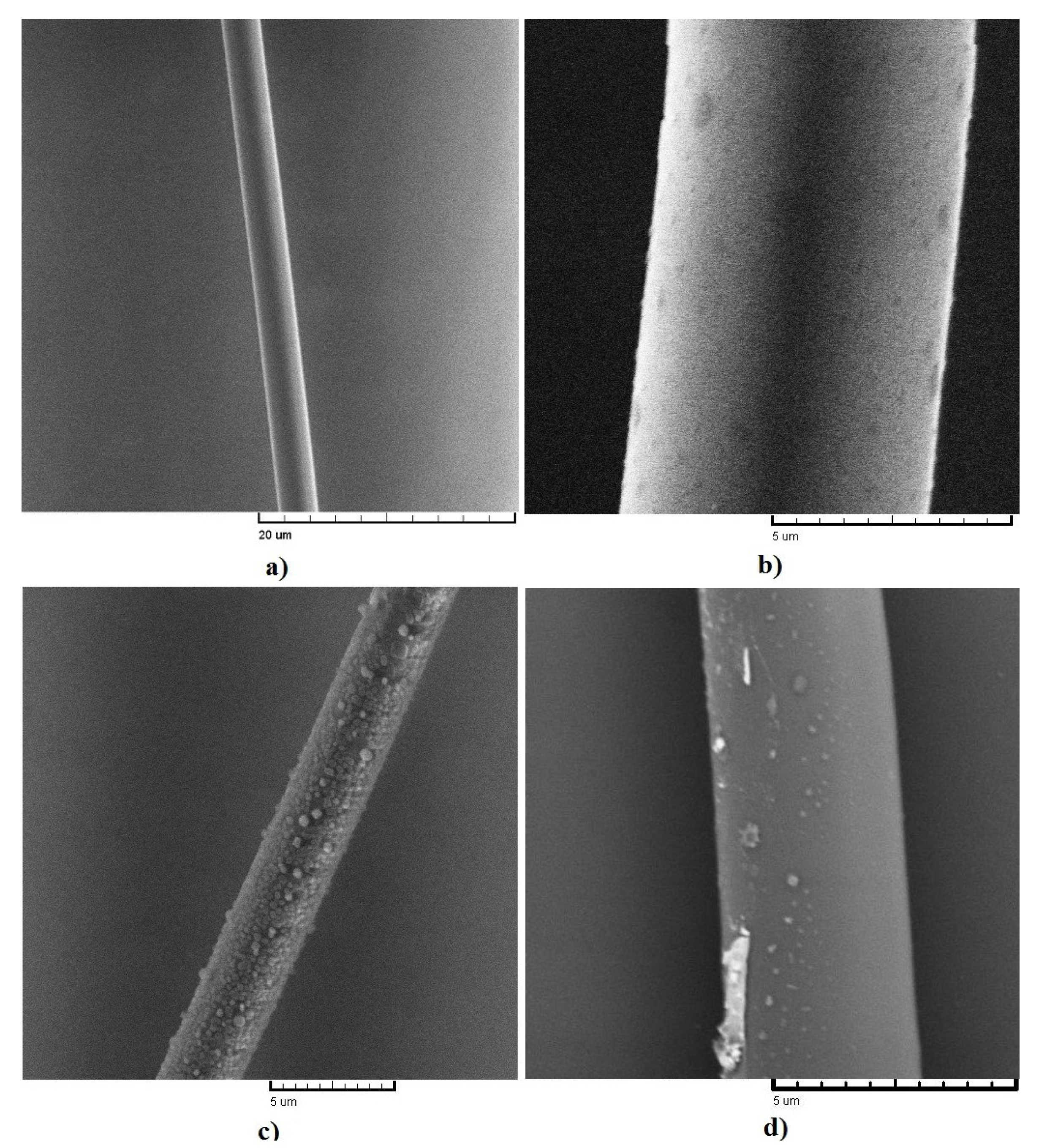
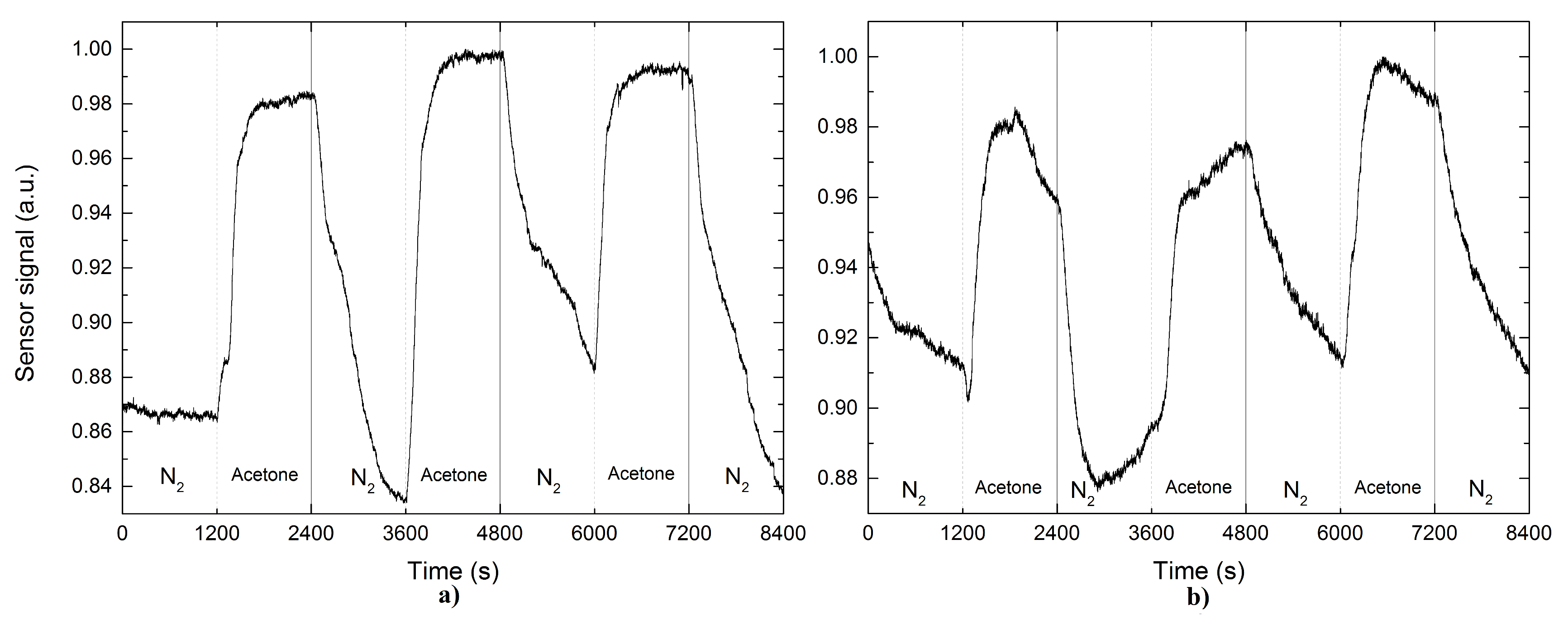
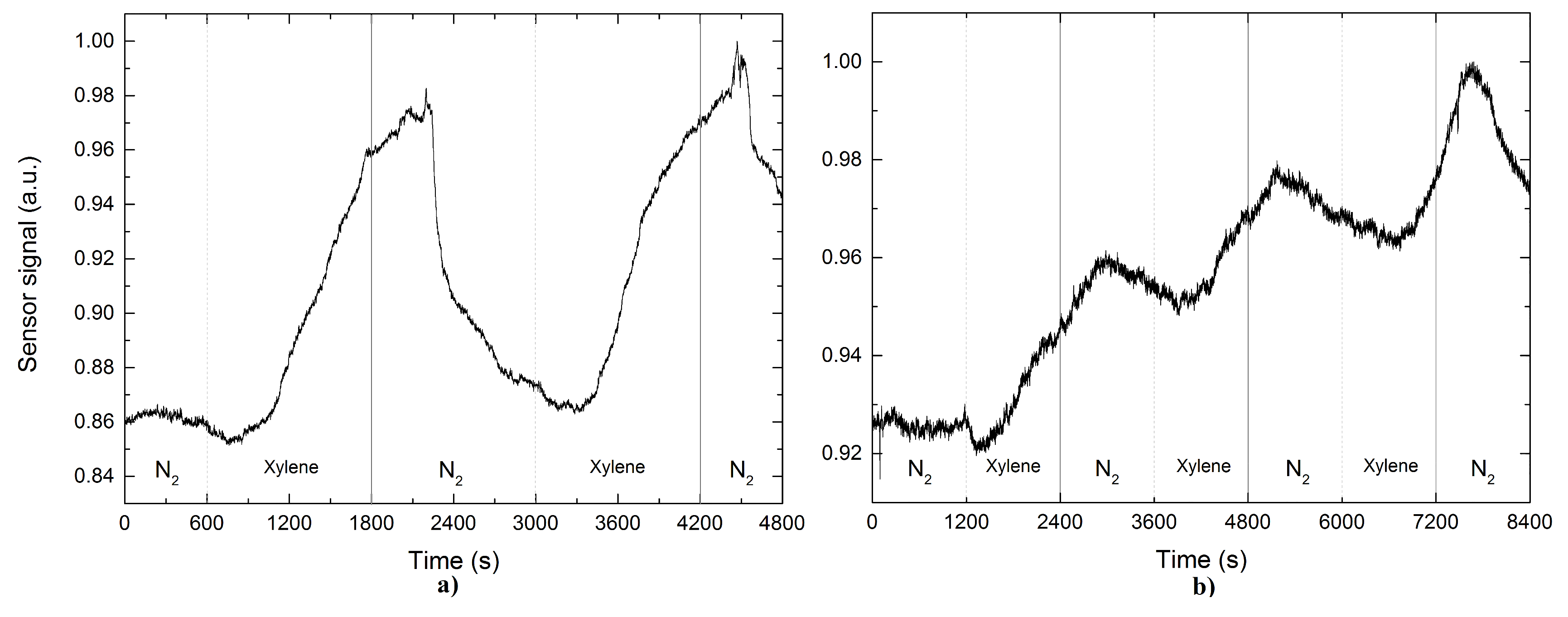
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, J.; Koppmann, R. Volatile Organic Compounds in the Atmosphere: An Overview. In Volatile Organic Compounds in the Atmosphere; John Wiley Sons: Hoboken, NJ, USA, 2007; pp. 1–32. [Google Scholar]
- Rodhe, H. A Comparison of the Contribution of Various Gases to the Greenhouse Effect. Science 1990, 248, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A Global Model of Natural Volatile Organic Compound Emissions. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Mølhave, L.; Bach, B.; Pedersen, O.F. Human Reactions to Low Concentrations of Volatile Organic Compounds. Environ. Int. 1986, 12, 167–175. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile Organic Compounds in Breath as Markers of Lung Cancer: A Cross-Sectional Study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The Analysis of Volatile Organic Compounds Biomarkers for Lung Cancer in Exhaled Breath, Tissues and Cell Lines. Cancer Biomarkers 2012, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The Future Energy Carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Udd, E.; Spillman, W.B., Jr. Fiber Optic Sensors: An Introduction for Engineers and Scientists; John Wiley Sons: Hoboken, NJ, USA, 2011; pp. 277–314. [Google Scholar]
