Ag Nanorods-Oxide Hybrid Array Substrates: Synthesis, Characterization, and Applications in Surface-Enhanced Raman Scattering
Abstract
:1. Introduction
2. Fabrication of AgNRs-Oxide Hybrid Array Substrates
2.1. Fabrication of AgNR Arrays
2.2. Fabrication of AgNRs-Oxide Hybrid Array Substrates with Different Oxide Layers
3. Characterization of AgNRs-Oxide Hybrid Array Substrates
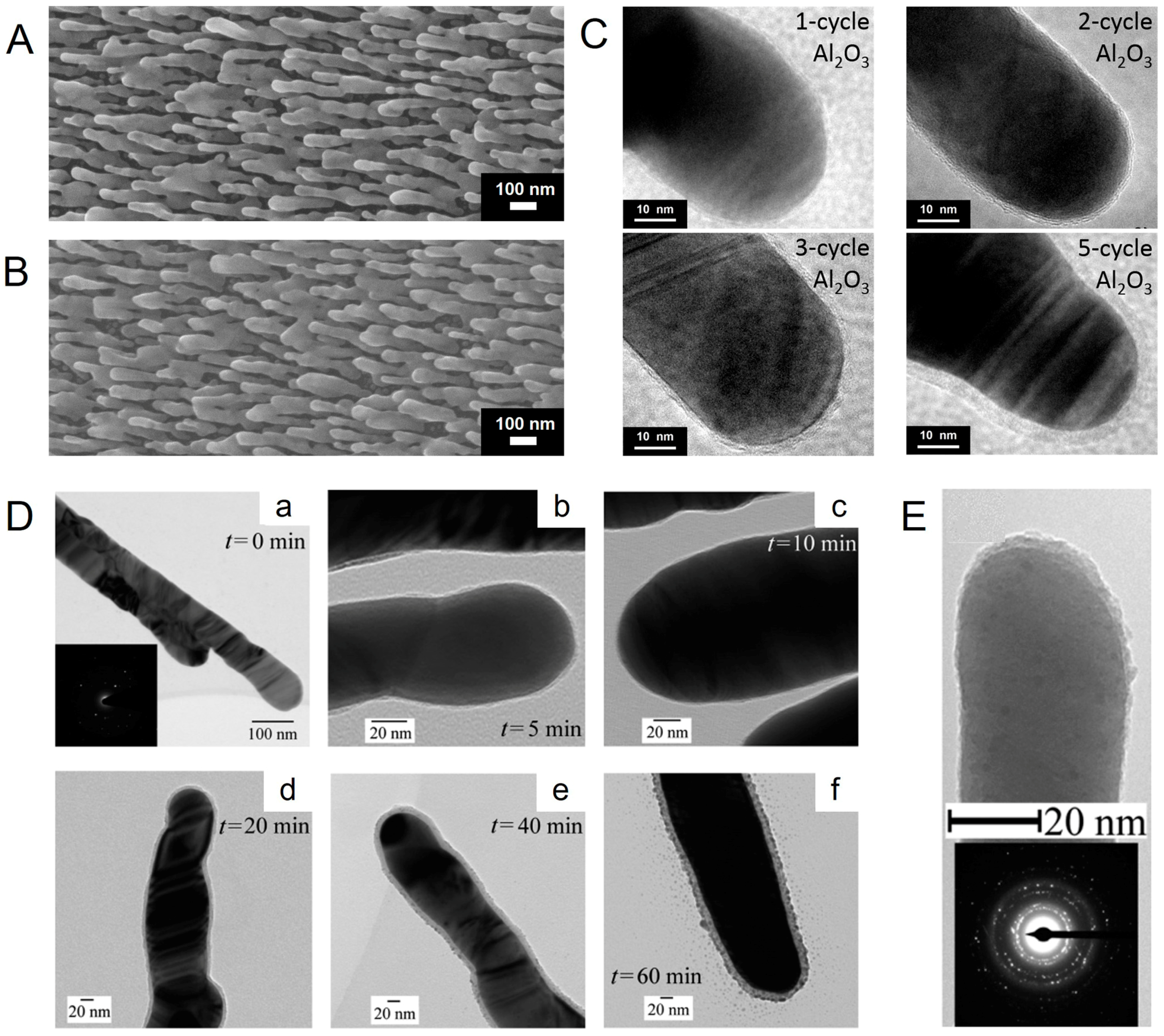
3.1. Morphology of AgNRs-Oxide Hybrid Array Substrates
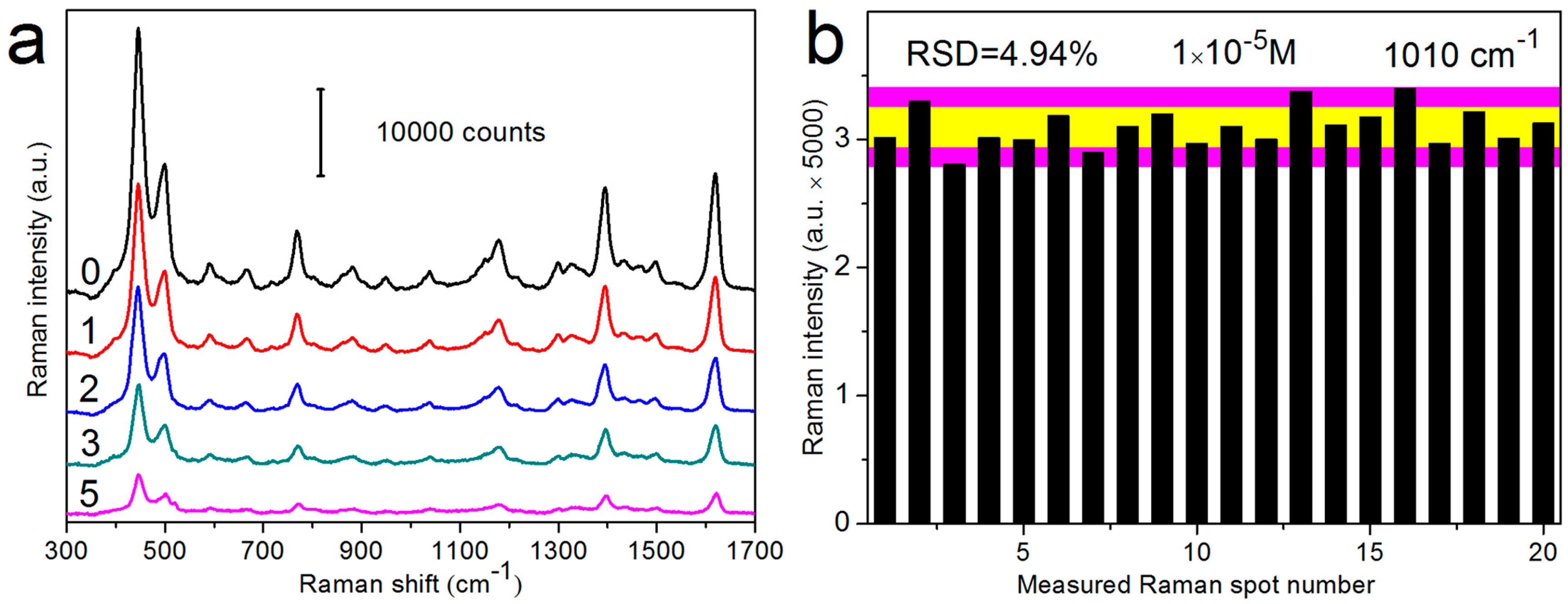
3.2. SERS Sensitivity and Reproducibility of AgNRs-Oxide Hybrid Array Substrates
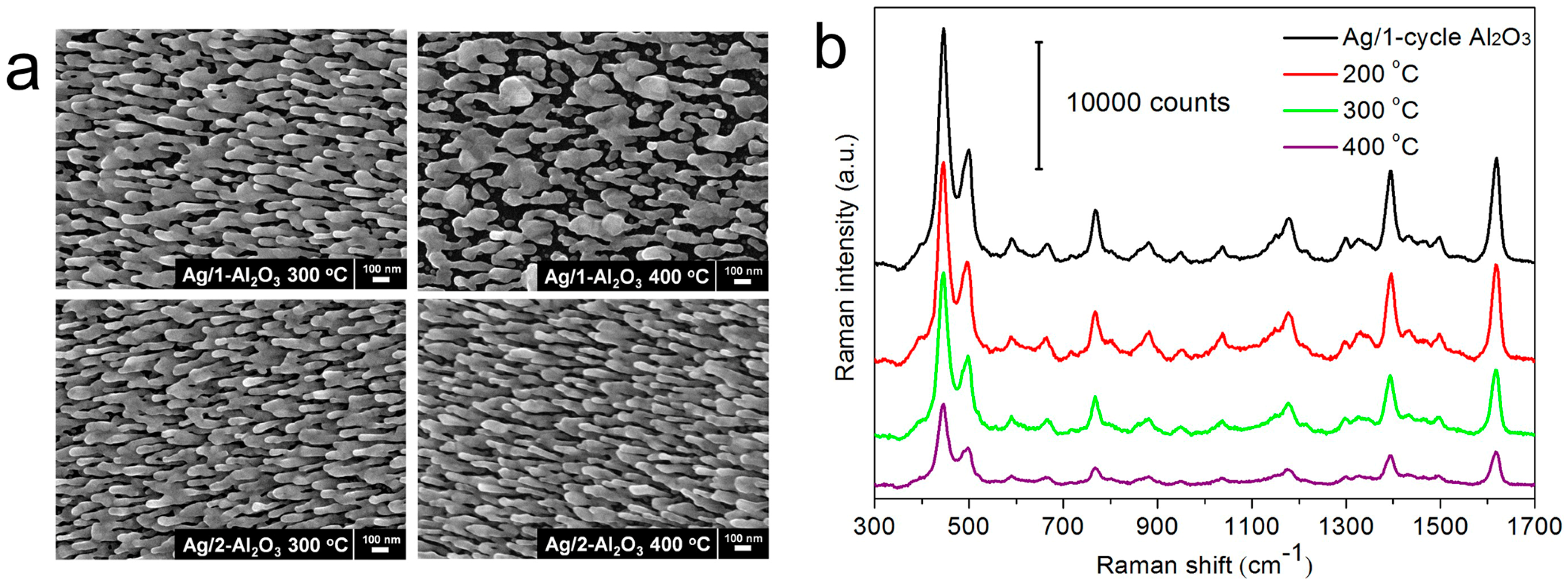
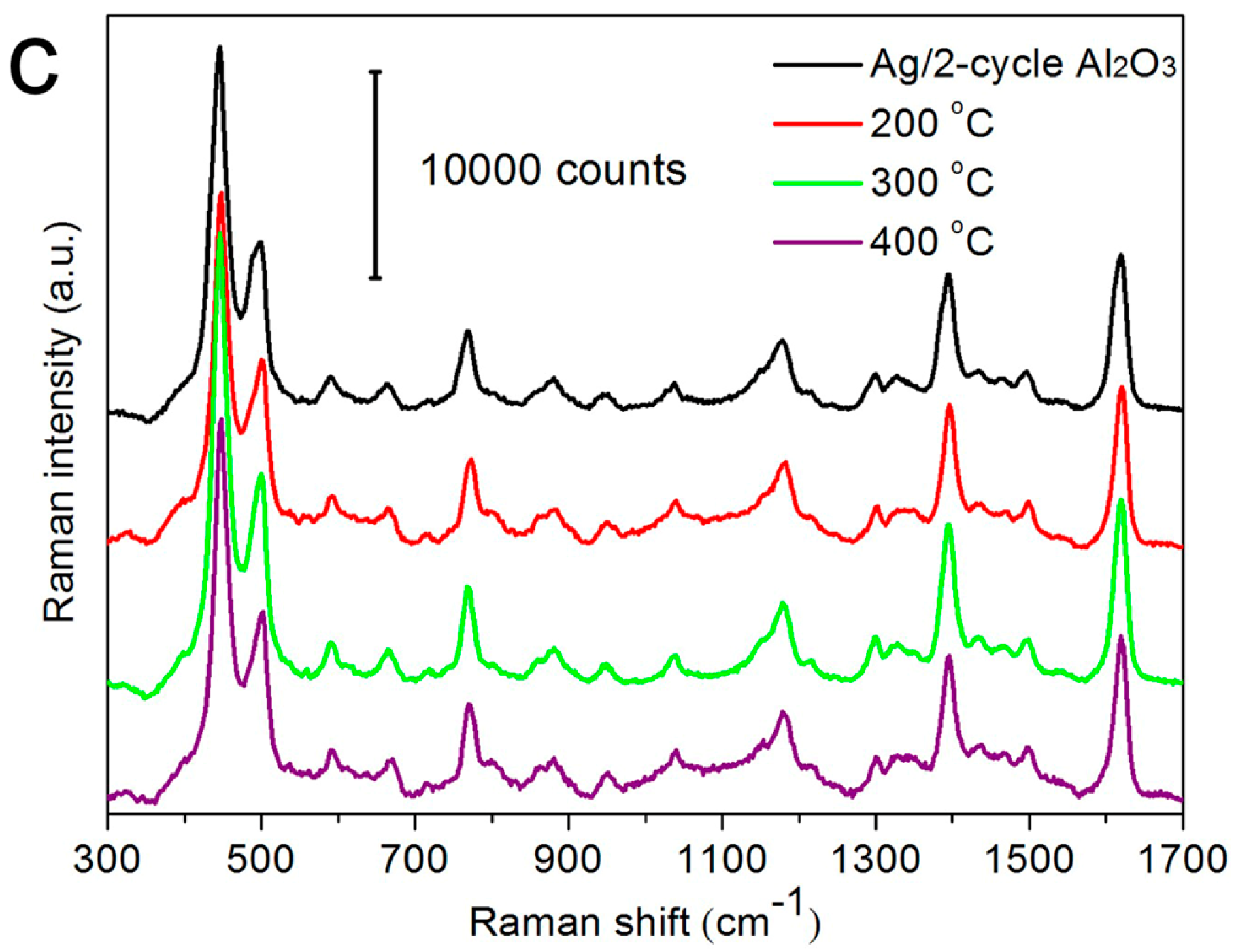
3.3. Thermal Stability of AgNRs-Oxide Hybrid Array Substrates
3.4. Temporal Stability of AgNRs-Oxide Hybrid Array Substrates
3.5. Chemical Stability of AgNRs-Oxide Hybrid Array Substrates
4. Applications of AgNRs-Oxide Hybrid Array Substrates
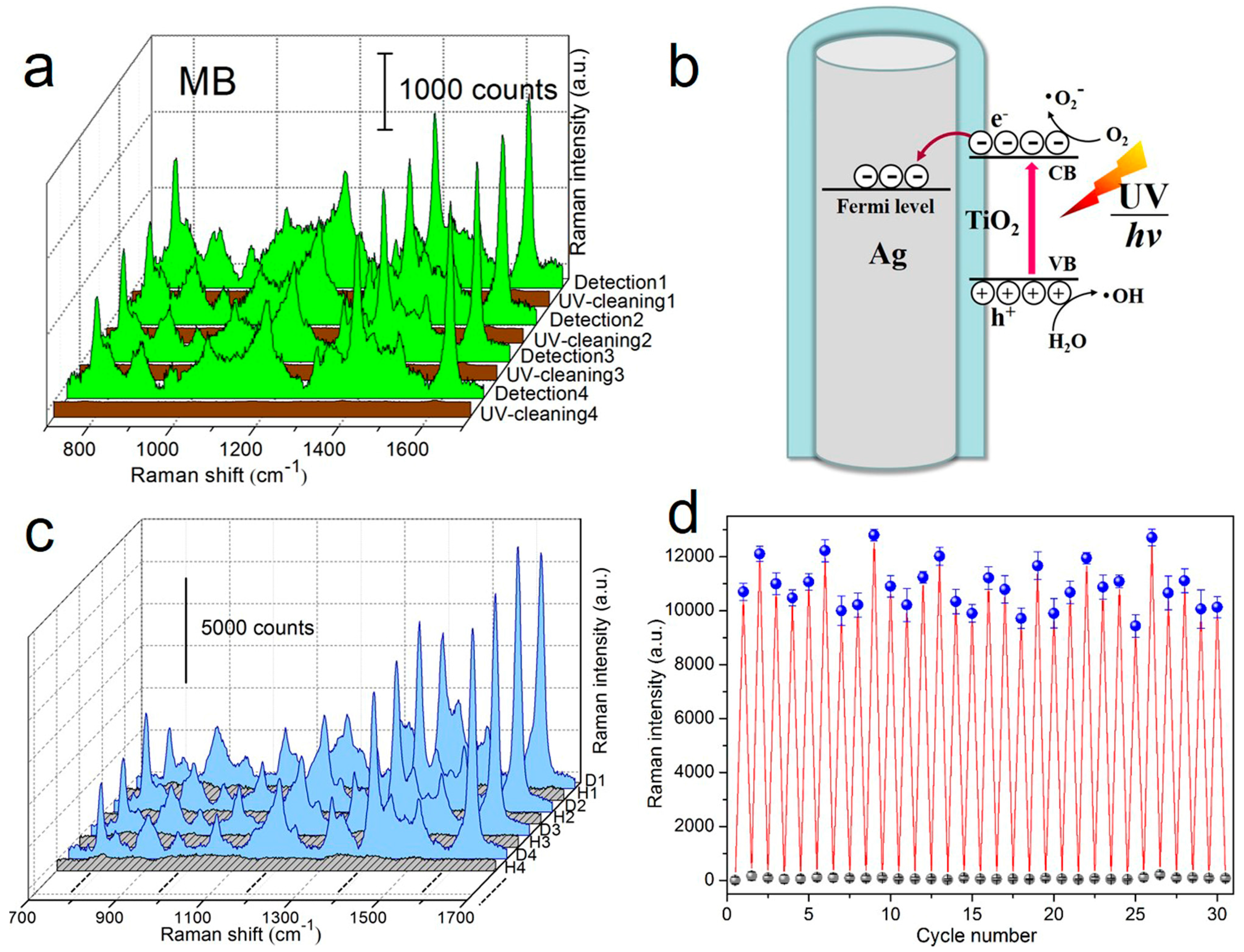
4.1. Reusable SERS Substrates
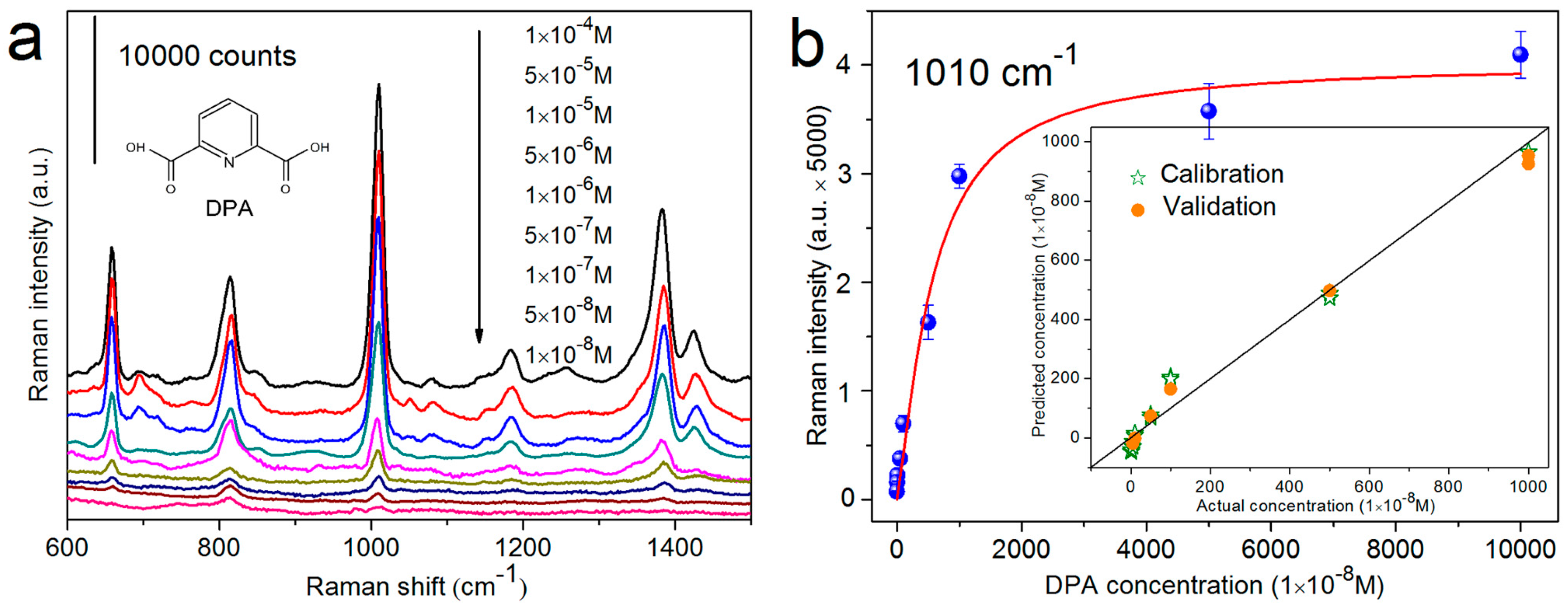
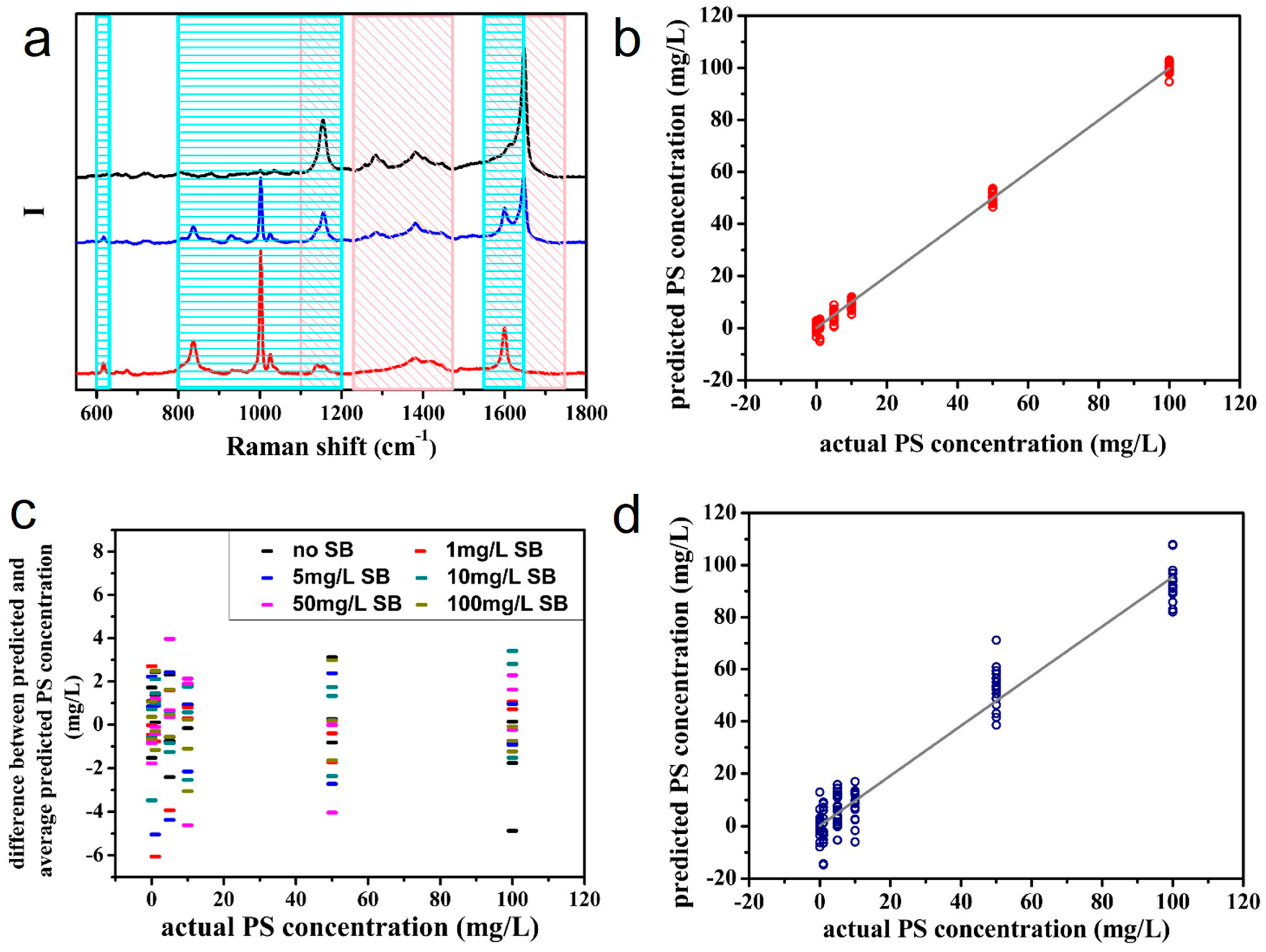
4.2. Qualitative and Quantitative SERS Analyses
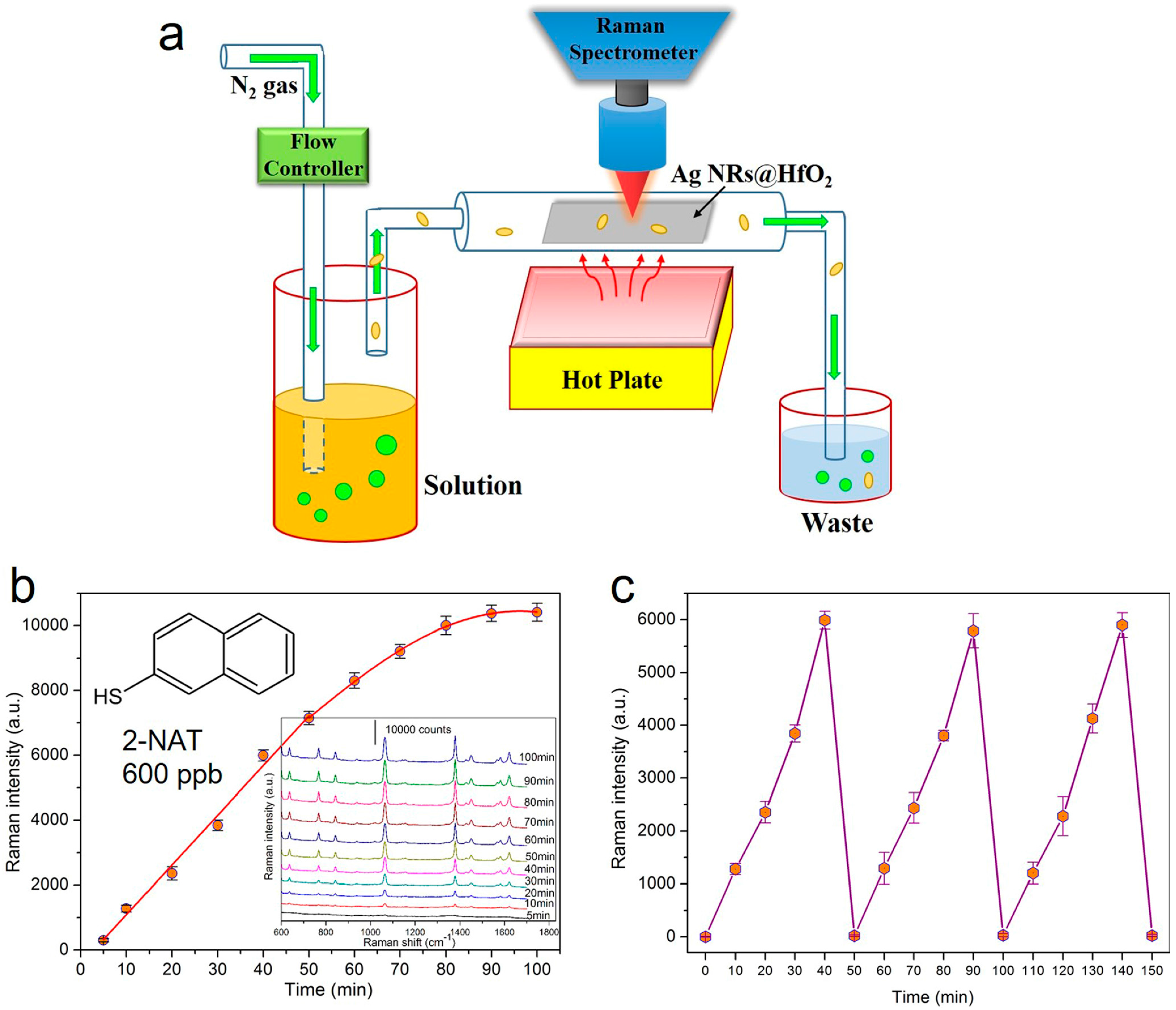
4.3. Vapor-Phase Molecule Sensing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Xu, L.; Yan, W.; Ma, W.; Kuang, H.; Wu, X.; Liu, L.; Zhao, Y.; Wang, L.; Xu, C. SERS encoded silver pyramids for attomolar detection of multiplexed disease biomarkers. Adv. Mater. 2015, 27, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Yang, Y.; Qin, D. Bifunctional Ag@Pd-Ag nanocubes for highly sensitive monitoring of catalytic reactions by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2015, 137, 7039–7042. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Sikdar, D.; Huang, X.; Si, K.J.; Xiong, W.; Gong, S.; Yap, L.W.; Premaratne, M.; Cheng, W. Plasmonic core-shell nanoparticles for SERS detection of the pesticide thiram: Size-and shape-dependent Raman enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Meng, L.; Lin, K.; Feng, J.; Huang, T.; Yang, Z.; Ren, B. Probing the location of hot spots by surface-enhanced Raman spectroscopy: Toward uniform substrates. ACS Nano 2013, 8, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Liao, F.; Cai, Q.; Li, Y.; Lee, S.; Shao, M. The effect of dielectric constants on noble metal/semiconductor SERS enhancement: FDTD simulation and experiment validation of Ag/Ge and Ag/Si substrates. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, L.; Hou, M.; Xie, Z.; Zhang, Z. Gradual plasmon evolution and huge infrared near-field enhancement of metallic bridged nanoparticle dimers. Phys. Chem. Chem. Phys. 2016, 18, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhan, Z.; Dou, J.; Zheng, X.; Xu, R.; Wu, M.; Lei, Y. Highly reproducible and sensitive SERS substrates with Ag inter-nanoparticle gaps of 5 nm fabricated by ultrathin aluminum mask technique. ACS Appl. Mater. Interfaces 2015, 7, 13322–13328. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Konzelmann, A.; Xu, F.; Gong, Y.; Liu, J.; Liu, Q.; Xin, M.; Hui, R.; Wu, J.Z. High sensitivity surface enhanced Raman spectroscopy of R6G on in situ fabricated Au nanoparticle/graphene plasmonic substrates. Carbon 2015, 86, 78–85. