The Role of Visual Noise in Influencing Mental Load and Fatigue in a Steady-State Motion Visual Evoked Potential-Based Brain-Computer Interface
Abstract
:1. Introduction
2. Materials and Methods
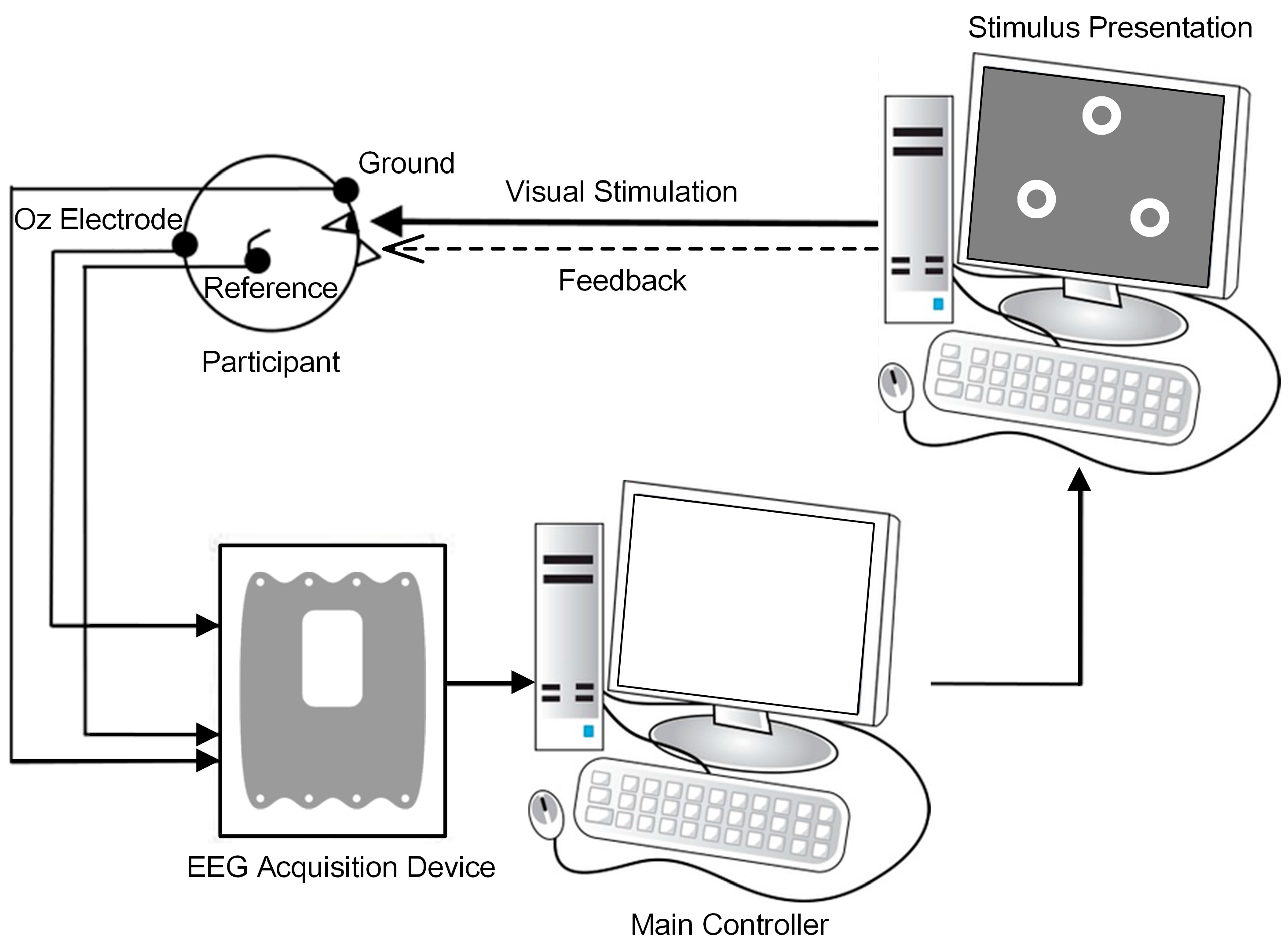
2.1. Participants and Recordings
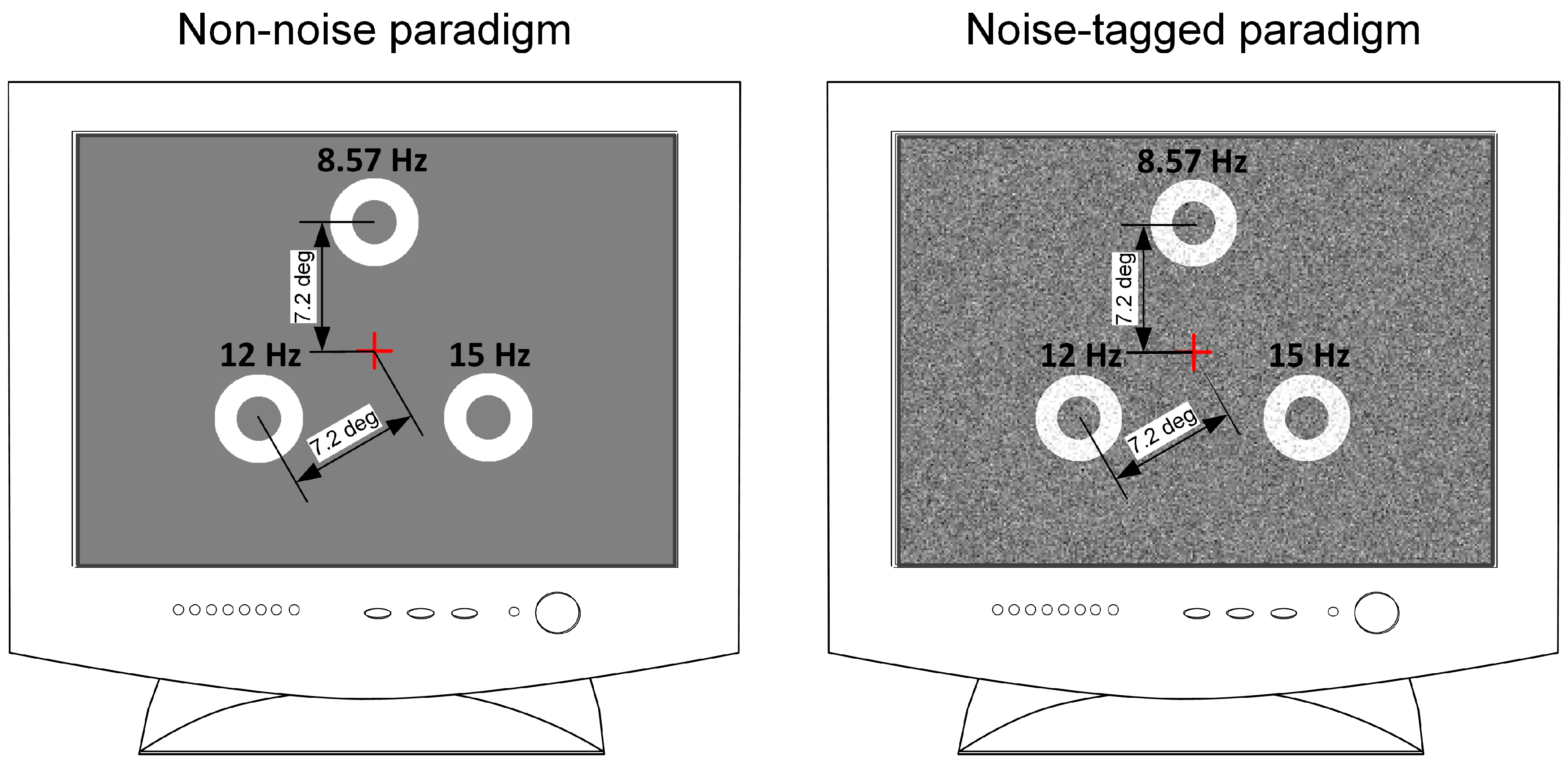
2.2. Stimulation Designs
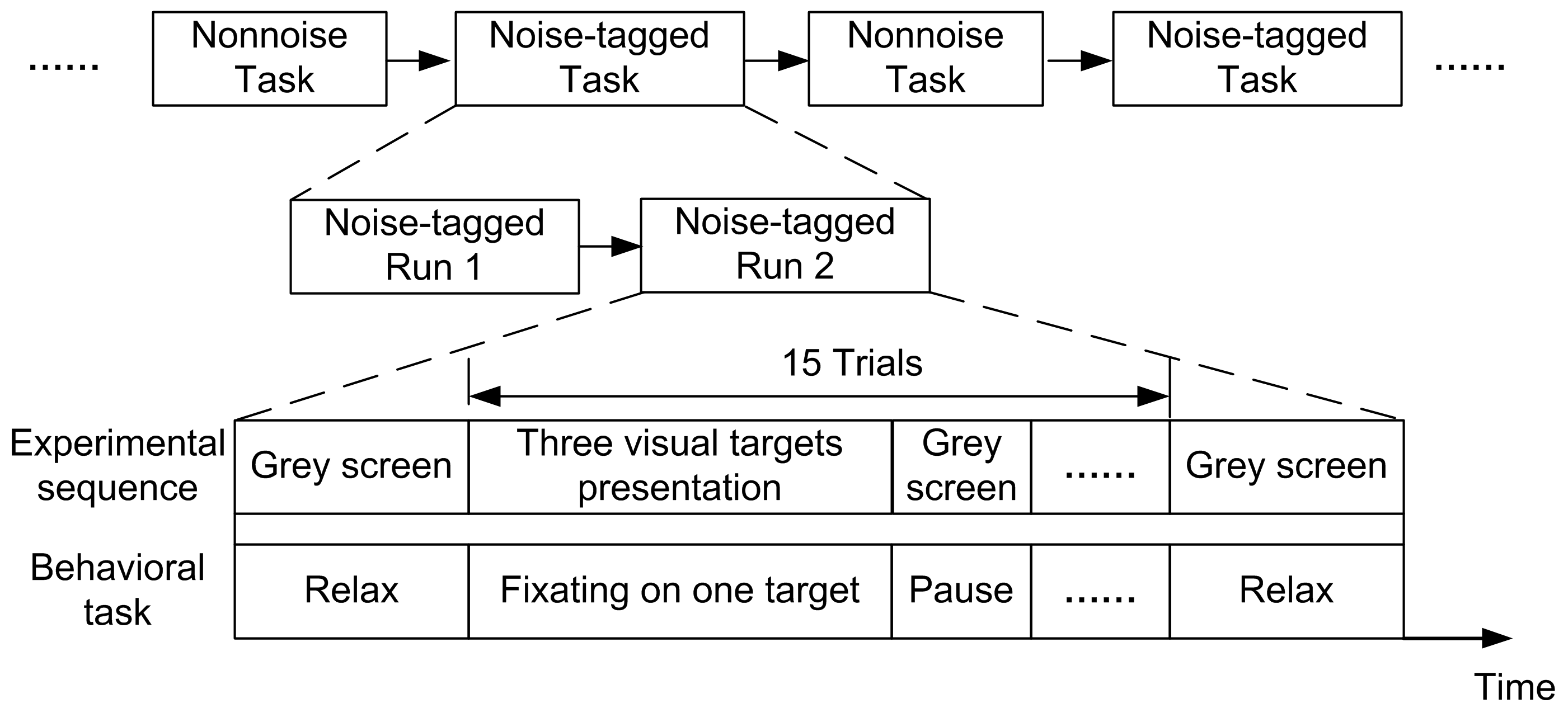
2.3. Online BCI Tasks
2.4. Online Target Identification
2.5. Statistical Analysis
3. Results
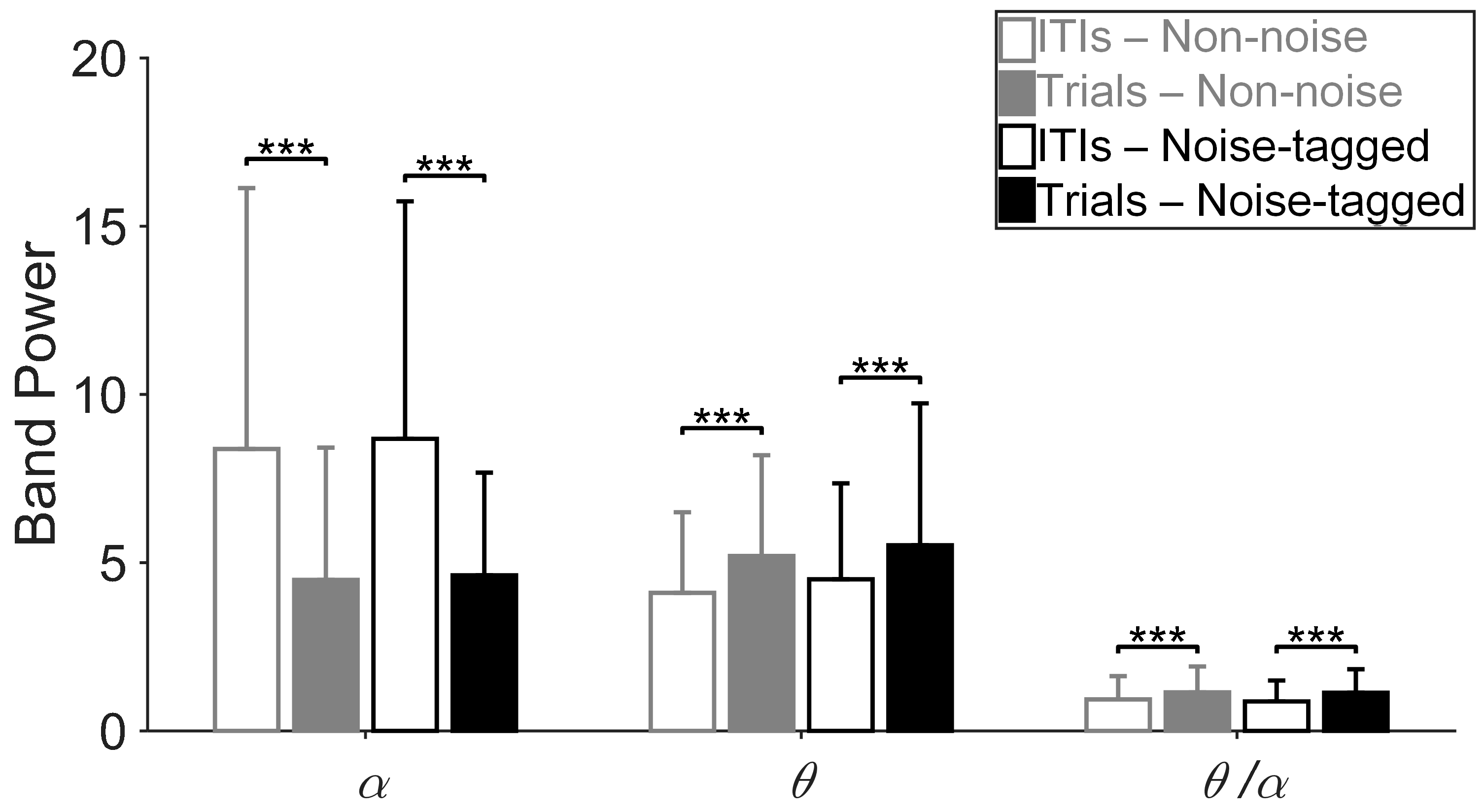
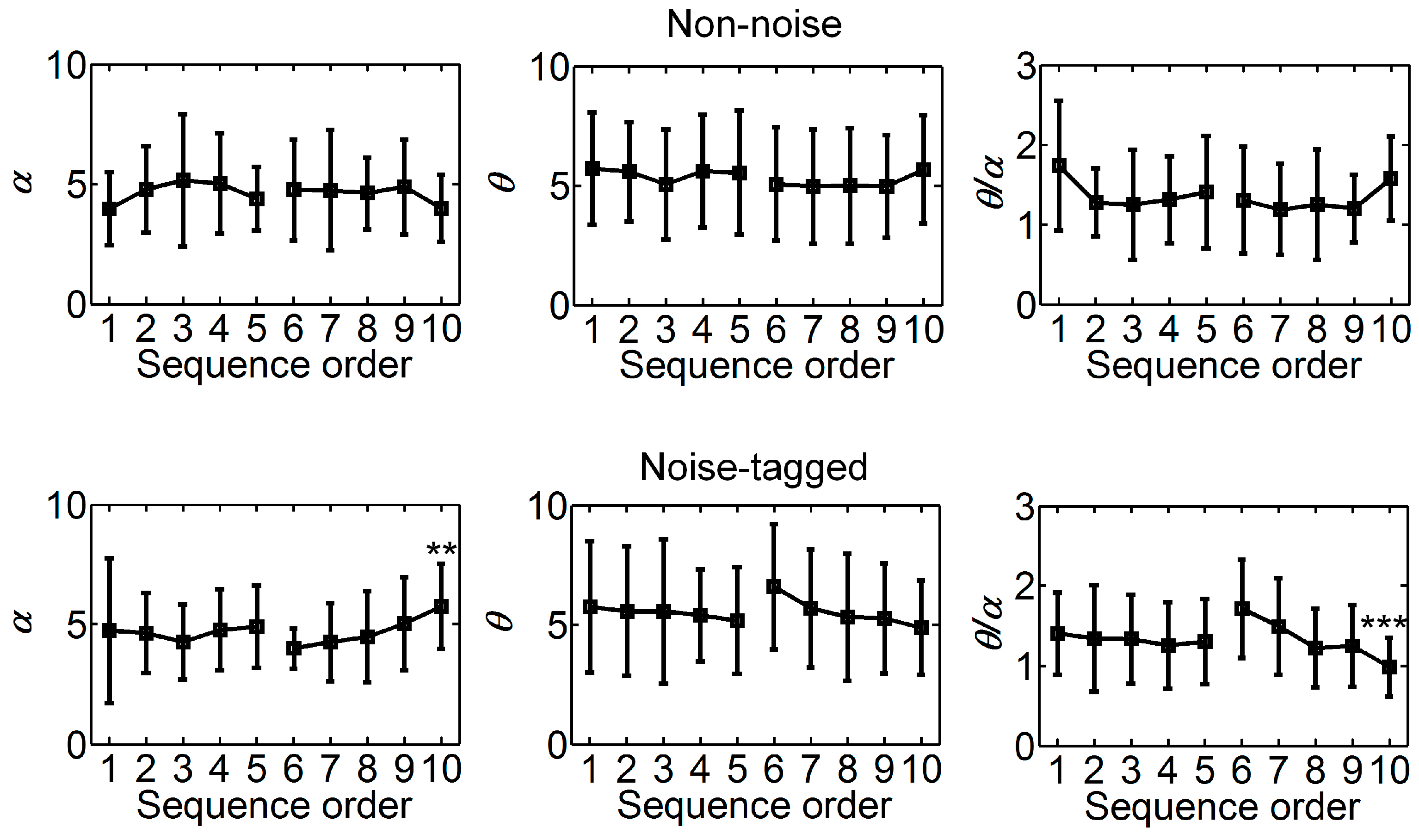
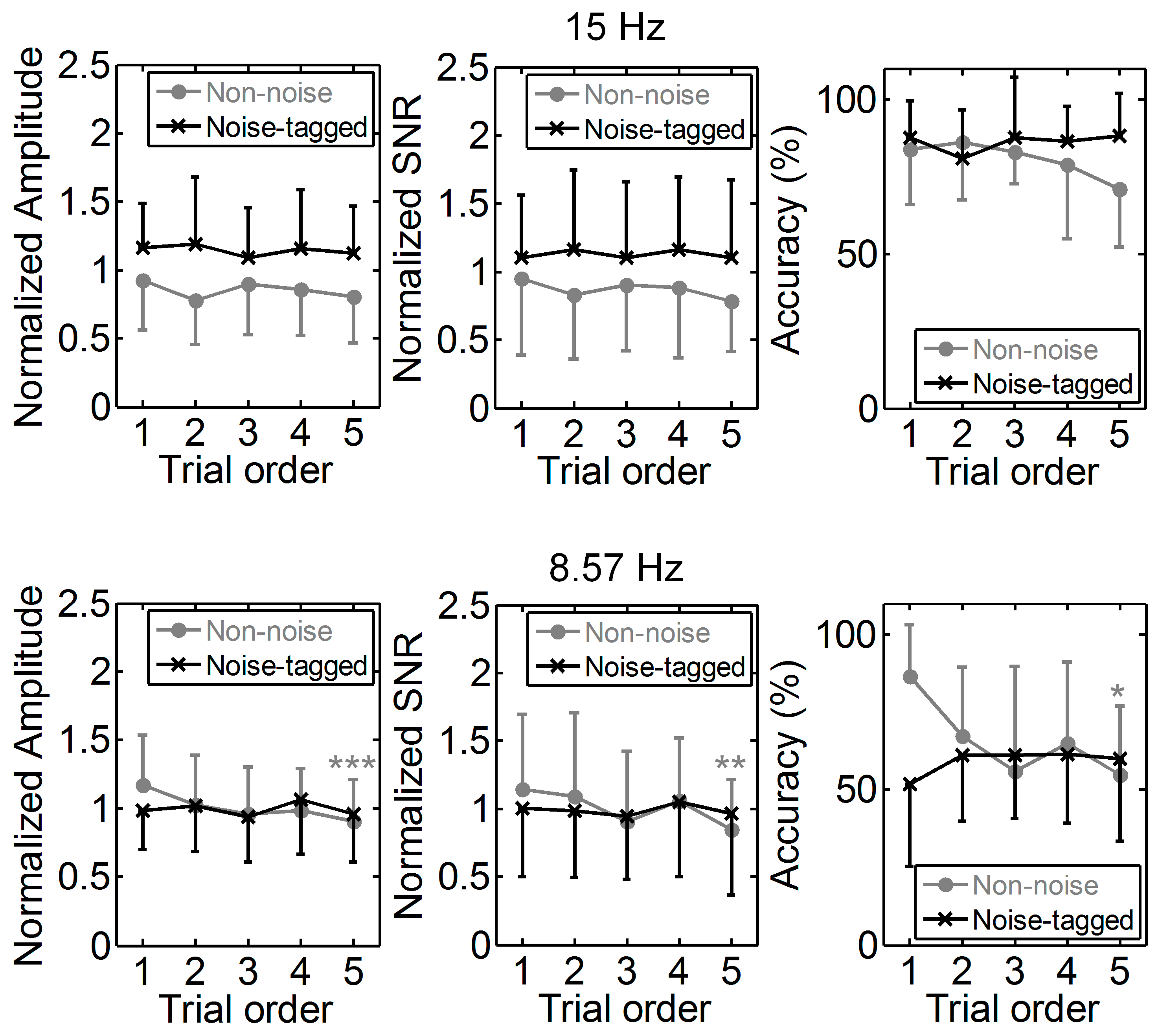
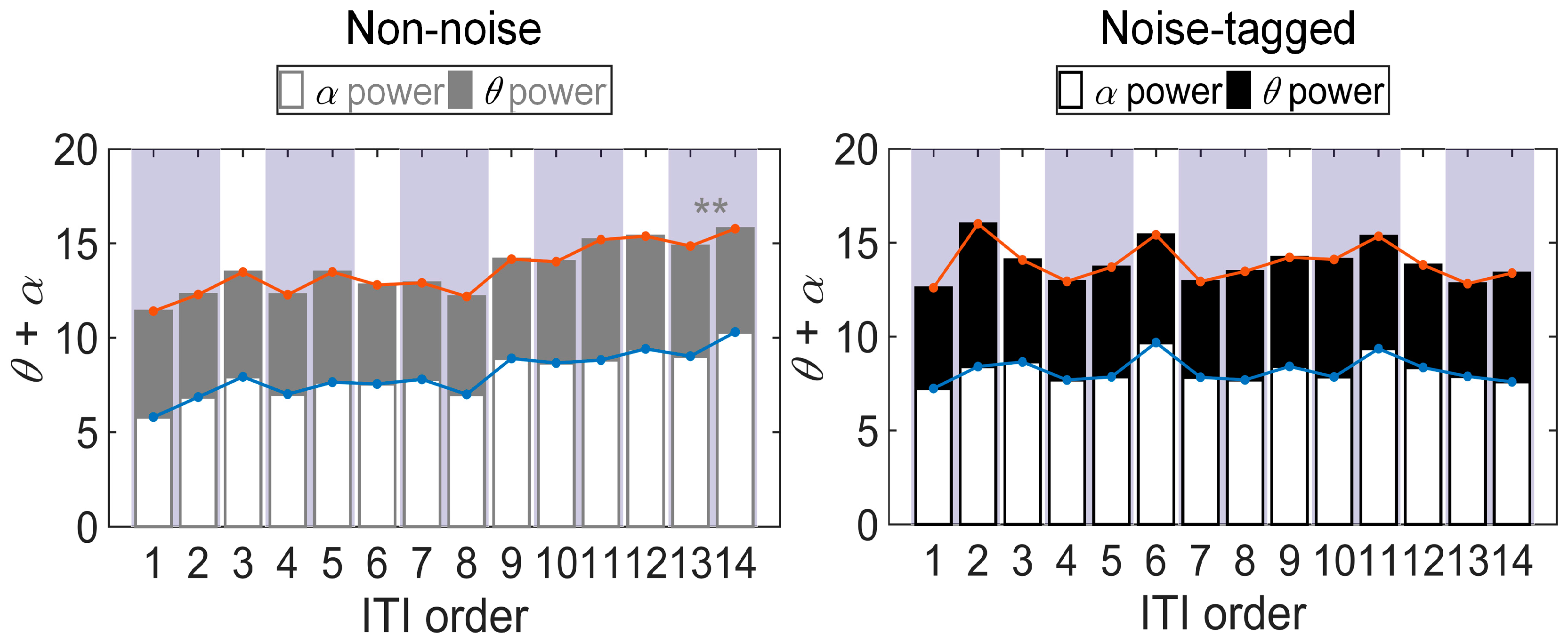
3.1. Influence of Visual Noise on Mental Load
3.2. Influence of Visual Noise on Fatigue
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mcfarland, D.J.; Wolpaw, J.R. Brain-computer interfaces for the operation of robotic and prosthetic devices. Adv. Comp. 2010, 79, 169–187. [Google Scholar]
- Mellinger, J.; Schalk, G.; Braun, C.; Preissl, H.; Rosenstiel, W.; Birbaumer, N.; Kübler, A. An MEG-based brain–computer interface (BCI). Neuroimage 2007, 36, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, N.; Mathiak, K.; Bock, S.W.; Scharnowski, F.; Veit, R.; Grodd, W.; Goebel, R.; Birbaumer, N. Principles of a brain-computer interface (BCI) based on real-time functional magnetic resonance imaging (fMRI). IEEE Trans. Biomed. Eng. 2004, 51, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Hong, K.S. Passive BCI based on drowsiness detection: An fNIRS study. Biomed. Opt. Express 2015, 6, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Naseer, N.; Hong, K.S. fNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Hong, K.S. Detection and classification of three-class initial dips from prefrontal cortex. Biomed. Opt. Express 2017, 8, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Turnip, A.; Hong, K.S.; Jeong, M.Y. Real-time feature extraction