- Tong, L.; Gattass, R.R.; Ashcom, J.B.; He, S.; Lou, J.; Shen, M.; Maxwell, I.; Mazur, E. Subwavelength-Diameter Silica Wires for Low-Loss Optical Wave Guiding. Nature 2003, 426, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.; Satija, J.; Mukherji, S. Evanescent Wave Absorption Based Fiber-Optic Sensor-Cascading of Bend and Tapered Geometry for Enhanced Sensitivity. In Sensing Technology: Current Status and Future Trends III; Springer: Berlin/Heidelberg, Germany, 2015; pp. 25–45. [Google Scholar]
- Brambilla, G. Optical Fibre Nanotaper Sensors. Opt. Fiber Technol. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Corres, J.M.; Arregui, F.J.; Matías, I.R. Sensitivity Optimization of Tapered Optical Fiber Humidity Sensors by Means of Tuning the Thickness of Nanostructured Sensitive Coatings. Sens. Actuators B Chem. 2007, 122, 442–449. [Google Scholar] [CrossRef]
- Griessen, R.; Strohfeldt, N.; Giessen, H. Thermodynamics of the Hybrid Interaction of Hydrogen with Palladium Nanoparticles. Nat. Mater. 2015, 15, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.F.; Coelho, L.; Frazão, O.; Santos, J.L.; Malcata, F.X. A Review of Palladium-Based Fiber-Optic Sensors for Molecular Hydrogen Detection. IEEE Sens. J. 2012, 12, 93–102. [Google Scholar] [CrossRef]
- Frazier, G.A.; Glosser, R. Characterization of Thin Films of the Palladium-Hydrogen System. J. Less-Common Met. 1980, 74, 89–96. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Monzón-Hernández, D. Effect of the Pd-Au Thin Film Thickness Uniformity on the Performance of an Optical Fiber Hydrogen Sensor. Appl. Surf. Sci. 2007, 253, 8615–8619. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Hydrogen Sensing with Palladium-Coated Optical Fibers. J. Appl. Phys. 1988, 64, 3706–3712. [Google Scholar] [CrossRef]
