A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus and Reagents
2.2. Procedures
2.2.1. Fabrication of PEDOT:PSS/20%IL/SPCE
2.2.2. Sessile Contact Angle Measurement
3. Results and Discussion
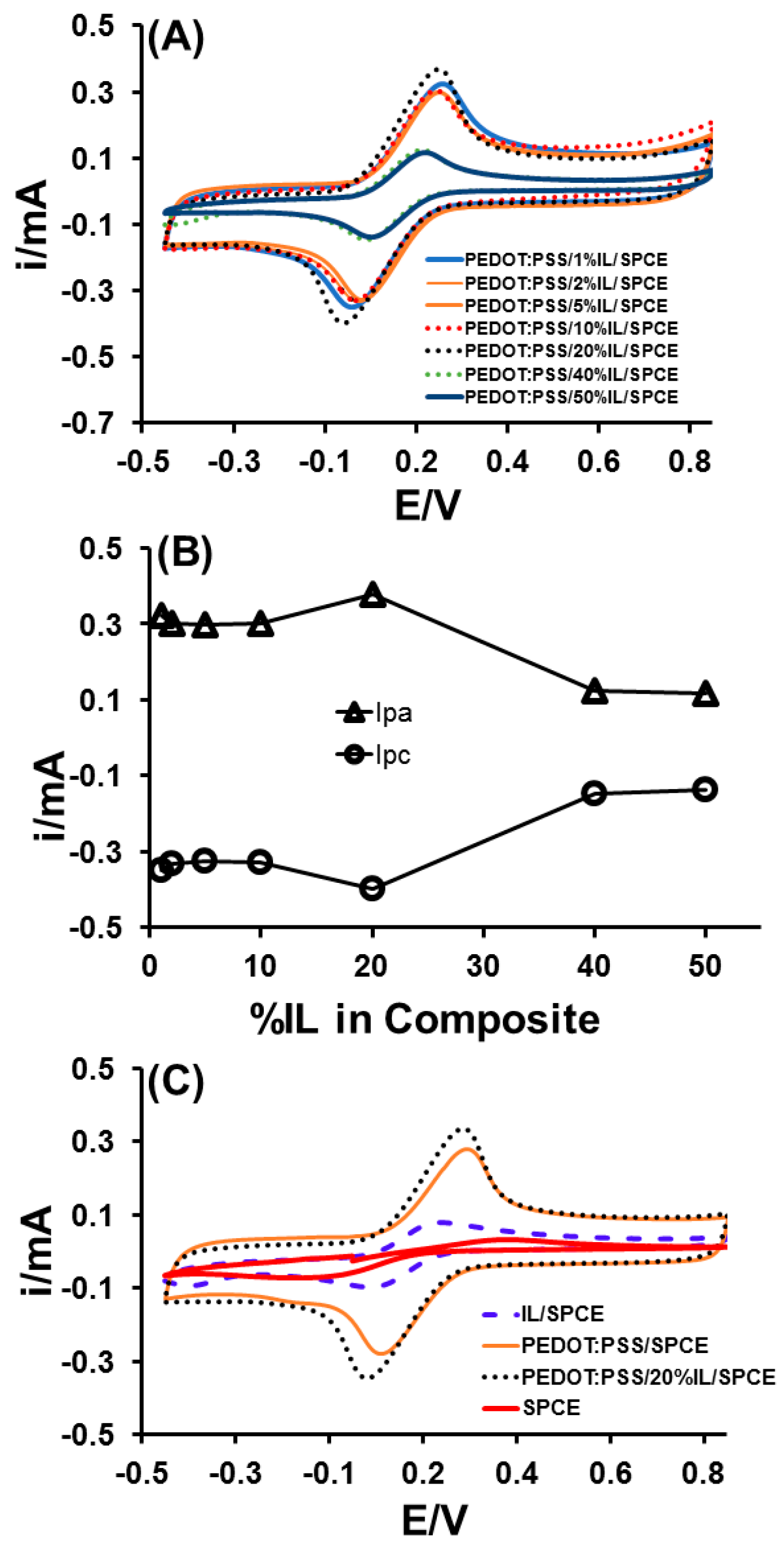
3.1. Optimisation of the Percentage of IL in PEDOT:PSS/IL Composite
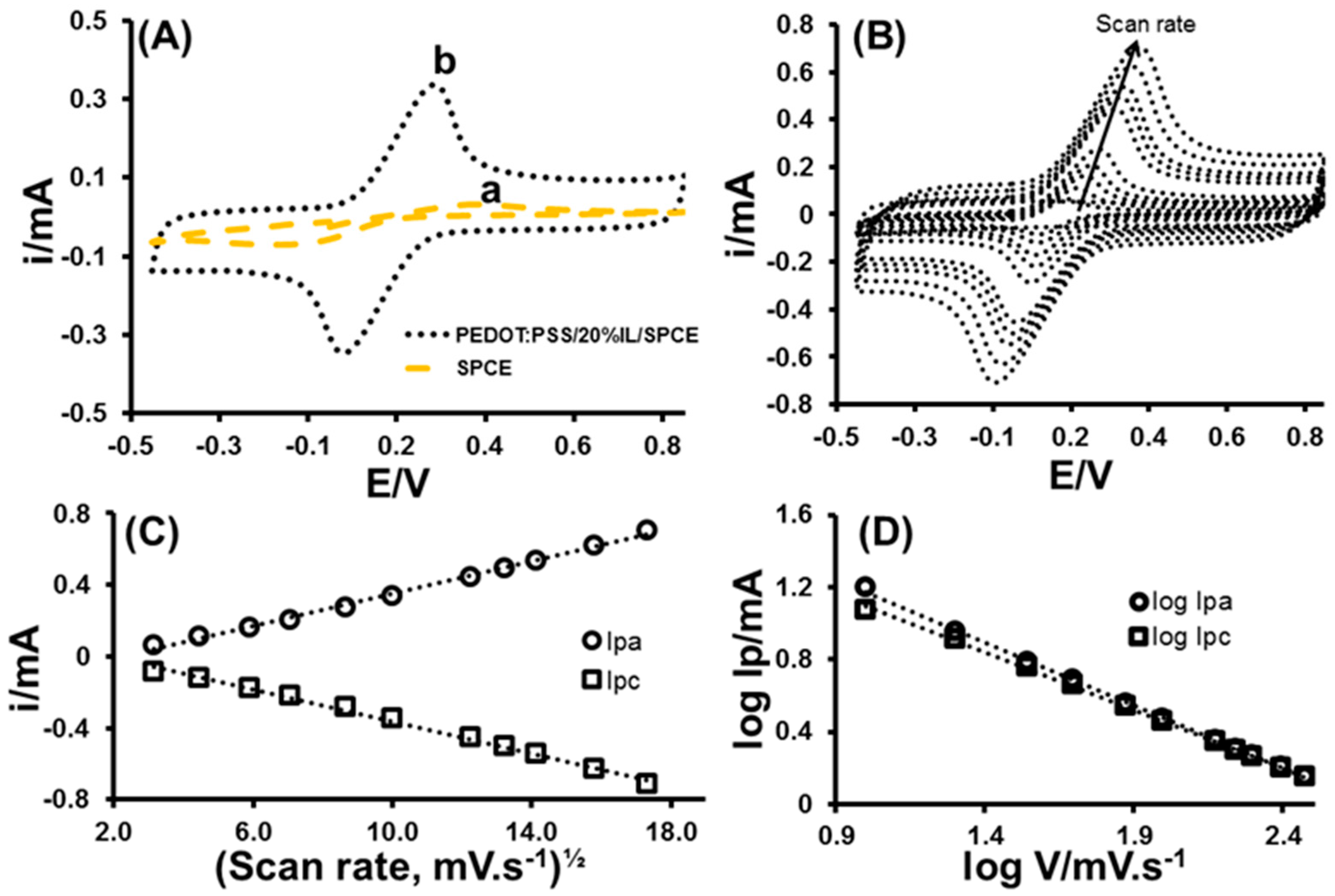
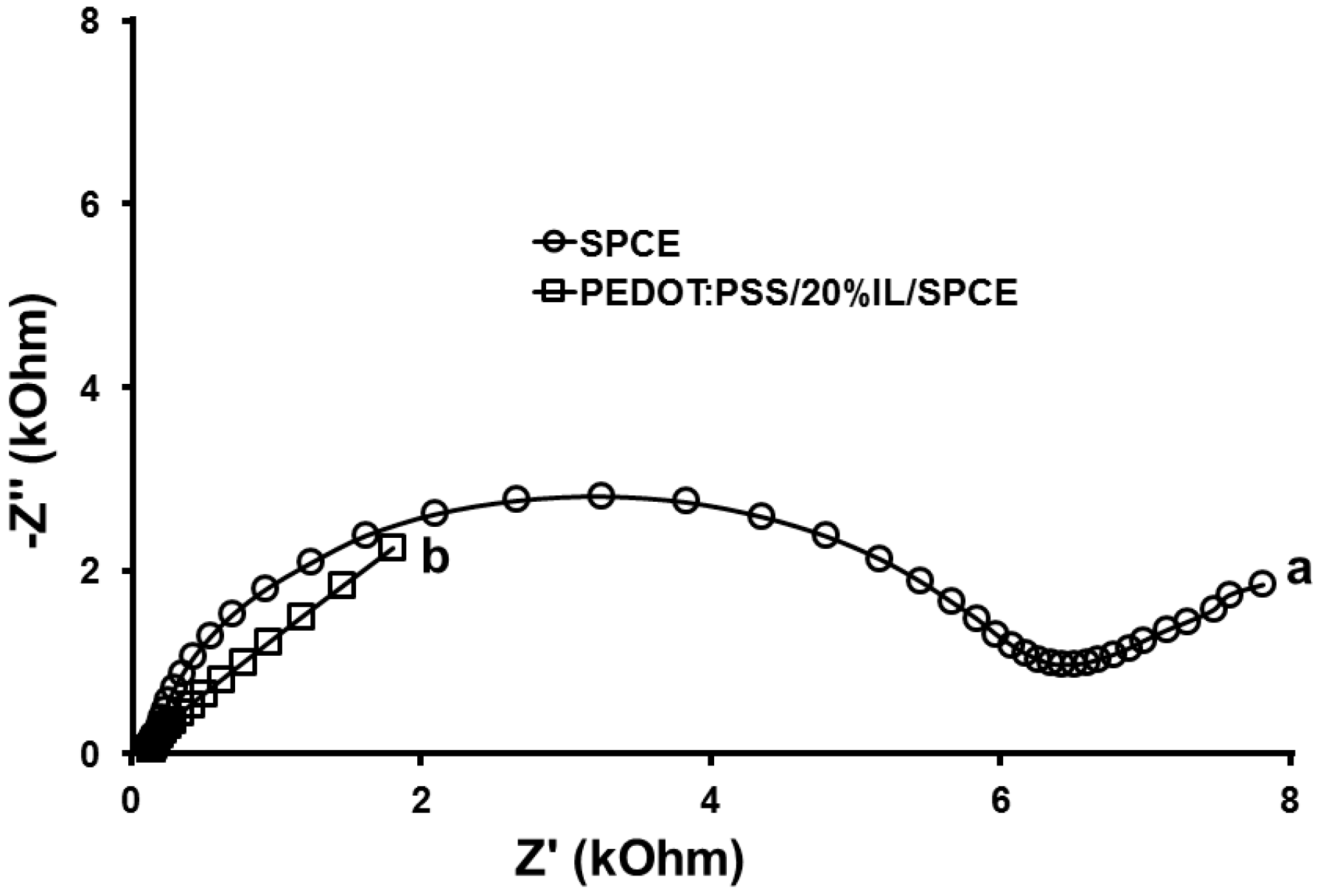