of P300 component using adaptive nonlinear principal component analysis. Biomed. Eng. Online 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Turnip, A.; Hong, K.S. Classifying mental activities from EEG-P300 signals using adaptive neural network. Int. J. Innov. Comp. Inf. Control 2012, 8, 6429–6443. [Google Scholar]
- Bi, L.; Lian, J.; Jie, K.; Lai, R.; Liu, Y. A speed and direction-based cursor control system with P300 and SSVEP. Biomed. Signal Process. Control 2014, 14, 126–133. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Cichocki, A.; Matsuno, F. A novel EOG/EEG hybrid human-machine interface adopting eye movements and ERPs: Application to robot control. IEEE Trans. Biomed. Eng. 2015, 62, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kubo, O.; Kitamura, M.; Yoshikawa, H. Neural network for human cognitive state estimation. In Proceedings of the 1994 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Munich, Germany, 12–16 September 1994; pp. 2176–2183. [Google Scholar]
- Kaseda, Y.; Jiang, C.; Kurokawa, K.; Mimori, Y.; Nakamura, S. Objective evaluation of fatigue by event-related potentials. J. Neurol. Sci. 1998, 158, 96–100. [Google Scholar] [CrossRef]
- Murata, A.; Uetake, A.; Takasawa, Y. Evaluation of mental fatigue using feature parameter extracted from event-related potential. Int. J. Ind. Ergon. 2005, 35, 761–770. [Google Scholar] [CrossRef]
- Cao, T.; Wan, F.; Wong, C.M.; da Cruz, J.N.; Hu, Y. Objective evaluation of fatigue by EEG spectral analysis in steady-state visual evoked potential-based brain-computer interfaces. Biomed. Eng. Online 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, S.; Park, M.C.; Park, S.; Whang, M. SSVEP and ERP measurement of cognitive fatigue caused by stereoscopic 3D. Neurosci. Lett. 2012, 525, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Jensen, O.; Gelfand, J.; Kounios, J.; Lisman, J.E. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex 2002, 12, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Peers, P.V.; Valdés-Sosa, M.; Galán, L.; García, L.; Martínez-Montes, E. Hemispheric modulations of alpha-band power reflect the rightward shift in attention induced by enhanced attentional load. Neuropsychologia 2009, 47, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schober, F.; Schellenberg, R.; Dimpfel, W. Reflection of mental exercise in the dynamic quantitative topographical EEG. Neuropsychobiology 1995, 31, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Stipacek, A.; Grabner, R.H.; Neuper, C.; Fink, A.; Neubauer, A.C. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci. Lett. 2003, 353, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Zatorre, R.J.; Hofle, N.; Caramanos, Z.; Gotman, J.; Petrides, M.; Evans, A.C. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J. Cognit. Neurosci. 1997, 9, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Käthner, I.; Wriessnegger, S.C.; Müller-Putz, G.R.; Kübler, A.; Halder, S. Effects of mental workload and fatigue on the P300, alpha and theta band power during operation of an ERP (P300) brain–computer interface. Biol. Psychol. 2014, 102, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Wascher, E.; Rasch, B.; Sänger, J.; Hoffmann, S.; Schneider, D.; Rinkenauer, G.; Heuer, H.; Gutberlet, I. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 2014, 96, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.A.; O’Hara, R.; Uijtdehaage, S.H.; Mandelkern, M.; Leuchter, A.F. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 408–414. [Google Scholar] [CrossRef]
- Laufs, H.; Kleinschmidt, A.; Beyerle, A.; Eger, E.; Salek-Haddadi, A.; Preibisch, C.; Krakow, K. EEG-correlated fMRI of human alpha activity. Neuroimage 2003, 19, 1463–1476. [Google Scholar] [CrossRef]