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, H.; Barrow, C.J.; Yang, W. Electrochemical synthesis of fractal bimetallic Cu/Ag nanodendrites for efficient surface enhanced Raman spectroscopy. Chem. Commun. 2016, 52, 10968–10971. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Aizpurua, J.; Käll, M.; Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys. Rev. E 2000, 62, 4318–4324. [Google Scholar] [CrossRef]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Liao, J.; Liu, B.H.; Yao, C.; Luo, S. Ag nanoclusters on ZnO nanodome array as hybrid SERS-active substrate for trace detection of malachite green. Sens. Actuators B Chem. 2015, 207, 430–436. [Google Scholar] [CrossRef]
- Hrelescu, C.; Sau, T.K.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Single gold nanostars enhance Raman scattering. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Tanaka, T.; Nogami, M. Self-assembled silver nanochains for surface-enhanced Raman scattering. Langmuir 2007, 23, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruan, W.; Zhang, J.; Yang, B.; Xu, W.; Zhao, B.; Lombardi, J.R. Direct observation of surface-enhanced Raman scattering in ZnO nanocrystals. J. Raman Spectrosc. 2009, 40, 1072–1077. [Google Scholar] [CrossRef]
- Vlasko-Vlasov, V.; Joshi-Imre, A.; Bahns, J.T.; Chen, L.; Ocola, L.; Welp, U. Liquid cell with plasmon lenses for surface enhanced Raman spectroscopy. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Rycenga, M.; Kim, M.H.; Camargo, P.H.; Cobley, C.; Li, Z.; Xia, Y. Surface-enhanced Raman scattering: Comparison of three different molecules on single-crystal nanocubes and nanospheres of silver. J. Phys. Chem. A 2009, 113, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, J.; Xu, C.; Zeng, Y.; Chen, Y.; Li, L.; Huang, Z.; Li, B.; Chen, R. Differentiation of digestive system cancers by using serum protein-based surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2017, 48, 16–21. [Google Scholar] [CrossRef]
- Murdoch, B.J.; Portoles, J.F.; Tardio, S.; Barlow, A.J.; Fletcher, I.W.; Cumpson, P.J. Visible wavelength surface-enhanced Raman spectroscopy from In-InP nanopillars for biomolecule detection. Appl. Phys. Lett. 2016, 109. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.; Wang, A.; Huang, H.; Feng, J. L-Arginine-assisted one-pot synthesis of hierarchical Ag1Pt2 nanocorallines for surface-enhanced Raman spectroscopy. J. Colloid Interface Sci. 2017, 498, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiao, X.; Chen, K.; Yin, W.; Li, Q.; Wang, P.; Lu, Z.; Ma, J.; Han, H. Ultrasensitive SERS detection of Bacillus thuringiensis special gene based on Au@Ag NRs and magnetic beads. Biosens. Bioelectron. 2017, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Boardman, A.K.; Wong, W.S.; Premasiri, W.R.; Ziegler, L.D.; Lee, J.C.; Miljkovic, M.; Klapperich, C.M.; Sharon, A.; Sauer-Budge, A.F. Rapid detection of bacteria from blood with surface-enhanced Raman spectroscopy. Anal. Chem. 2016, 88, 8026–8035. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.L.; Choi, J.; Shin, D.; Shin, K.S. Effect of volatile organic chemicals on surface-enhanced Raman scattering of 4-aminobenzenethiol on Ag: Comparison with the potential dependence. Phys. Chem. Chem. Phys. 2011, 13, 15603–15609. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Tang, D.; Liu, L.; Wang, Y.; Zhou, Q.; Su, S.; Hu, D.; Han, B.; Jin, M.; Ao, X. Highly reproducible surface-enhanced Raman scattering substrate for detection of phenolic pollutants. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qiu, L.; Tian, Y.; Lee, Y.; Hou, X.; Wu, L. Surface-enhanced Raman scattering using monolayer graphene-encapsulated Ag nanoparticles as a substrate for sensitive detection of 2, 4, 6-trinitrotoluene. Anal. Methods 2017, 9, 3105–3113. [Google Scholar] [CrossRef]
- Mbah, J.; Moorer, K.; Pacheco Londoño, L.; Hernandez Rivera, S.; Cruz, G. A rapid technique for synthesis of metallic nanoparticles for surface enhanced Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 723–726. [Google Scholar] [CrossRef]
- Nuntawong, N.; Eiamchai, P.; Wong-ek, B.; Horprathum, M.; Limwichean, K.; Patthanasettakul, V.; Chindaudom, P. Shelf time effect on SERS effectiveness of silver nanorod prepared by OAD technique. Vacuum 2013, 88, 23–27. [Google Scholar] [CrossRef]
- Driskell, J.D.; Shanmukh, S.; Liu, Y.; Chaney, S.B.; Tang, X.; Zhao, Y.; Dluhy, R.A. The use of aligned silver nanorod arrays prepared by oblique angle deposition as surface enhanced Raman scattering substrates. J. Phys. Chem. C 2008, 112, 895–901. [Google Scholar] [CrossRef]
- Chaney, S.B.; Shanmukh, S.; Dluhy, R.A.; Zhao, Y. Aligned silver nanorod arrays produce high sensitivity surface-enhanced Raman spectroscopy substrates. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Sun, K.; Meng, G.; Huang, Q.; Zhao, X.; Zhu, C.; Huang, Z.; Qian, Y.; Wang, X.; Hu, X. Gap-tunable Ag-nanorod arrays on alumina nanotip arrays as effective SERS substrates. J. Mater. Chem. C 2013, 1, 5015–5022. [Google Scholar] [CrossRef]
- Gu, G.H.; Suh, J.S. Silver nanorods used to promote SERS as a quantitative analytical tool. J. Raman Spectrosc. 2010, 41, 624–627. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Huang, Q.; Chen, B.; Zhu, C.; Zhang, Z. Large-area Ag nanorod array substrates for SERS: AAO template-assisted fabrication, functionalization, and application in detection PCBs. J. Raman Spectrosc. 2013, 44, 240–246. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- He, Y.; Fu, J.; Zhao, Y. Oblique angle deposition and its applications in plasmonics. Front. Phys. 2014, 9, 47–59. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Z.; Yang, Y.; Zhang, Z. Arrays of aligned, single crystalline silver nanorods for trace amount detection. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Zhou, Q.; He, Y.; Abell, J.; Zhang, Z.; Zhao, Y. Optical Properties and Surface Enhanced Raman Scattering of L-Shaped Silver Nanorod Arrays. J. Phys. Chem. C 2011, 115, 14131–14140. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, X.; Huang, Y.; Li, Z.; Zhao, Y.; Zhang, Z. Enhanced surface-enhanced Raman scattering performance by folding silver nanorods. Appl. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Jen, Y.J.; Chan, S.; Huang, J.W.; Jheng, C.Y.; Liu, W.C. Self-Shadowing Deposited Pure Metal Nanohelix Arrays and SERS Application. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; He, Y.; Abell, J.; Zhang, Z.; Zhao, Y. Surface-enhanced Raman scattering from helical silver nanorod arrays. Chem. Commun. 2011, 47, 4466–4468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chu, H.Y.; Zhao, Y. Silver nanorod array substrates fabricated by oblique angle deposition: Morphological, optical, and SERS characterizations. J. Phys. Chem. C 2010, 114, 8176–8183. [Google Scholar] [CrossRef]
- Chu, H.; Huang, Y.; Zhao, Y. Silver nanorod arrays as a surface-enhanced Raman scattering substrate for foodborne pathogenic bacteria detection. Appl. Spectrosc. 2008, 62, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.E.; Bottomley, L.A. Surface-enhanced Raman scattering of bacterial cell culture growth media. Appl. Spectrosc. 2010, 64, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.; Tripp, R.A.; Rota, P.; Dluhy, R.A. Identification of individual genotypes of measles virus using surface enhanced Raman spectroscopy. Analyst 2010, 135, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Cao, Z.; Deng, J.; Huang, Z.; Xu, Z.; Fu, J.; Yobas, L. Microfluidic-based metal enhanced fluorescence for capillary electrophoresis by Ag nanorod arrays. Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Leverette, C.L.; Villa-Aleman, E.; Jokela, S.; Zhang, Z.; Liu, Y.; Zhao, Y.; Smith, S.A. Trace detection and differentiation of uranyl (VI) ion cast films utilizing aligned Ag nanorod SERS substrates. Vib. Spectrosc. 2009, 50, 143–151. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Xie, Z.; Zhang, Z. Silver nanorods wrapped with ultrathin Al2O3 layers exhibiting excellent SERS sensitivity and outstanding SERS stability. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Bachenheimer, L.; Elliott, P.; Stagon, S.; Huang, H. Enhanced thermal stability of Ag nanorods through capping. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Bao, L.; Mahurin, S.M.; Dai, S. Controlled Layer-By-Layer formation of ultrathin TiO2 on silver island films via a surface sol-gel method for surface-enhanced Raman scattering measurement. Anal. Chem. 2004, 76, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- John, J.F.; Mahurin, S.; Dai, S.; Sepaniak, M.J. Use of atomic layer deposition to improve the stability of silver substrates for in situ, high-temperature SERS measurements. J. Raman Spectrosc. 2010, 41, 4–11. [Google Scholar] [CrossRef]
- Im, H.; Lindquist, N.C.; Lesuffleur, A.; Oh, S. Atomic layer deposition of dielectric overlayers for enhancing the optical properties and chemical stability of plasmonic nanoholes. ACS Nano 2010, 4, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Ma, L.; Cao, Y.; Li, D.; Liu, Z.; Wang, Z.; Sun, Z. Stable Ag@oxides nanoplates for surface-enhanced Raman spectroscopy of amino acids. ACS Appl. Mater. Interfaces 2014, 6, 8853–8858. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Yang, J.; Xing, X.; Li, J.; Wang, L. Evidence of significant covalent bonding in Au (CN)2−. J. Am. Chem. Soc. 2009, 131, 16368–16370. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, Y.; Hou, M.; Li, J.; Xie, Z.; Zhang, Z. Pinhole-containing, subnanometer-thick Al2O3 shell-coated Ag nanorods as practical substrates for quantitative surface-enhanced Raman scattering. J. Phys. Chem. C 2015, 120, 606–615. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Xie, Z.; Zhang, Z. Ag nanorods coated with ultrathin TiO2 shells as stable and recyclable SERS substrates. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chen, J.; Abell, J.L.; Cui, Y.; Zhao, Y. Ag-SiO2 core-shell nanorod arrays: Morphological, optical, SERS, and wetting properties. Langmuir 2011, 28, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, Y.; Hou, M.; Li, J.; Zhang, Z. Pinhole effect on the melting behavior of Ag@Al2O3 SERS substrates. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, H.; Huang, Y.; Zou, S.; Li, J.; Zhang, Z. High-performance real-time SERS detection with recyclable ag nanorods@HfO2 substrates. ACS Appl. Mater. Interfaces 2016, 8, 27162–27168. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Huang, Y.; Ma, L.; Zhang, Z. Quantitative analysis of single and mix food antiseptics basing on SERS spectra with PLSR method. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, Y.; Hsu, T.; Juang, M. Strategy to improve stability of surface-enhanced Raman scattering-active Ag substrates. J. Mater. Chem. 2010, 20, 7530–7535. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Bao, L.; Dai, S. Controlled layer-by-layer formation of ultrathin oxide films on silver island films for surface-enhanced Raman scattering measurement. Isr. J. Chem. 2006, 46, 329–336. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, L.; Hou, M.; Li, J.; Xie, Z.; Zhang, Z. Hybridized plasmon modes and near-field enhancement of metallic nanoparticle-dimer on a mirror. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Guziewicz, E.; Kowalik, I.A.; Godlewski, M.; Kopalko, K.; Osinniy, V.; Wójcik, A.; Yatsunenko, S.; Łusakowska, E.; Paszkowicz, W.; Guziewicz, M. Extremely low temperature growth of ZnO by atomic layer deposition. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Muraki, N. In situ monitoring of thermal crystallization of ultrathin Tris(8-Hydroxyquinoline) aluminum films using surface-enhanced Raman scattering. Appl. Spectrosc. 2014, 68, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Formo, E.V.; Wu, Z.; Mahurin, S.M.; Dai, S. In situ high temperature surface enhanced Raman spectroscopy for the study of interface phenomena: Probing a solid acid on alumina. J. Phys. Chem. C 2011, 115, 9068–9073. [Google Scholar] [CrossRef]
- Li, X.; Lee, J.; Blinn, K.S.; Chen, D.; Yoo, S.; Kang, B.; Bottomley, L.A.; El-Sayed, M.A.; Park, S.; Liu, M. High-temperature surface enhanced Raman spectroscopy for in situ study of solid oxide fuel cell materials. Energ. Environ. Sci. 2014, 7, 306–310. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, R.; Cao, W.; Zeng, H.; Su, Y.; Gui, X.; Wu, T.; Maruyama, S.; Tang, Z. Is it possible to enhance Raman scattering of single-walled carbon nanotubes by metal particles during chemical vapor deposition? Carbon 2014, 80, 311–317. [Google Scholar] [CrossRef]
