A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of AgNPs/MoS2 Modified Electrodes
2.3. Electrochemical Measurements
2.4. Materials Characterization
2.5. Real Sample Collection and Preparation
3. Results
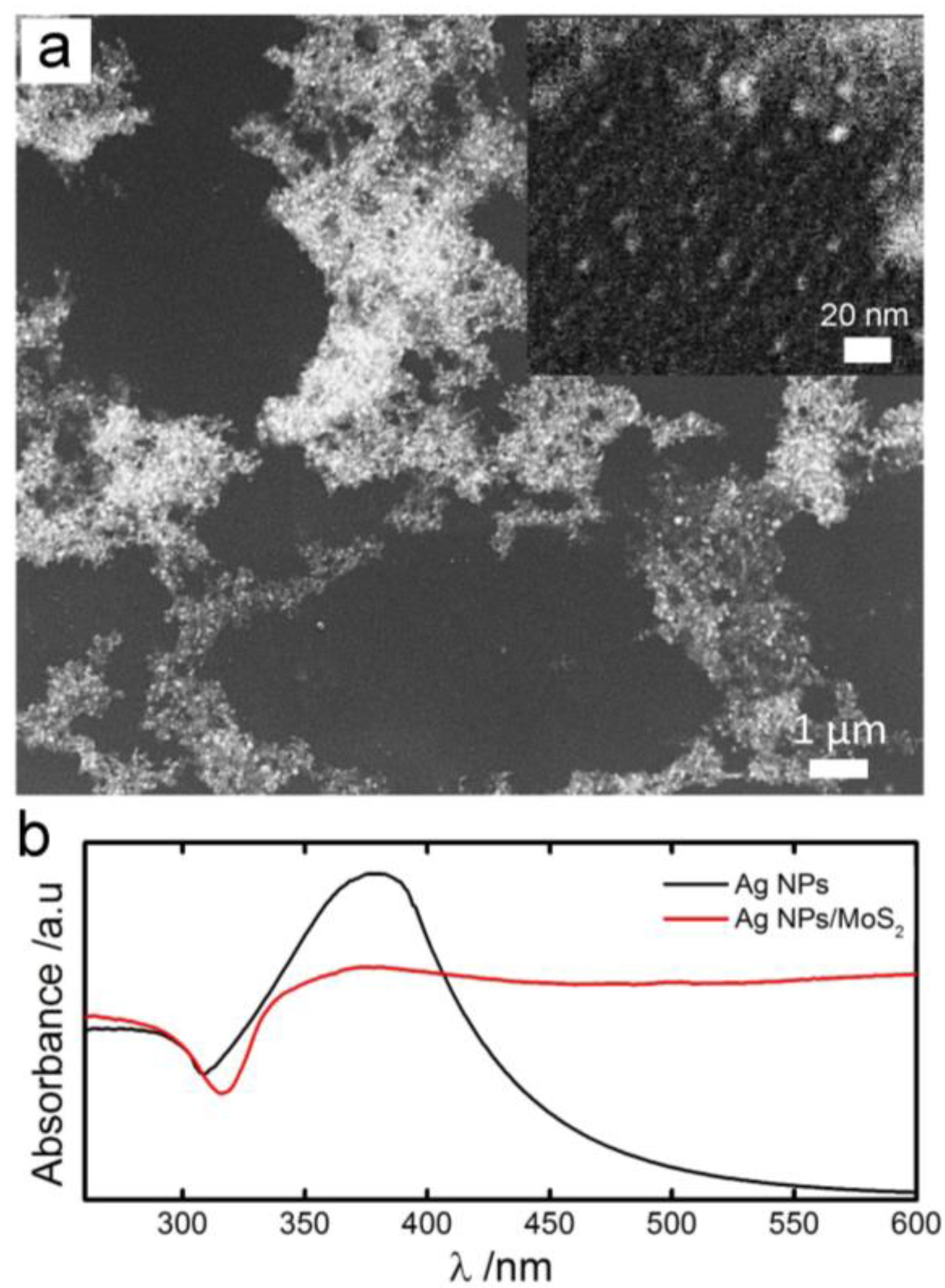
3.1. Materials Characterization of the Biosensor
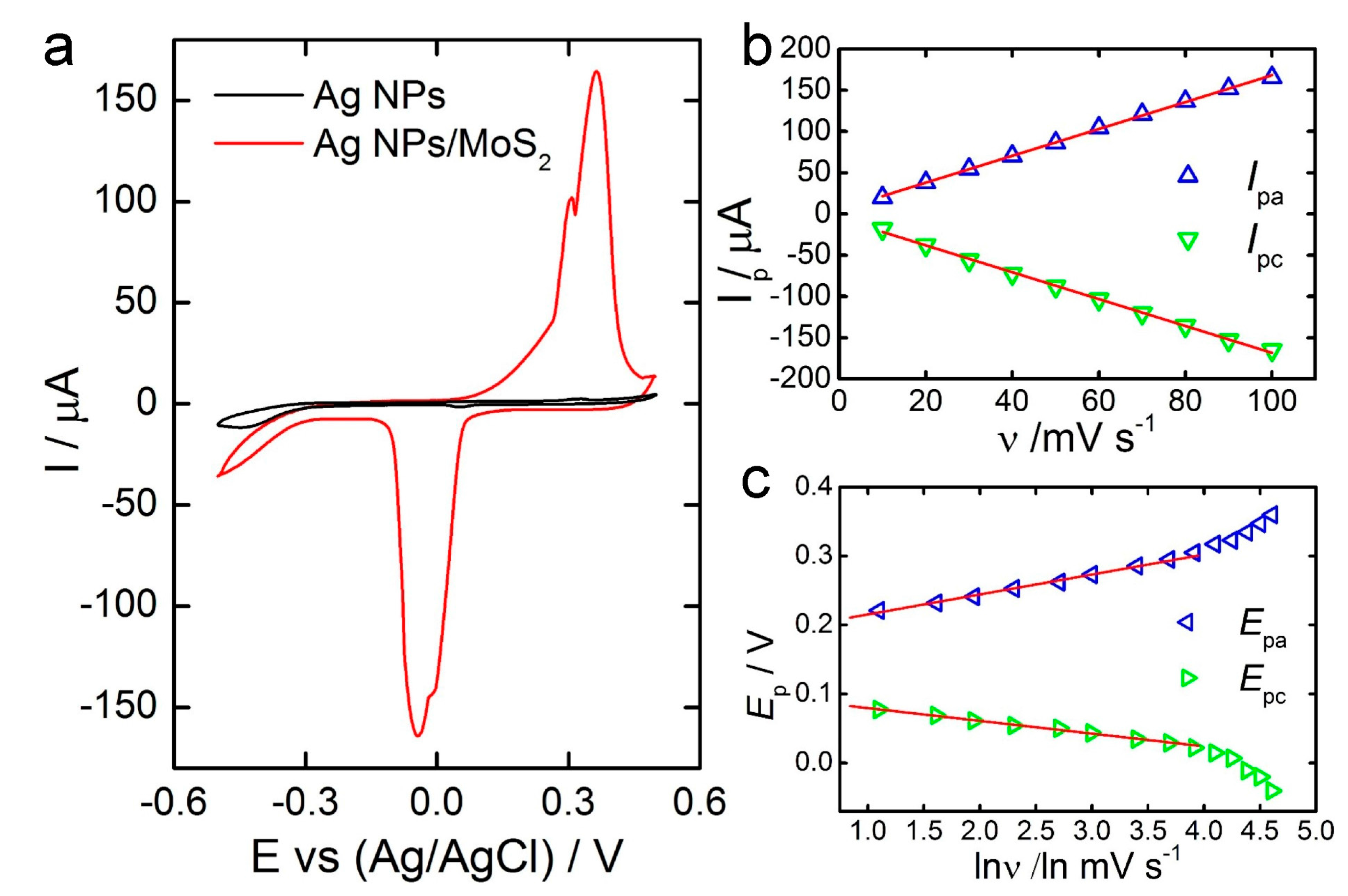
3.2. Electrochemical Behavior
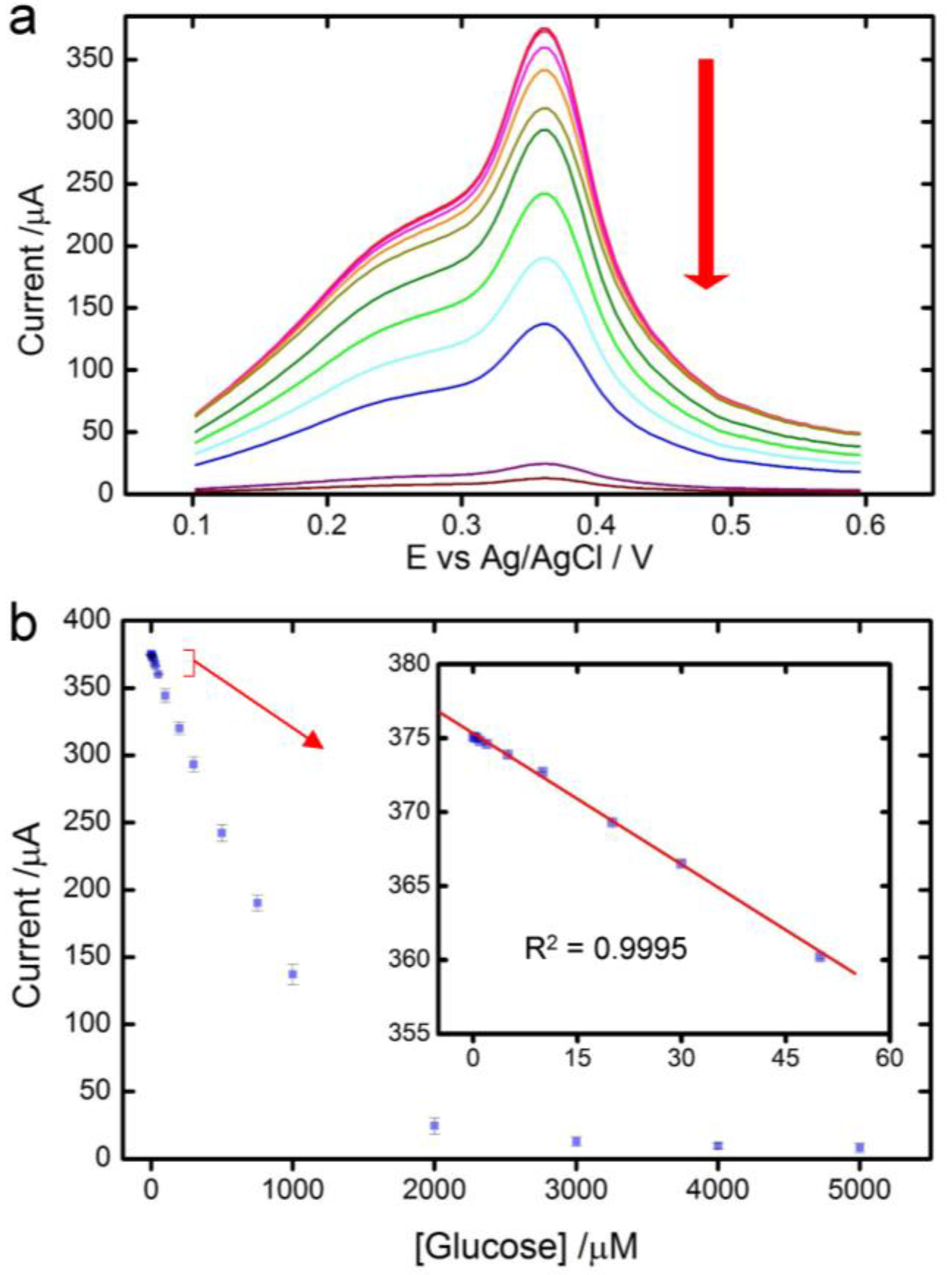
3.3. Glucose Detection
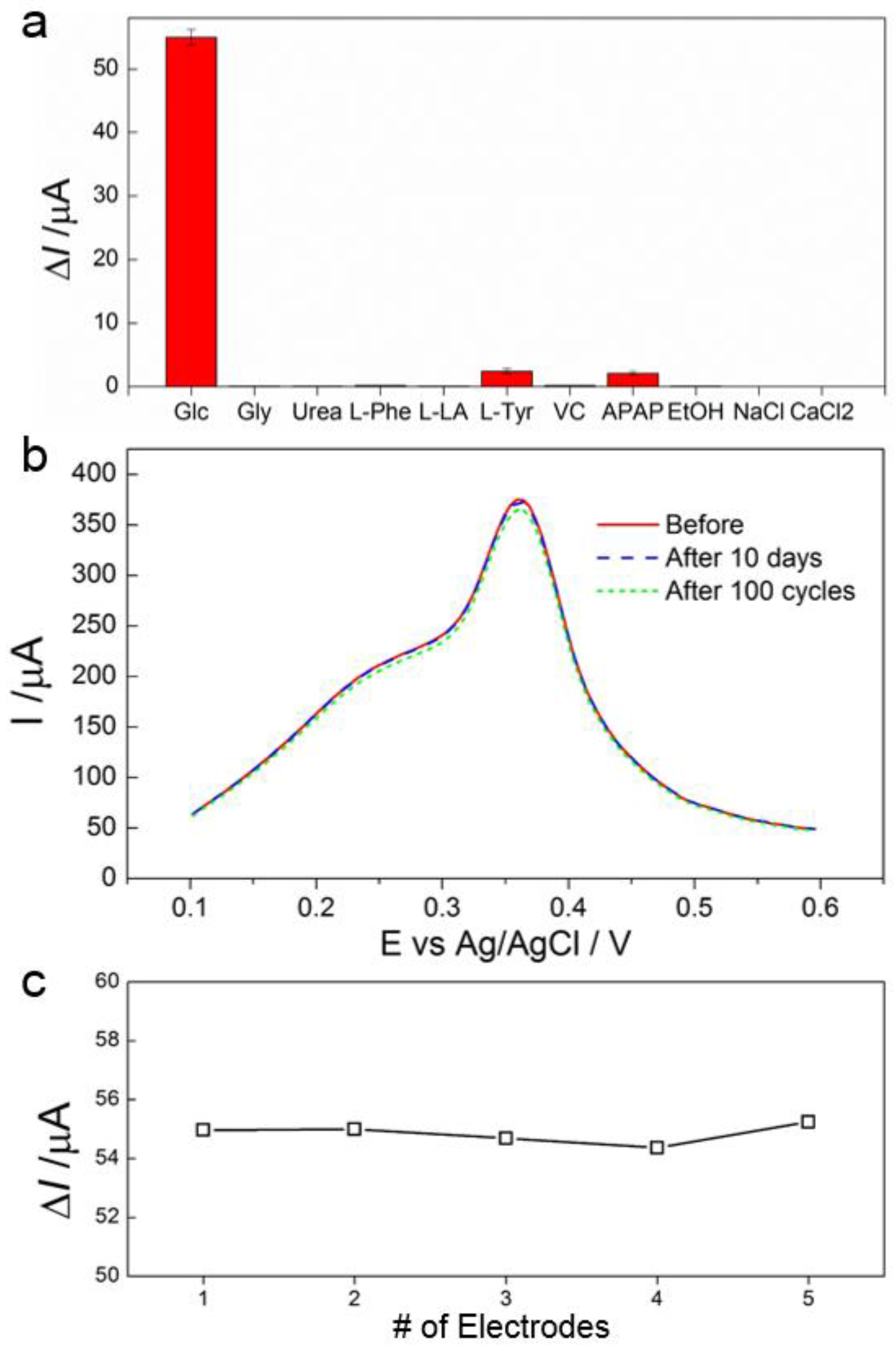
3.4. Selectivity, Stability and Reproducibility of the Biosensor
3.5. Glucose Detection in Real Samples
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Israel Defense Forces. International Diabetes Federation Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Xue, Z.; Huang, J.; Joseph, W. Electrochemical Sensors, Biosensors and Their Biomedical Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tao, Y.; Yang, Y.; Xiang, Y.; Li, G. In vitro analysis of DNA—Protein interactions in gene transcription using dnazyme-based electrochemical assay. Anal. Chem. 2017, 89, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Wu, S.; Feng, C.; Li, G. Highly sensitive protein detection based on dnazyme cycling activated surface assembly of peptide decorated nanoparticles. Electrochem. Commun. 2016, 71, 84–88. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, L.; Mao, X.; Li, G. Highly sensitive protein detection based on smart hybrid nanocomposite-controlled switch of DNA polymerase activity. ACS Appl. Mater. Interfaces 2016, 8, 28202–28207. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Mao, X.; Yang, Y.; Zhu, X.; Yin, Y.; Li, G. Rolling circle amplification in electrochemical biosensor with biomedical applications. J. Electroanal. Chem. 2016, 781, 223–232. [Google Scholar] [CrossRef]