- Eryürek, M.; Karadag, Y.; Taşaltin, N.; Kilinç, N.; Kiraz, A. Optical Sensor for Hydrogen Gas Based on a Palladium-Coated Polymer Microresonator. Sens. Actuators B Chem. 2015, 212, 78–83. [Google Scholar] [CrossRef]
- Zalvidea, D.; Díez, A.; Cruz, J.L.; Andrés, M.V. Hydrogen Sensor Based on a Palladium-Coated Fibre-Taper with Improved Time-Response. Sens. Actuators B Chem. 2006, 114, 268–274. [Google Scholar] [CrossRef]
- Villatoro, J.; Monzón-Hernández, D. Fast Detection of Hydrogen with Nano Fiber Tapers Coated with Ultra Thin Palladium Layers. Opt. Express 2005, 13, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Birks, T.A.; Li, Y.W. The Shape of Fiber Tapers. J. Light. Technol. 1992, 10, 432–438. [Google Scholar] [CrossRef]
- Chávez, F.; Pérez-Sánchez, G.F.; Goiz, O.; Zaca-Morán, P.; Peña-Sierra, R.; Morales-Acevedo, A.; Soledad-Priego, M. Sensing Performance of Palladium-Functionalized WO3 Nanowires by a Drop-Casting Method. Appl. Surf. Sci. 2013, 275, 28–35. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Yamashita, S. Deposition of Carbon Nanotubes around Microfiber via Evanascent Light. Opt. Express 2009, 17, 18364–18370. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.R.J.; Jones, B.J.S.; Barton, J.S.; McCulloch, S.; Allsop, T.; Jones, J.D.C.; Bennion, I. Fibre Optics in Palladium-Based Hydrogen Sensing. J. Opt. A Pure Appl. Opt. 2007, 45, S45–S59. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry, 16th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Bévenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clément, M. Hydrogen Leak Detection Using an Optical Fibre Sensor for Aerospace Applications. Sens. Actuators B Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K. Sensors Characteristics. Sensors: An Introductory Course; Springer: Berlin/Heidelberg, Germany, 2013; pp. 11–28. [Google Scholar]
- Villatoro, J.; Luna-Moreno, D.; Monzón-Hernández, D. Optical Fiber Hydrogen Sensor for Concentrations below the Lower Explosive Limit. Sens. Actuators B Chem. 2005, 110, 23–27. [Google Scholar] [CrossRef]
- Barmenkov, Y.O.; Ortigosa-Blanch, A; Diez, A; Cruz, J.L.; Andrés, M.V. Time-Domain Fiber Laser Hydrogen Sensor. Opt. Lett. 2004, 29, 2461–2463. [Google Scholar] [CrossRef]
- Villatoro, J.; Díez, A.; Cruz, J.L.; Andrés, M.V. In-Line Highly Sensitive Hydrogen Sensor Based on Palladium-Coated Single-Mode Tapered Fibers. IEEE Sens. J. 2003, 3, 533–537. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen Sensing Using Titania Nanotubes. Sens. Actuators B Chem. 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Lopez, S.; Hernaez, M.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Resonance-Based Refractometric Response of Cladding-Removed Optical Fibers with Sputtered Indium Tin Oxide Coatings. Sens. Actuators B Chem. 2012, 175, 106–110. [Google Scholar] [CrossRef]