- Moss, F.; Ward, L.M.; Sannita, W.G. Stochastic resonance and sensory information processing: A tutorial and review of application. Clin. Neurophysiol. 2004, 115, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.; Wiersema, J.R.; van der Meere, J.J.; Roeyers, H. Context-dependent dynamic processes in attention deficit/hyperactivity disorder: Differentiating common and unique effects of state regulation deficits and delay aversion. Neuropsychol. Rev. 2010, 20, 86–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, M.D.; Abbott, D. What is stochastic resonance? definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 2009, 5, e1000348. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.D.; Ward, L.M. The benefits of noise in neural systems: Bridging theory and experiment. Nat. Rev. Neurosci. 2011, 12, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Benzi, R.; Sutera, A.; Vulpiani, A. The mechanism of stochastic resonance. J. Phys. A Math. Gen. 1981, 14, L453–L457. [Google Scholar] [CrossRef]
- Söderlund, G.B.; Sikström, S.; Loftesnes, J.M.; Sonuga-Barke, E.J. The effects of background white noise on memory performance in inattentive school children. Behav. Brain Funct. 2010, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglass, J.K.; Wilkens, L.; Pantazelou, E.; Moss, F. Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 1993, 365, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Miller, J.P. Broadband neural encoding in the cricket cereal sensory system enhanced by stochastic resonance. Nature 1996, 380, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; MacLean, S.E.; Kirschner, A. Stochastic resonance modulates neural synchronization within and between cortical sources. PLoS ONE 2010, 5, e14371. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.D.; Stocks, N.G.; Abbott, D. Optimal stimulus and noise distributions for information transmission via suprathreshold stochastic resonance. Phys. Rev. E 2007, 75, 061105. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, G.; Wang, J.; Zhang, S.; Zhang, F.; Li, Y.; Han, C.; Li, L. Addition of visual noise boosts evoked potential-based brain-computer interface. Sci. Rep. 2014, 4, 4953. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.; Selen, L.P.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008, 9, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Meigen, T. Do’s and don’ts in Fourier analysis of steady-state potentials. Doc. Ophthalmol. 1999, 99, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Palaniappan, R. Analogue mouse pointer control via an online steady state visual evoked potential (SSVEP) brain–computer interface. J. Neural Eng. 2011, 8, 025026. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.P.; Bach, M. Adaptation characteristics of steady-state motion visual evoked potentials. Clin. Neurophysiol. 2003, 114, 1359–1366. [Google Scholar] [CrossRef]
- Ng, K.B.; Bradley, A.P.; Cunnington, R. Stimulus specificity of a steady-state visual-evoked potential-based brain-computer interface. J. Neural Eng. 2012, 9, 036008. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, G.; Wang, J.; Zhang, F.; Zhang, Y. Steady-state motion visual evoked potentials produced by oscillating newton’s rings: Implications for brain-computer interfaces. PLoS ONE 2012, 7, e39707. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G. The video toolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, I.; Davila, C.E.; Srebro, R. A new multi-harmonic statistic for the detection of steady-state evoked potentials. In Proceedings of the 1997 Sixteenth Southern Biomedical Engineering Conference, Biloxi, MS, USA, 4–6 April 1997; pp. 441–444. [Google Scholar]