3.2. Characterisation of SPCE and PEDOT:PSS/20%IL/SPCE Sensor
3.2.1. Cyclic Voltammetry
3.2.2. Electrochemical Impedance Spectroscopy
3.2.3. Scanning Electron Spectroscopy and Profilometry
3.2.4. Sessile Contact Angle Measurements
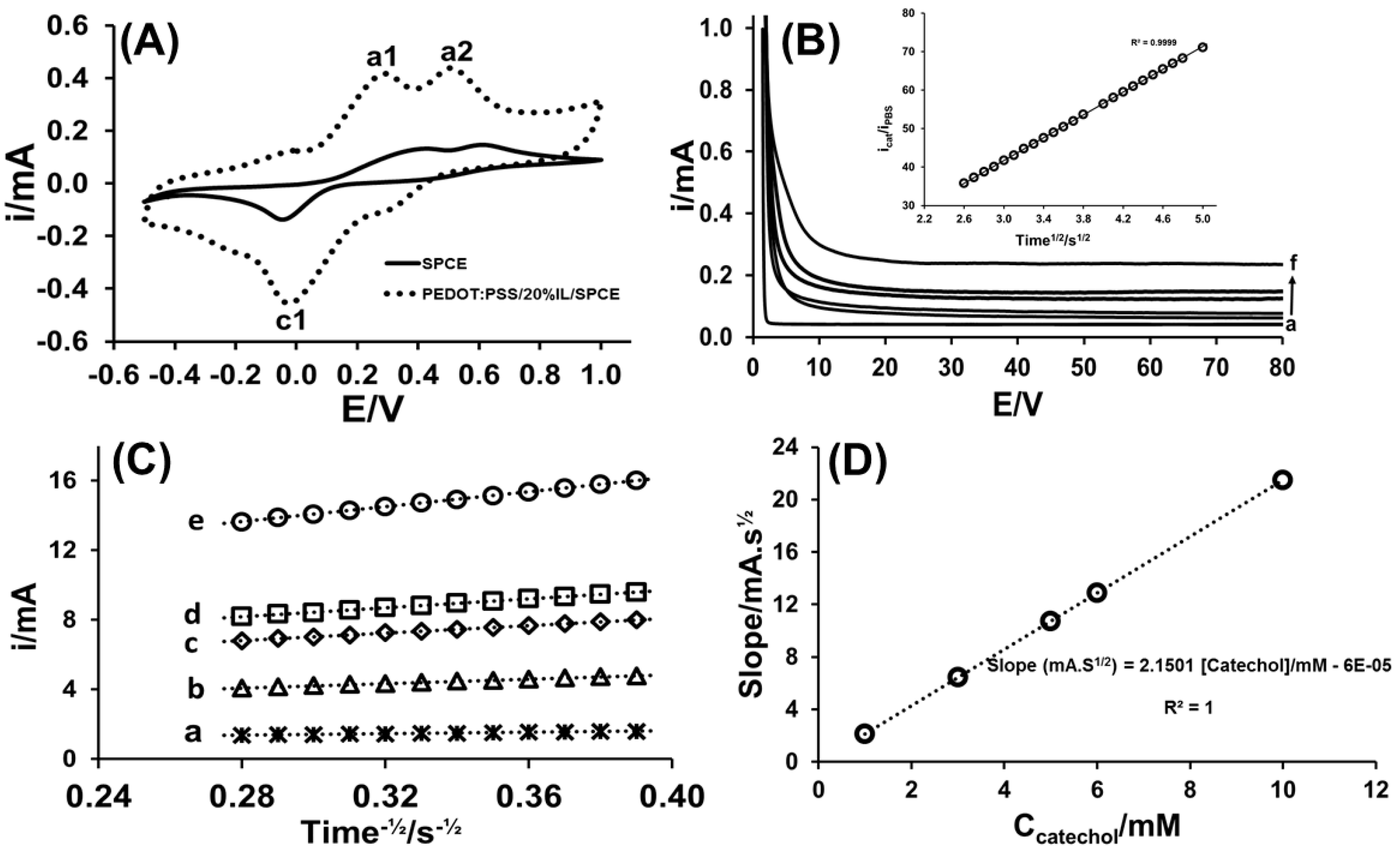
3.3. Application of PEDOT:PSS/20%IL/SPCE to Catechol Analysis
3.3.1. Cyclic Voltammetry
3.3.2. Chronoamperometry