- An, J.; Tang, B.; Zheng, X.; Zhou, J.; Dong, F.; Xu, S.; Wang, Y.; Zhao, B.; Xu, W. Sculpturing effect of chloride ions in shape transformation from triangular to discal silver nanoplates. J. Phys. Chem. C 2008, 112, 15176–15182. [Google Scholar] [CrossRef]
- Song, C.; Abell, J.L.; He, Y.; Murph, S.H.; Cui, Y.; Zhao, Y. Gold-modified silver nanorod arrays: Growth dynamics and improved SERS properties. J. Mater. Chem. 2012, 22, 1150–1159. [Google Scholar] [CrossRef]
- Hong, B.H.; Bae, S.C.; Lee, C.; Jeong, S.; Kim, K.S. Ultrathin single-crystalline silver nanowire arrays formed in an ambient solution phase. Science 2001, 294, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhu, J.; Cao, F.; Lu, Y.; Li, H. In situ encapsulation of Au nanoparticles in mesoporous core-shell TiO2 microspheres with enhanced activity and durability. Chem. Commun. 2009, 3789–3791. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.W.; Parkin, I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalton Trans. 2011, 40, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sasaki, T.; Shimizu, Y.; Koshizaki, N. Hexagonal-close-packed, hierarchical amorphous TiO2 nanocolumn arrays: Transferability, enhanced photocatalytic activity, and superamphiphilicity without UV irradiation. J. Am. Chem. Soc. 2008, 130, 14755–14762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, L.; Xi, M.; Feng, Q.; Jiang, C.; Fong, H. Electrospun TiO2 nanofelt surface-decorated with Ag nanoparticles as sensitive and UV-cleanable substrate for surface enhanced Raman scattering. ACS Appl. Mater. Interfaces 2014, 6, 5759–5767. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core-shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Y.; Liu, X.; Dai, J.; Wu, Y.; Tsang, Y.H.; Lei, D.Y. In situ SERS monitoring of photocatalytic organic decomposition using recyclable TiO2-coated Ag nanowire arrays. Appl. Surf. Sci. 2014, 301, 351–357. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Lopato, L.M. Phase equilibria in the Hafnia-Yttria-Lanthana system. J. Am. Ceram. Soc. 2001, 84, 2415–2420. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Whitney, A.V.; Elam, J.W.; Van Duyne, R.P. Ultrastable substrates for surface-enhanced Raman spectroscopy: Al2O3 overlayers fabricated by atomic layer deposition yield improved anthrax biomarker detection. J. Am. Chem. Soc. 2006, 128, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Allara, D.L.; Nuzzo, R.G. Spontaneously organized molecular assemblies. 1. Formation, dynamics, and physical properties of n-alkanoic acids adsorbed from solution on an oxidized aluminum surface. Langmuir 1985, 1, 45–52. [Google Scholar] [CrossRef]
- Cheng, H.; Huan, S.; Wu, H.; Shen, G.; Yu, R. Surface-enhanced Raman spectroscopic detection of a bacteria biomarker using gold nanoparticle immobilized substrates. Anal. Chem. 2009, 81, 9902–9912. [Google Scholar] [CrossRef] [PubMed]