- Sternberg, R.; Barrau, M.-B.; Gangiotti, L.; Thévenot, D.R.; Bindra, D.S.; Wilson, G.S.; Velho, G.; Froguel, P.; Reach, G. Study and development of multilayer needle-type enzyme-based glucose microsensors. Biosensors 1989, 4, 27–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilson, G.S. In vitro and in vivo evaluation of oxygen effects on a glucose oxidase based implantable glucose sensor. Anal. Chim. Acta 1993, 281, 513–520. [Google Scholar] [CrossRef]
- Liu, S.; Ju, H. Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidal gold modified carbon paste electrode. Biosens. Bioelectron. 2003, 19, 177–183. [Google Scholar] [CrossRef]
- Feng, X.; Cheng, H.; Pan, Y.; Zheng, H. Development of glucose biosensors based on nanostructured graphene-conducting polyaniline composite. Biosens. Bioelectron. 2015, 70, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shao, C.; Liang, B.; Liu, A. Genetically engineered phage-templated MnO2 nanowires: synthesis and their application in electrochemical glucose biosensor operated at neutral pH condition. ACS Appl. Mater. Interfaces 2016, 8, 13768–13776. [Google Scholar] [CrossRef] [PubMed]
- Chaichi, M.J.; Ehsani, M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol–H2O2–gold nanoparticle chemiluminescence detection system. Sens. Actuators B Chem. 2016, 223, 713–722. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, B.; Fang, L.; Ma, G.; Yang, G.; Zhu, Q.; Chen, S.; Ye, X. Antifouling zwitterionic coating via electrochemically mediated atom transfer radical polymerization on enzyme-based glucose sensors for long-time stability in 37 °C serum. Langmuir 2016, 32, 11763–11770. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sheng, Q.; Shen, Y.; Zheng, J. Enhanced direct electron transfer of glucose oxidase based on gold nanoprism and its application in biosensing. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 113–118. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Simanaityte, R.; Ramanaviciene, A.; Glumbokaite, L.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on a graphite rod electrode layer-by-layer modified with 1, 10-phenanthroline-5, 6-dione and glucose oxidase. Talanta 2017, 171, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xia, X.; Du, Y.; Ye, W.; Wang, C. Amperometric glucose biosensor based on aupd modified reduced graphene oxide/polyimide film with glucose oxidase. J. Electrochem. Soc. 2017, 164, B285–B291. [Google Scholar] [CrossRef]
- Mehmeti, E.; Stanković, D.M.; Chaiyo, S.; Zavasnik, J.; Žagar, K.; Kalcher, K. Wiring of glucose oxidase with graphene nanoribbons: An electrochemical third generation glucose biosensor. Microchim. Acta 2017, 184, 1127–1134. [Google Scholar] [CrossRef]
- Gong, C.; Chen, J.; Song, Y.; Sun, M.; Song, Y.; Guo, Q.; Wang, L. A glucose biosensor based on the polymerization of aniline induced by a bio-interphase of glucose oxidase and horseradish peroxidase. Anal. Methods 2016, 8, 1513–1519. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Panchbhai, A.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Res. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Jurysta, C.; Bulur, N.; Oguzhan, B.; Satman, I.; Yilmaz, T.M.; Malaisse, W.J.; Sener, A. Salivary glucose concentration and excretion in normal and diabetic subjects. BioMed Res. Int. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, I.V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Li, H.; Li, H.; Jiang, L.; Shi, Y.; Sun, Y.; Lu, G.; Zhang, Q.; Chen, X.; Zhang, H. Single-Layer MoS2 Phototransistors. ACS Nano 2011, 6, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Amaratunga, G.A. Thin Films of Fullerene-Like MoS2 Nanoparticles with Ultra-Low Friction and Wear. Nature 2000, 407, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, L.; Lee, Y.-H.; Shi, Y.; Hsu, A.; Chin, M.L.; Li, L.-J.; Dubey, M.; Kong, J.; Palacios, T. Integrated circuits based on bilayer MoS2 transistors. Nano Lett. 2012, 12, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1t phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lee, G.-H.; Kim, Y.D.; Arefe, G.; Huang, P.Y.; Lee, C.-H.; Chenet, D.A.; Zhang, X.; Wang, L.; Ye, F. Multi-terminal transport measurements of MoS2 using a van der Waals heterostructure device platform. Nat. Nanotechnol. 2015, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Jariwala, D.; Kim, I.S.; Chen, K.-S.; Marks, T.J.; Lauhon, L.J.; Hersam, M.C. Gate-Tunable Memristive Phenomena Mediated by Grain Boundaries in Single-Layer MoS2. Nat. Nanotechnol. 2015, 10, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Fullon, R.; Yang, J.; Silva, C.C.C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kong, D.; Hsu, P.-C.; Yuan, H.; Lee, H.-W.; Liu, Y.; Wang, H.; Wang, S.; Yan, K.; Lin, D. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 2016, 11, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Woollam, J.A.; Somoano, R.B. Physics and chemistry of MoS2 intercalation compounds. Mater. Sci. Eng. 1977, 31, 289–295. [Google Scholar] [CrossRef]
- Benavente, E.; Santa Ana, M.; Mendizábal, F.; González, G. Intercalation chemistry of molybdenum disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Wang, J.Z.; Lu, L.; Lotya, M.; Coleman, J.N.; Chou, S.L.; Liu, H.K.; Minett, A.I.; Chen, J. Development of MoS2–CNT composite thin film from layered MoS2 for lithium batteries. Adv. Energy Mater. 2013, 3, 798–805. [Google Scholar] [CrossRef]
- Yu, H.; Ma, C.; Ge, B.; Chen, Y.; Xu, Z.; Zhu, C.; Li, C.; Ouyang, Q.; Gao, P.; Li, J. Three-Dimensional Hierarchical Architectures Constructed by Graphene/MoS2 Nanoflake Arrays and Their Rapid Charging/Discharging properties as Lithium-Ion Battery Anodes. Chem. Eur. J. 2013, 19, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, Y.T.; Xie, X.M.; Zhu, X.D. Coordination-driven hierarchical assembly of silver nanoparticles on MoS2 nanosheets for improved lithium storage. Chem Asian J. 2014, 9, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, T.; Gu, S.; Zhi, J.; Yang, J.; Li, G. An electrochemical biosensor for the assay of alpha-fetoprotein-L3 with practical applications. Biosens. Bioelectron. 2017, 87, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Baset, S.; Akbari, H.; Zeynali, H.; Shafie, M. Size measurement of metal and semiconductor nanoparticles via UV-Vis absorption spectra. Dig. J. Nanomater. Biostruct. 2011, 6, 709–716. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Mitsumori, M.; Kano, Y. Noninvasively measuring blood glucose using saliva. IEEE Eng. Med. Biol. 1998, 17, 59–63. [Google Scholar] [CrossRef]