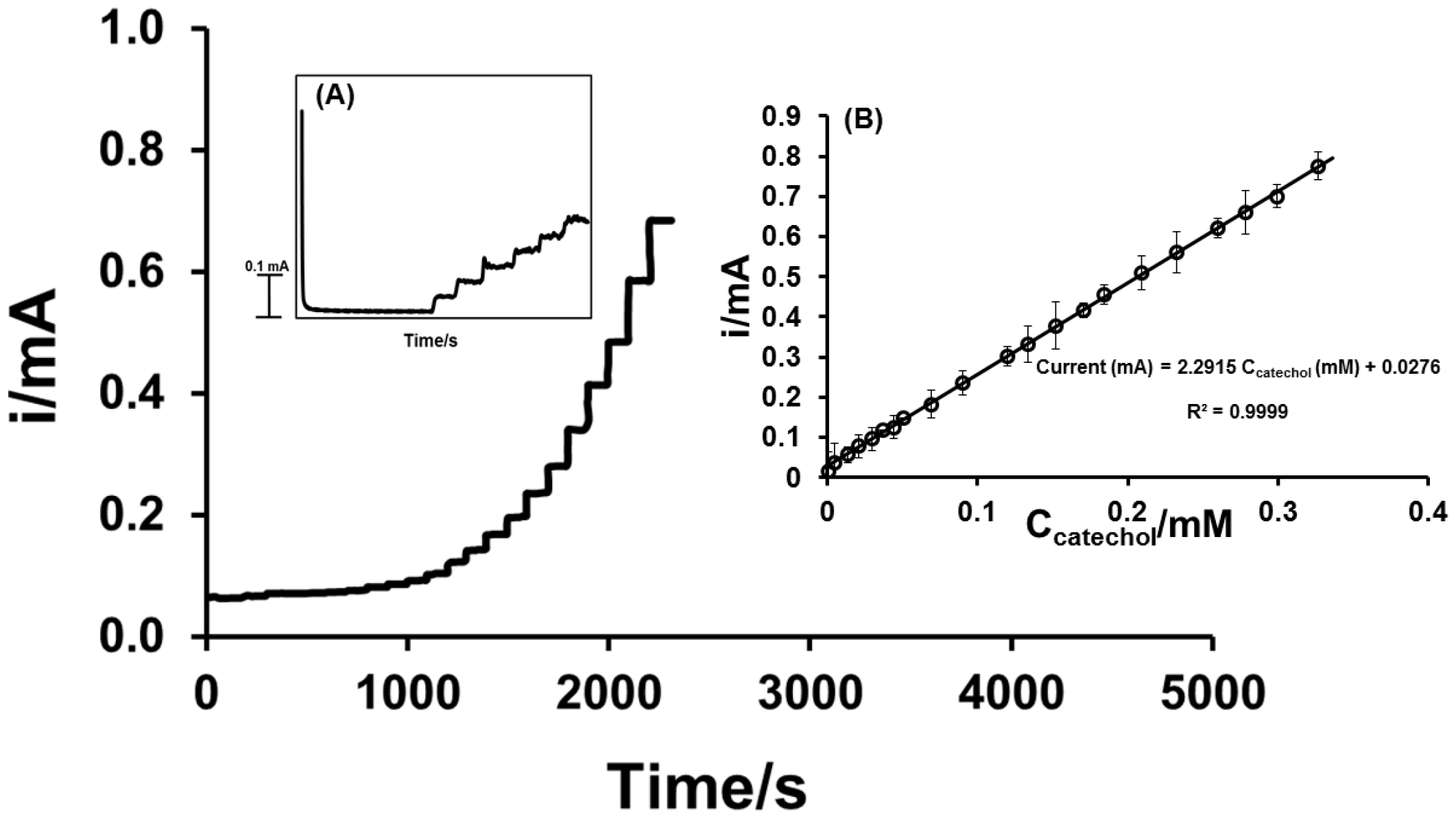
3.3.3. Amperometry in Stirred Solution
3.3.4. Stability of PEDOT:PSS/20%IL/SPCE Sensor
3.3.5. Analysis of Natural Water Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Bysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Vad, N.M.; Kandala, P.K.; Srivastava, S.K.; Moridani, M.Y. Structure-toxicity relationship of phenolic analogs as anti-melanoma agents: An enzyme directed prodrug approach. Chem. Biol. Interact. 2010, 183, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Draths, K.M.; Frost, J.W. Conversion of D-glucose into catechol: The not-so-common pathway of aromatic biosynthesis. J. Am. Chem. Soc. 1991, 113, 9361–9363. [Google Scholar] [CrossRef]
- Draths, K.M.; Frost, J.W. Evironmentally compatible synthesis of catechol from D-gluose. J. Am. Chem. Soc. 1995, 117, 2395–2400. [Google Scholar] [CrossRef]
- European Union Environment Framework. Directive 2008/105/EC. Available online: http://ec.europa.eu/environment/water/water-framework/priority_substances.htm (accessed on 14 March 2017).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 14 March 2017).
