Electrical Detection of Pneumococcus through the Nanoparticle Decoration Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Functionalization of Microgap Devices
2.4. Preparation of AuNP@PnC Antibody Probes
2.5. The Bacteria Detection through the Nanoparticle Decoration Method
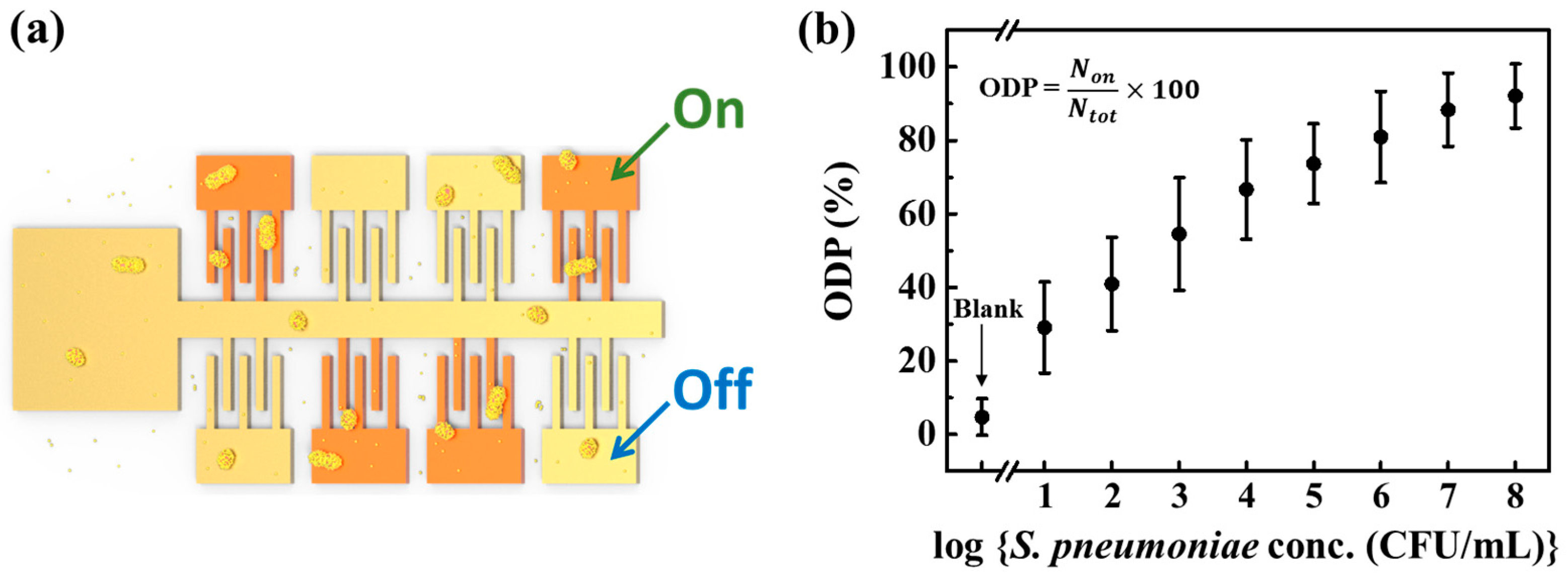
2.6. The Measurement of the On-Device Percentage (ODP) of the Sensor Chip
3. Results and Discussion
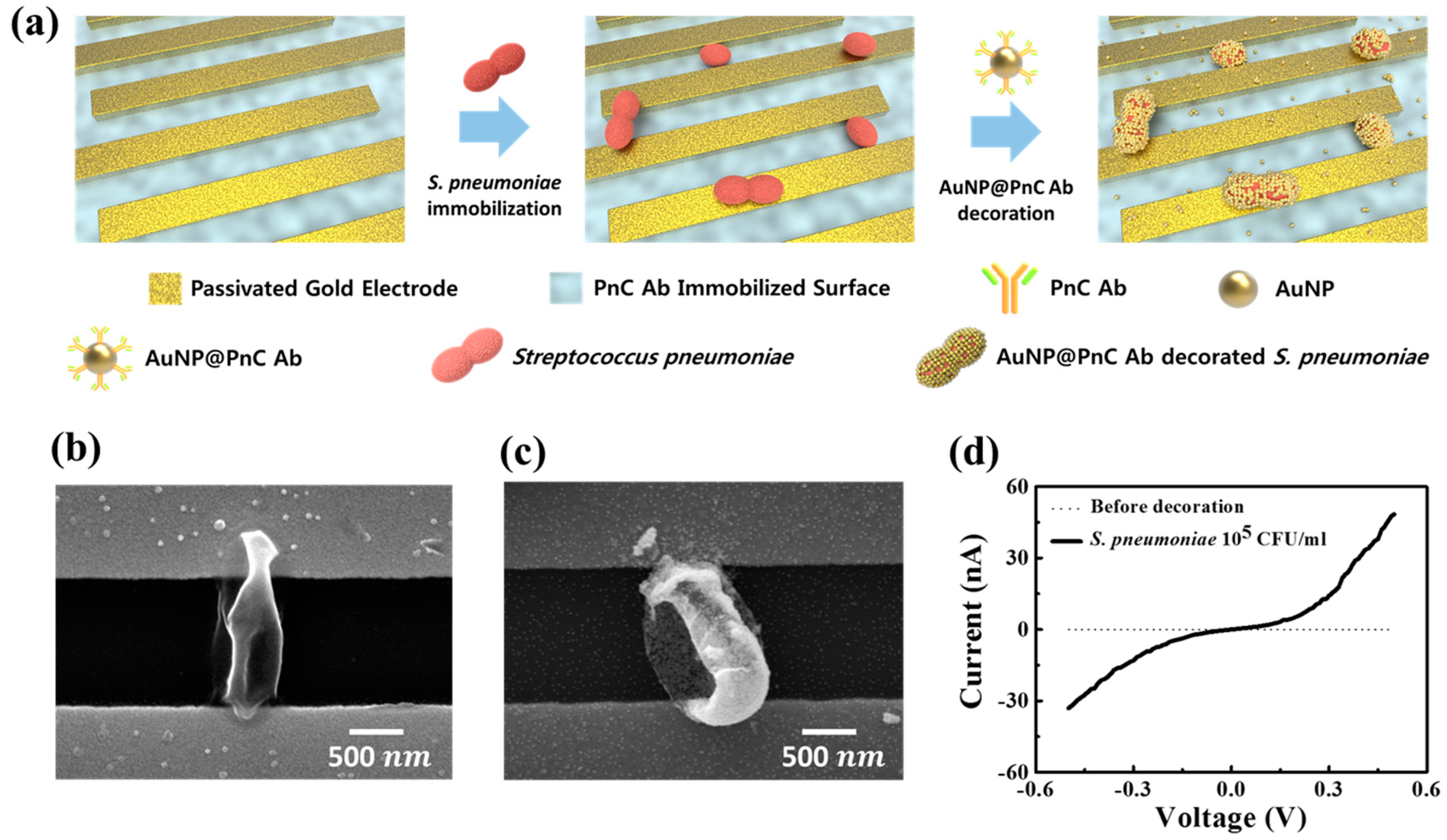
3.1. The Concept of Nanoparticle Decoration Method
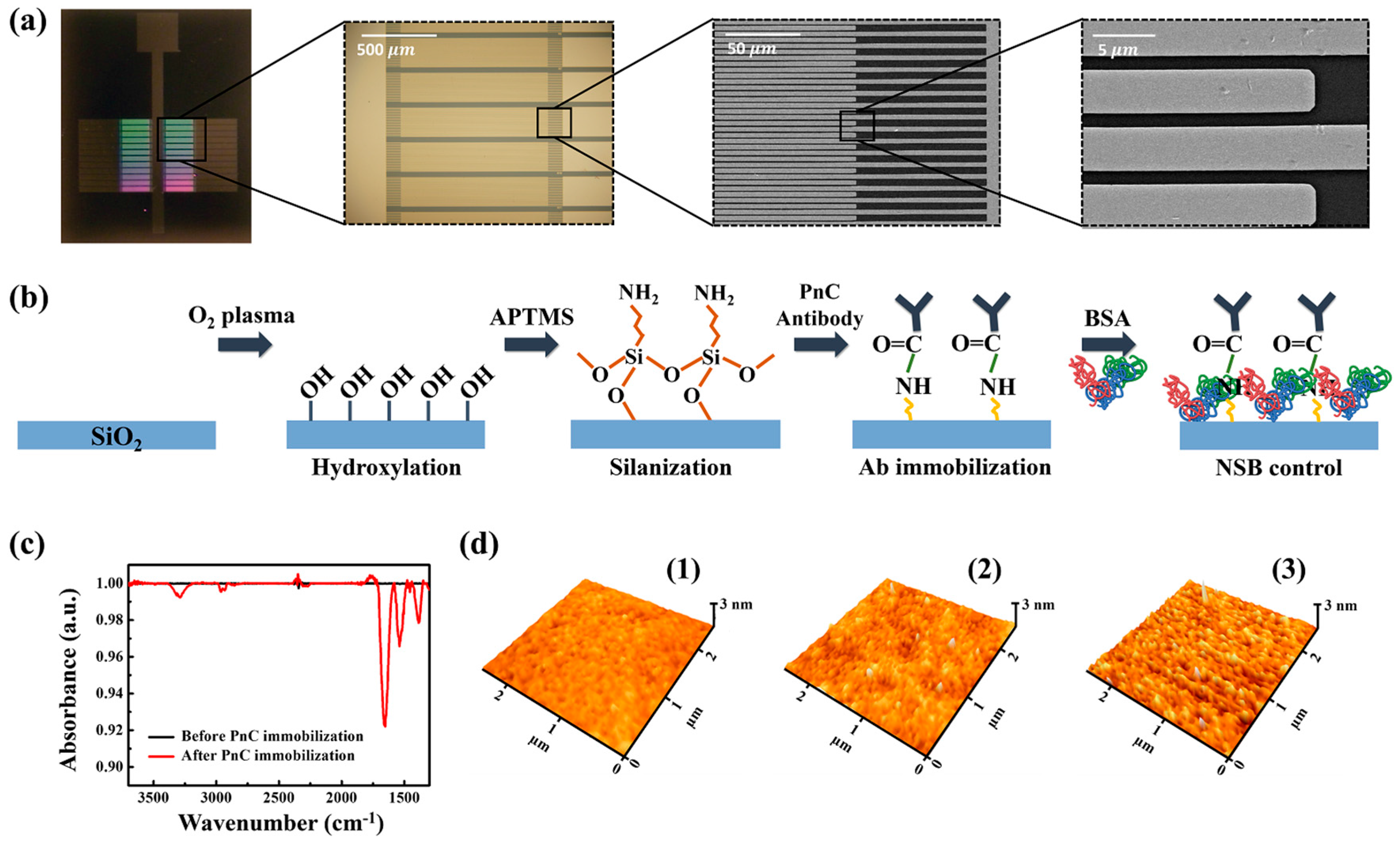
3.2. The Structure of the IDE Device and the Surface Functionalization of Silicon Oxide Surface
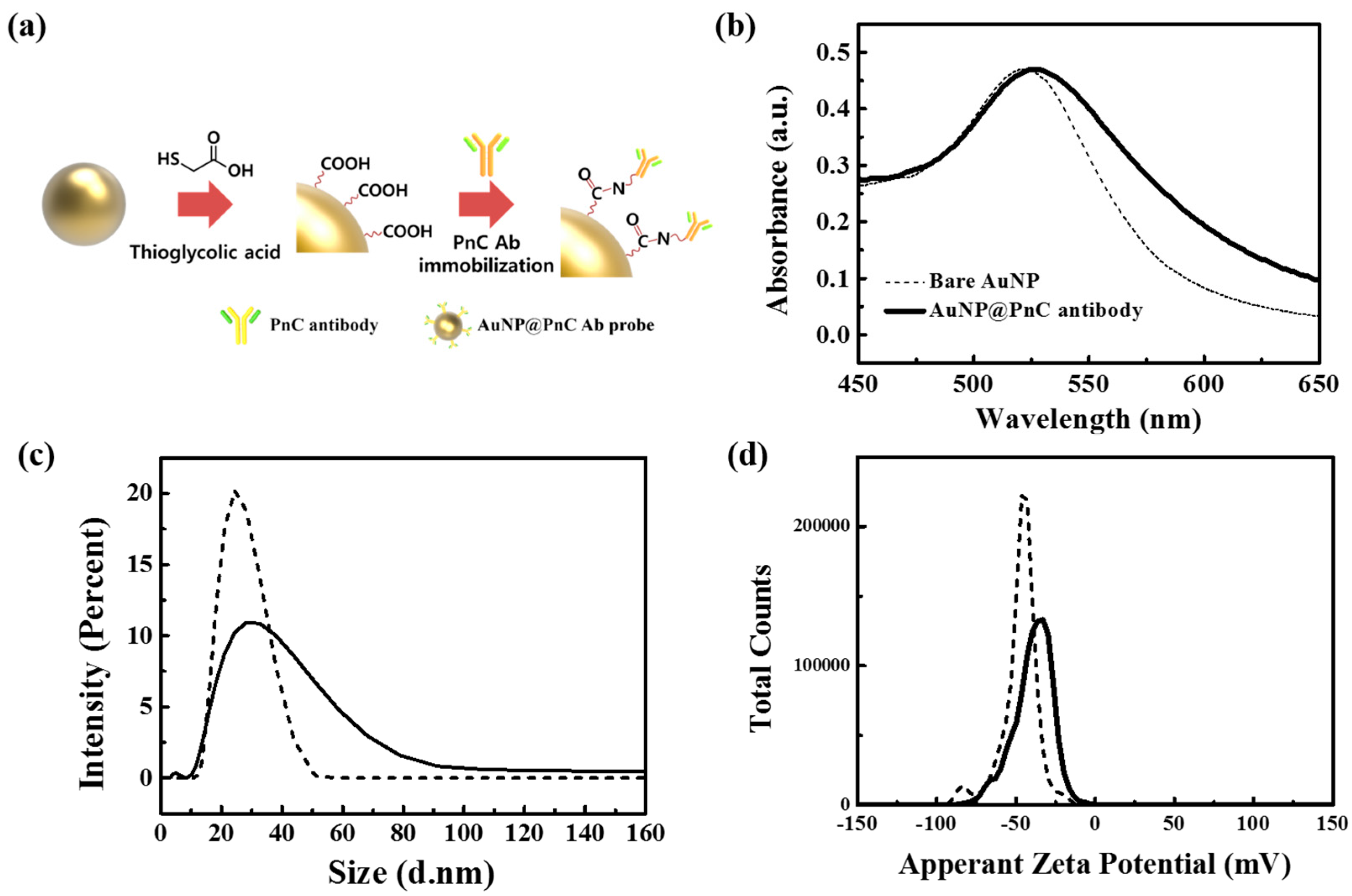
3.3. AuNP@PnC Antibody Probes