| Gas | Gas or Vapor Pulse | Sensor Performance Parameters | |||||
|---|---|---|---|---|---|---|---|
| Response (%) | Response Time (min) | Recovery Time (min) | |||||
| Acetone | 1 | 13.6 | 7.8 | 6.3 | 5.9 | 10.1 | 9.5 |
| 2 | 19.4 | 8.9 | 5.4 | 12.1 | 24.2 | 16.6 | |
| 3 | 12.5 | 9.2 | 4.4 | 6.5 | 10.4 | 15.8 | |
| 2-Propanol | 1 | - | 2.8 | 18.4 | 6.3 | 5.8 | 4.5 |
| 2 | 3.7 | 2.6 | 18.2 | 7.4 | 4.2 | 4.3 | |
| 3 | 3.6 | 2.5 | 18.9 | 6.5 | 5 | 4.2 | |
| Xylene | 1 | 11.6 | - | 18.5 | - | 15.5 | - |
| 2 | 11.1 | - | 17.1 | - | 19.5 | - | |
| Hydrogen | 1 | 1.1 | 0.8 | 16.2 | 2.76 | 15.1 | 10.3 |
| 2 | - | 1.2 | - | 2.6 | - | 10.2 | |
| 3 | - | 1.06 | - | 3.3 | - | 12 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Sierra, N.E.; Gómez-Pavón, L.D.C.; Pérez-Sánchez, G.F.; Luis-Ramos, A.; Zaca-Morán, P.; Muñoz-Pacheco, J.M.; Chávez-Ramírez, F. Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes. Sensors 2017, 17, 2039. https://doi.org/10.3390/s17092039
González-Sierra NE, Gómez-Pavón LDC, Pérez-Sánchez GF, Luis-Ramos A, Zaca-Morán P, Muñoz-Pacheco JM, Chávez-Ramírez F. Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes. Sensors. 2017; 17(9):2039. https://doi.org/10.3390/s17092039
Chicago/Turabian StyleGonzález-Sierra, Nancy Elizabeth, Luz Del Carmen Gómez-Pavón, Gerardo Francisco Pérez-Sánchez, Arnulfo Luis-Ramos, Plácido Zaca-Morán, Jesús Manuel Muñoz-Pacheco, and Fernando Chávez-Ramírez. 2017. "Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes" Sensors 17, no. 9: 2039. https://doi.org/10.3390/s17092039