- Palanisamy, S.; Karuppiah, C.; Chen, S.M.; Yang, C.Y.; Periakaruppan, P. Simultaneous and selective electrochemical determination of dihydroxybenzene isomers at a reduced graphene oxide and copper nanoparticles composite modified glassy carbon electrode. Anal. Methods 2014, 6, 4271–42785. [Google Scholar] [CrossRef]
- Nambiar, S.R.; Aneesh, P.K.; Rao, T.P. Ultrasensitive voltammetric determination of catechol at a gold atomic cluster/poly(3,4-ethylenedioxythiophene) nanocomposite electrode. Analyst 2013, 138, 5031–5038. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-G.; Cui, H.; Li, Y.-H.; Lin, X.-Q. Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta 2000, 53, 661–666. [Google Scholar] [CrossRef]
- Pistonesi, M.F.; Di Nezio, M.S.; Centurion, M.E.; Palomeque, M.E.; Lista, A.G.; Band, B.S.F. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least-squares (PLS). Talanta 2006, 69, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 2001, 55, 1039–1046. [Google Scholar] [CrossRef]
- Govindhan, M.; Lafleur, T.; Adhikari, B.R.; Chen, A. Electrochemical sensor based on carbon nanotubes for the simultaneous detection of phenolic pollutants. Electroanalysis 2015, 27, 902–909. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Fabrication and electrochemical characterization of polydopamine redox polymer modified screen-printed carbon electrode for the detection of guanine. Sens. Actuators B Chem. 2016, 233, 528–534. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. A non-enzymatic sensor based on the redox of ferrocene carboxylic acid on ionic liquid film-modified screen-printed graphite electrode for the analysis of hydrogen peroxide residues in milk. J. Electroanal. Chem. 2016, 766, 147–151. [Google Scholar] [CrossRef]
- Kanyong, P.; Huges, G.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Amperometric screen-printed galactose biosensor for cell toxicity applications. Anal. Lett. 2016, 49, 236–244. [Google Scholar] [CrossRef]
- Quan, Y.L.; Xue, Z.; Shi, H.; Zhou, X.; Du, J.; Liu, X.; Lu, X. A high-performance and simple method for rapid and simultaneous determination of dihydroxybenzene isomers. Analyst 2012, 137, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ye, J.; Zhang, J.; Huang, X.; Yu, C.A. Voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2011, 650, 209–213. [Google Scholar] [CrossRef]
- Bai, J.; Guo, L.P.; Ndamanisha, J.C.; Qi, B. Electrochemical properties and simultaneous determination of dihydroxybenzene isomers at ordered mesoporous carbon-modified electrode. J. Appl. Electrochem. 2009, 39, 2497–2503. [Google Scholar] [CrossRef]
- Li, D.W.; Li, Y.T.; Song, W.; Long, Y.T. Simultaneous determination of dihydroxybenzene isomers using disposable screen-printed electrode modified by multiwalled carbon nanotubes and gold nanoparticles. Anal. Methods 2010, 2, 837–843. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Zhang, F.; Wang, M.; Lin, X.; Li, H. Simultaneous detection of hydroquinone and catechol on electrochemical-activated glassy carbon electrode by simple anodic and cathodic polarization. J. Solid State Electrochem. 2017, 21, 735–745. [Google Scholar] [CrossRef]
- Terasawa, N.; Asaka, K. High-performance PEDOT:PSS/Single-Walled Carbon Nanotube/Ionic Liquid actuators combining electrostatic double-layer and faradaic capacitors. Langmuir 2016, 32, 7210–7218. [Google Scholar] [CrossRef] [PubMed]
- Elschner, A.; Kirchmeyer, S.; Lövenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Shahinpoor, M. Ionic polymer−conductor composites as biomimetic sensors, robotic actuators and artificial muscles—A review. Electrochim. Acta 2003, 48, 2343–2353. [Google Scholar] [CrossRef]