3.4. Detection of AuNP@PnC Antibody Decorated S. pneumoniae
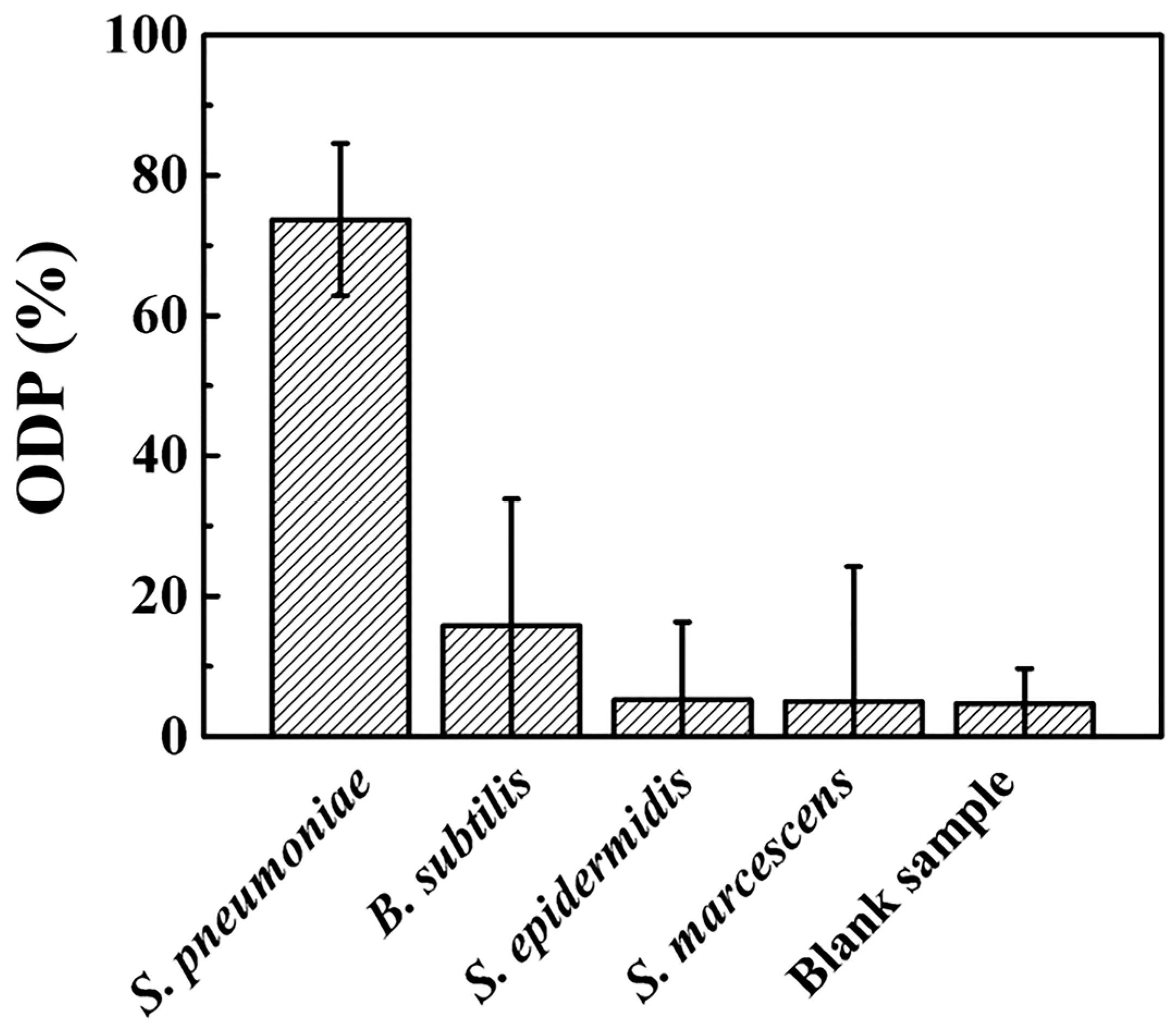
3.5. Selectivity Test of S. pnuemoniae Detection through the Nanoparticlce Decoration Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Zemanick, E.T.; Wagner, B.D.; Sagel, S.D.; Stevens, M.J.; Accurso, F.J.; Harris, J.K. Reliability of Quantitative Real-Time PCR for Bacterial Detection in Cystic Fibrosis Airway Specimens. PLoS ONE 2010, 5, e15101. [Google Scholar] [CrossRef] [PubMed]
- Edouard, S.; Prudent, E.; Gautret, P.; Memish, Z.A.; Raoult, D. Cost-effective pooling of DNA from nasopharyngeal swab samples for large-scale detection of bacteria by real-time PCR. J. Clin. Microbiol. 