- Wang, Y.; Wang, R.; Gao, X.; Hong, B.; Gao, S. A practical VEP-based brain-computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Vialatte, F.B.; Maurice, M.; Dauwels, J.; Cichocki, A. Steady state visual evoked potentials in the delta range (0.5–5 Hz). In Proceedings of the 2008 15th International Conference on Neural Information Processing (ICONIP), Auckland, New Zealand, 25–28 November 2008; pp. 400–407. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, A.M.; Hogervorst, M.A.; Van Erp, J.B.; Heffelaar, T.; Zimmerman, P.H.; Oostenveld, R. Estimating workload using EEG spectral power and ERPs in the n-back task. J. Neural Eng. 2012, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.; Xu, S.; Zhu, S.; Fang, Z.; Spaeth, A.M.; Xin, Y.; Feng, T.; Rao, H. Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. Neuroimage 2015, 120, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.P.; Bach, M. Adaptation dynamics in pattern-reversal visual evoked potentials. Doc. Ophthalmol. 2001, 102, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Boksem, M.A.; Meijman, T.F.; Lorist, M.M. Effects of mental fatigue on attention: An ERP study. Cognit. Brain Res. 2005, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cordo, P.; Inglis, J.T.; Verschueren, S.; Collins, J.J. Noise in human muscle spindles. Nature 1996, 383, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Simonotto, E.; Riani, M.; Seife, C.; Roberts, M.; Twitty, J.; Moss, F. Visual perception of stochastic resonance. Phys. Rev. Lett. 1997, 78, 1186–1189. [Google Scholar] [CrossRef]
- Piana, M.; Canfora, M.; Riani, M. Role of noise in image processing by the human perceptive system. Phys. Rev. E 2000, 62, 1104–1109. [Google Scholar] [CrossRef]
- Hidaka, I.; Nozaki, D.; Yamamoto, Y. Functional stochastic resonance in the human brain: Noise induced sensitization of baroreflex system. Phys. Rev. Lett. 2000, 85, 3740–3743. [Google Scholar] [CrossRef] [PubMed]