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based noninvasive diabetes sensing technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.K.; Sadhukhan, M.; Barman, S. Ordered assemblies of silver nanoparticles on carbon nitride sheets and their application in the nonenzymatic sensing of hydrogen peroxide and glucose. J. Mater. Chem. B 2015, 3, 1289–1300. [Google Scholar] [CrossRef]
- Fang, B.; Gu, A.; Wang, G.; Wang, W.; Feng, Y.; Zhang, C.; Zhang, X. Silver oxide nanowalls grown on Cu substrate as an enzymeless glucose sensor. ACS Appl. Mater. Interfaces 2009, 1, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Vaseem, M.; Tripathy, N.; Hahn, Y.-B. Wide linear-range detecting nonenzymatic glucose biosensor based on CuO nanoparticles inkjet-printed on electrodes. Anal. Chem. 2013, 85, 10448–10454. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ma, Z. Amperometric glucose biosensor based on a triangular silver nanoprisms/chitosan composite film as immobilization matrix. Biosens. Bioelectron. 2010, 26, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuwen, L.; Han, Y.; Tian, J.; Zhu, X.; Weng, L.; Wang, L. Reduced graphene oxide/pamam-silver nanoparticles nanocomposite modified electrode for direct electrochemistry of glucose oxidase and glucose sensing. Biosens. Bioelectron. 2012, 36, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Krishnan, S.; Chakaravarthy, S.; Hernandez-Rangel, A.; Prokhorov, E.; Luna-Bárcenas, G.; Esparza, R.; Meyyappan, M. Chitosan supported silver nanowires as a platform for direct electrochemistry and highly sensitive electrochemical glucose biosensing. RSC Adv. 2016, 6, 20102–20108. [Google Scholar] [CrossRef]