- Cowcher, D.P.; Xu, Y.; Goodacre, R. Portable, quantitative detection of Bacillus bacterial spores using surface-enhanced Raman scattering. Anal. Chem. 2013, 85, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ella-Menye, J.; Galvan, D.D.; Bai, T.; Hung, H.; Chou, Y.; Zhang, P.; Jiang, S.; Yu, Q. Stealth surface modification of surface-enhanced Raman scattering substrates for sensitive and accurate detection in protein solutions. ACS Nano 2015, 9, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Anema, J.R.; Yang, Z.; Huang, Y.; Ding, Y.; Wu, Y.; Zhou, X.; Wu, D.; Ren, B. Synthesis and characterization of gold nanoparticles coated with ultrathin and chemically inert dielectric shells for SHINERS applications. Appl. Spectrosc. 2011, 65, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, E.V.; Myund, L.A.; Dem Yanchuk, E.M.; Makarov, A.A.; Denisova, A.S. Adsorption of acridine on silver electrode: SERS spectra potential dependence as a probe of adsorbate state. J. Mol. Struct. 2013, 1034, 19–21. [Google Scholar] [CrossRef]
- Gao, J.; Guo, L.; Wu, J.; Feng, J.; Wang, S.; Lai, F.; Xie, J.; Tian, Z. Simple and sensitive detection of cyanide using pinhole shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Raman Spectrosc. 2014, 45, 619–626. [Google Scholar] [CrossRef]
- Würgler, F.E.; Schlatter, J.; Maier, P. The genotoxicity status of sorbic acid, potassium sorbate and sodium sorbate. Mutat. Res. Lett. 1992, 283, 107–111. [Google Scholar] [CrossRef]
- Tremblay, G.C.; Qureshi, I.A. The biochemistry and toxicology of benzoic acid metabolism and its relationship to the elimination of waste nitrogen. Pharmacol. Ther. 1993, 60, 63–90. [Google Scholar] [CrossRef]
- Wilson, S.C.; Brasel, T.L.; Martin, J.M.; Wu, C.; Andriychuk, L.; Douglas, D.R.; Cobos, L.; Straus, D.C. Efficacy of chlorine dioxide as a gas and in solution in the inactivation of two trichothecene mycotoxins. Int. J. Toxicol. 2005, 24, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.R.; Singh, R.K.; Arya, S.S. Chemistry of sorbates—A basic perspective. Food Rev. Int. 1994, 10, 71–91. [Google Scholar] [CrossRef]
- Zhang, W. Automotive fuels from biomass via gasification. Fuel Process. Technol. 2010, 91, 866–876. [Google Scholar] [CrossRef]
- Menezes, H.C.; Amorim, L.C.; Cardeal, Z.L. Sampling and analytical methods for determining VOC in air by biomonitoring human exposure. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1–39. [Google Scholar] [CrossRef]
- Yang, L.; Ma, L.; Chen, G.; Liu, J.; Tian, Z.Q. Ultrasensitive SERS detection of TNT by imprinting molecular recognition using a new type of stable substrate. Chem.-Eur. J. 2010, 16, 12683–12693. [Google Scholar] [CrossRef] [PubMed]
- Demeritte, T.; Kanchanapally, R.; Fan, Z.; Singh, A.K.; Senapati, D.; Dubey, M.; Zakar, E.; Ray, P.C. Highly efficient SERS substrate for direct detection of explosive TNT using popcorn-shaped gold nanoparticle-functionalized SWCNT hybrid. Analyst 2012, 137, 5041–5045. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Wu, C.; Mercer-Smith, A.R.; Dodson, R.A.; Moersch, T.L.; Koonath, P.; Pipino, A.C.; Lu, H.; Yang, Y.; Sapirstein, V.S. Raman system for sensitive and selective identification of volatile organic compounds. Sens. Actuators B Chem. 2015, 220, 491–499. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H. Detection of volatile organic compounds using surface enhanced Raman spectroscopy substrates mounted on a thermoelectric cooler. Anal. Chim. Acta 2003, 488, 15–23. [Google Scholar] [CrossRef]