- Soma, R.; Nozaki, D.; Kwak, S.; Yamamoto, Y. 1/f noise outperforms white noise in sensitizing baroreflex function in the human brain. Phys. Rev. Lett. 2003, 91, 078101. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Psychophysics of stochastic resonance. Fluct. Noise. Lett. 2004, 4, L11–L21. [Google Scholar] [CrossRef]
- Söderlund, G.; Sikström, S.; Smart, A. Listen to the noise: Noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry 2007, 48, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Kitajo, K.; Yamanaka, K.; Ward, L.M.; Yamamoto, Y. Stochastic resonance in attention control. Europhys. Lett. 2006, 76, 1029–1035. [Google Scholar] [CrossRef]
- Walther, D.B.; Caddigan, E.; Fei-Fei, L.; Beck, D.M. Natural scene categories revealed in distributed patterns of activity in the human brain. J. Neurosci. 2009, 29, 10573–10581. [Google Scholar] [CrossRef] [PubMed]
- Kitajo, K.; Nozaki, D.; Ward, L.M.; Yamamoto, Y. Behavioral stochastic resonance within the human brain. Phys. Rev. Lett. 2003, 90, 218103. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.B.; Gossen, E.R.; Jones, K.E. Neuronal variability: Noise or part of the signal? Nat. Rev. Neurosci. 2005, 6, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Womelsdorf, T.; Fries, P. The role of neuronal synchronization in selective attention. Curr. Opin. Neurobiol. 2007, 17, 154–160. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.D. Information capacity of stochastic pooling networks is achieved by discrete inputs. Phys. Rev. E 2009, 79, 041107. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav. Brain Sci. 2000, 23, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Varshney, L.R.; Varshney, P.K. Noise-enhanced information systems. Proc. IEEE 2014, 102, 1607–1621. [Google Scholar] [CrossRef]
- Khan, M.J.; Hong, M.J.; Hong, K.S. Decoding of four movement directions using hybrid NIRS-EEG brain-computer interface. Front. Hum. Neurosci. 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Hong, K.S. Hybrid EEG-fNIRS-based eight-command decoding for BCI: Application to quadcopter control. Front. Neurorob. 2017, 11, 6. [Google Scholar] [CrossRef] [PubMed]