2015, 53, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.F.; Curry, J.I. Detection and Identification of Mycobacterium tuberculosis Directly from Sputum Sediments by Ligase Chain Reaction. J. Clin. Microbiol. 1998, 36, 1028–1031. [Google Scholar] [PubMed]
- Arias, E.; Méndez, M.T.; Arias, E.; Moggio, I.; Ledezma, A.; Romero, J.; Margheri, G.; Giorgetti, E. Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups. Sensors 2017, 17, 1028. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Yim, C.; Kwon, D.; Lee, J.; Shin, H.H.; Cha, H.J.; Jeon, S. A facile and sensitive detection of pathogenic bacteria using magnetic nanoparticles and optical nanocrystal probes. Analyst 2012, 137, 3609–3612. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yao, C.; Xia, J.; Wang, J.; Chen, M.; Huang, J.; Chang, K.; Liu, C.; Pan, H.; Fu, W. Rapid parallelized and quantitative analysis of five pathogenic bacteria by ITS hybridization using QCM biosensor. Sens. Actuators B Chem. 2011, 155, 500–504. [Google Scholar] [CrossRef]
- Hao, R.; Wang, D.; Zhang, X.; Zuo, G.; Wei, H.; Yang, R.; Zhang, Z.; Cheng, Y.; Cui, Z.; Zhou, Y. Rapid detection of Bacillus anthracis using monoclonal antibody functionalized QCM sensor. Biosens. Bioelectron. 2009, 24, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.T.N.; Kim, A.-Y.; Kim, Y.-R. Identification of Pathogenic Factors in Klebsiella pneumoniae Using Impedimetric Sensor Equipped with Biomimetic Surfaces. Sensors 2017, 17, 1406. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Shang, L.; Marcus, M.S.; Hamers, R.J. Manipulation and Real-Time Electrical Detection of Individual Bacterial Cells at Electrode Junctions: A Model for Assembly of Nanoscale Biosystems. Nano Lett. 2005, 5, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chuang, Y.-S.; Chen, Y.-Y.; Shu, A.-C.; Hsu, H.-Y.; Chang, H.-Y.; Yew, T.-R. Bacteria detection utilizing electrical conductivity. Biosens. Bioelectron. 2008, 23, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Cooper, P.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, H.J.; Park, J.; Park, D.K.; Pyo, H.; Kim, S.C.; Yun, W.S. Quantification of antigen by digital domain analysis of integrated nanogap biosensors. Biosens. Bioelectron. 2017, 97, 273–277. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, H.; Lee, C.Y.; Kim, D.; Kim, G.; Lee, S.; Yun, W.S. Electrical Detection of Pneumococcus through the Nanoparticle Decoration Method. Sensors 2017, 17, 2012. https://doi.org/10.3390/s17092012
Pyo H, Lee CY, Kim D, Kim G, Lee S, Yun WS. Electrical Detection of Pneumococcus through the Nanoparticle Decoration Method. Sensors. 2017; 17(9):2012. https://doi.org/10.3390/s17092012
Chicago/Turabian StylePyo, Hannah, Cho Yeon Lee, Daehee Kim, Gyuhee Kim, Sangho Lee, and Wan Soo Yun. 2017. "Electrical Detection of Pneumococcus through the Nanoparticle Decoration Method" Sensors 17, no. 9: 2012. https://doi.org/10.3390/s17092012