- Pearman, W.F.; Fountain, A.W. Classification of chemical and biological warfare agent simulants by surface-enhanced Raman spectroscopy and multivariate statistical techniques. Appl. Spectrosc. 2006, 60, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.A.; Biggs, K.B.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy of half-mustard agent. Analyst 2006, 131, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Ma, X.; Dong, J.; Jiang, C.; Qian, W. A reproducible SERS substrate based on electrostatically assisted APTES-functionalized surface-assembly of gold nanostars. ACS Appl. Mater. Interfaces 2011, 3, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, A.R.; Cannistraro, S. SERS detection of thrombin by protein recognition using functionalized gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Liu, F.; Cohen, L.; Köllensperger, P.; Cass, T. SERS platforms for high density DNA arrays. Faraday Discuss. 2006, 132, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, Z.; Si, S.; Zhang, X.; Wu, W.; Deng, H.; Wang, F.; Xiao, X.; Jiang, C. Ultrasensitive SERS substrate integrated with uniform subnanometer scale “hot spots” created by a graphene spacer for the detection of mercury ions. Small 2017, 13. [Google Scholar] [CrossRef]
- Ouyang, L.; Hu, Y.; Zhu, L.; Cheng, G.J.; Irudayaraj, J. A reusable laser wrapped graphene-Ag array based SERS sensor for trace detection of genomic DNA methylation. Biosens. Bioelectron. 2017, 92, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, C.; Huang, Z.; Hu, X.; Zhu, X. In situ synthesis of pristine-graphene/Ag nanocomposites as highly sensitive SERS substrates. RSC Adv. 2016, 6, 91579–91583. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Y.; You, T.; Zhang, X.; Yang, N.; Wang, G.; Yin, P. Interfacial synthesis of three-dimensional hierarchical MoS2-NS@Ag-NPs nanocomposites as SERS nanosensor for ultrasensitive thiram detection. Nanoscale 2017, 9, 8879–8888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Guo, J.; Zhang, C.; Li, C.; Wang, M.; Li, Z.; Gao, S.; Chen, P.; Si, H.; Xu, S. A sensitive, uniform, reproducible and stable SERS substrate has been presented based on MoS2@Ag nanoparticles@pyramidal silicon. RSC Adv. 2017, 7, 5764–5773. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, J.; Zou, S.; Zhang, Z. Ag Nanorods-Oxide Hybrid Array Substrates: Synthesis, Characterization, and Applications in Surface-Enhanced Raman Scattering. Sensors 2017, 17, 1895. https://doi.org/10.3390/s17081895
Ma L, Li J, Zou S, Zhang Z. Ag Nanorods-Oxide Hybrid Array Substrates: Synthesis, Characterization, and Applications in Surface-Enhanced Raman Scattering. Sensors. 2017; 17(8):1895. https://doi.org/10.3390/s17081895
Chicago/Turabian StyleMa, Lingwei, Jianghao Li, Sumeng Zou, and Zhengjun Zhang. 2017. "Ag Nanorods-Oxide Hybrid Array Substrates: Synthesis, Characterization, and Applications in Surface-Enhanced Raman Scattering" Sensors 17, no. 8: 1895. https://doi.org/10.3390/s17081895