| Participants | 15 Hz | 8.57 Hz | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-Noise | Noise-Tagged | Non-Noise | Noise-Tagged | |||||
| TPR (%) | FPR (%) | TPR (%) | FPR (%) | TPR (%) | FPR (%) | TPR (%) | FPR (%) | |
| 1 | 95 | 40 | 100 | 0 | 88 | 36 | 56 | 0 |
| 2 | 90 | 15 | 95 | 5 | 85 | 35 | 92.5 | 2.5 |
| 3 | 90 | 35 | 85 | 25 | 65 | 20 | 70 | 5 |
| 4 | 90 | 40 | 96.67 | 13.33 | 56.67 | 23.33 | 66.67 | 3.33 |
| 5 | 86.67 | 20 | 93.33 | 13.33 | 70 | 20 | 50 | 15 |
| 6 | 76.67 | 33.33 | 86.67 | 23.33 | 84 | 52 | 68 | 8 |
| 7 | 53.33 | 26.67 | 80 | 20 | 53.33 | 46.67 | 80 | 20 |
| 8 | 80 | 36 | 96 | 20 | 70 | 20 | 100 | 0 |
| 9 | 73.33 | 43.33 | 100 | 16.67 | 66.67 | 26.67 | 56.67 | 6.67 |
| 10 | 60 | 30 | 100 | 25 | 35 | 40 | 65 | 10 |
| 11 | 62.5 | 37.5 | 80 | 27.5 | 62.5 | 77.5 | 60 | 27.5 |
| 12 | 60 | 24 | 72 | 12 | 80 | 23.33 | 53.33 | 6.67 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Xu, G.; Luo, A.; Li, M.; Zhang, S.; Han, C.; Yan, W. The Role of Visual Noise in Influencing Mental Load and Fatigue in a Steady-State Motion Visual Evoked Potential-Based Brain-Computer Interface. Sensors 2017, 17, 1873. https://doi.org/10.3390/s17081873
Xie J, Xu G, Luo A, Li M, Zhang S, Han C, Yan W. The Role of Visual Noise in Influencing Mental Load and Fatigue in a Steady-State Motion Visual Evoked Potential-Based Brain-Computer Interface. Sensors. 2017; 17(8):1873. https://doi.org/10.3390/s17081873
Chicago/Turabian StyleXie, Jun, Guanghua Xu, Ailing Luo, Min Li, Sicong Zhang, Chengcheng Han, and Wenqiang Yan. 2017. "The Role of Visual Noise in Influencing Mental Load and Fatigue in a Steady-State Motion Visual Evoked Potential-Based Brain-Computer Interface" Sensors 17, no. 8: 1873. https://doi.org/10.3390/s17081873