| Sensor | LoD (μM) | Sensitivity (μA·mM−1·cm−2) | Linear Range (μM) | Reference |
|---|---|---|---|---|
| GC/Colloidal AgNPs/MoS2 | 0.03 | 9044.6 | 0.1–1000 | This work |
| GC/Ag-CNx | 0.6 | 97 | 1–100 | [44] |
| GC/Cu-Ag2O NWs | 10 | 298.2 | 200–3200 | [45] |
| CuNCs-DLEG | 0.25 | 4532 | 25–4500 | [46] |
| CuO NPs/Ag/Si | 0.5 | 2762.5 | 50–18,450 | [47] |
| Pt/AgTNPs/CHIT/GOx | 1 | 67.17 | 3–3000 | [48] |
| rGO/PAMAM/Ag/GOx | 4.5 | 75.72 | 32–1890 | [49] |
| AgNWs/CS/GOx | 2.1 | 16.72 | 1000–15,000 | [50] |
| Samples | Glucose Concentration of Samples (μM) | Glucose Added (μM) | Glucose Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Sweat | 80.71 | 7 | 86.54 | 98.6 | 1.1 |
| 100 | 179.82 | 99.5 | 1.1 | ||
| Saliva | 7.42 | 3 | 10.11 | 97.0 | 1.7 |
| 20 | 27.02 | 98.5 | 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, K.; Poulter, B.; Dudgeon, J.; Li, S.-E.; Ma, X. A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2. Sensors 2017, 17, 1807. https://doi.org/10.3390/s17081807
Anderson K, Poulter B, Dudgeon J, Li S-E, Ma X. A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2. Sensors. 2017; 17(8):1807. https://doi.org/10.3390/s17081807
Chicago/Turabian StyleAnderson, Kash, Benjamin Poulter, John Dudgeon, Shu-En Li, and Xiang Ma. 2017. "A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2" Sensors 17, no. 8: 1807. https://doi.org/10.3390/s17081807