- Moczko, E.; Istamboulie, G.; Calas-Blanchard, C.; Rouillon, R.; Nogue, T. Biosensor employing screen-printed PEDOT:PSS for sensitive detection of phenolic compounds in water. Polymer Chem. 2012, 50, 2286–2292. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Xu, G.; Xu, S.; Wang, S.; Luo, X. Cost-effective preparation and sensing application of conducting polymer PEDOT/ionic liquid nanocomposite with excellent electrochemical properties. RSC Adv. 2015, 5, 20741–20746. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Gold nanoparticle modified screen-printed carbon arrays for the simultaneous electrochemical analysis of lead and copper in tap water. Microchim. Acta 2016, 183, 2361–2368. [Google Scholar] [CrossRef]
- Morrin, A.; Killard, A.J.; Smyth, M.R. Electrochemical characterization of commercial and home-made screen-printed carbon electrodes. Anal. Lett. 2003, 36, 2021–2039. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry—Simulation and Analysis of Reaction Mechanisms; VCH: New York, NY, USA, 1993. [Google Scholar]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, D.; Dehdashtian, S. Electrochemical oxidation of catechol in the presence of indole: A facile and one-pot method for the synthesis of trisindolyl-0-benzoquinone. Tetrahedron Lett. 2008, 49, 645–649. [Google Scholar] [CrossRef]
- Khalafi, L.; Rafiee, M.; Shahbak, M.; Shirmohammadi, H. Kinetic study of the oxidation of catehols in the presence of N-methylaniline. J. Chem. 2013, 2013, 497515. [Google Scholar] [CrossRef]
- Nematollahi, D.; Afkhami, A.; Mosaed, F.; Rafiee, M. Investigation of the electro-oxidation and oxidation of catechol in the presence of sulfanilic acid. Res. Chem. Intermed. 2004, 30, 299–309. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical system. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rotariu, L.; Zamfir, L.-G.; Bala, C. Low potential thiocholine oxidation at carbon nanotube-ionic liquid gel sensor. Sens. Actuators B Chem. 2010, 150, 73–79. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Simultaneous electrochemical determination of dopamine and 5-hydroxyindoleacetic acid in urine using screen-printed graphite electrode modified with gold nanoparticles. Anal. Bional. Chem. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanyong, P.; Rawlinson, S.; Davis, J. A voltammetric sensor based on chemically reduced graphene oxide-modified screen-printed carbon electrode for the simultaneous analysis of uric acid, ascorbic acid and dopamine. Chemosensors 2016, 4, 25. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of a sandwich format, amperometric screen-printed uric acid biosensor for urine analysis. Anal. Biochem. 2012, 428, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of an amperometric screen-printed galactose biosensor for serum analysis. Anal. Biochem. 2013, 435, 114–119. [Google Scholar] [CrossRef] [PubMed]



| Sample | [Catechol]/µM | Mean Recovery (%) | |
|---|---|---|---|
| Amount Added | Amount Found | ||
| Tap Water | . | ||
| Repeat 1 | 20 | 19.98 | |
| Repeat 2 | 20 | 19.96 | |
| Repeat 3 | 20 | 19.89 | |
| Mean | - | 19.943 | |
| SD | - | 0.035 | |
| CV (%) | - | 0.177 | |
| River Water | |||
| Repeat 1 | 20 | 19.76 | |
| Repeat 2 | 20 | 19.88 | |
| Repeat 3 | 20 | 19.79 | |
| Mean | - | 19.81 | |
| SD | - | 0.062 | |
| CV (%) | - | 0.315 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krampa, F.D.; Aniweh, Y.; Awandare, G.A.; Kanyong, P. A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples. Sensors 2017, 17, 1716. https://doi.org/10.3390/s17081716
Krampa FD, Aniweh Y, Awandare GA, Kanyong P. A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples. Sensors. 2017; 17(8):1716. https://doi.org/10.3390/s17081716
Chicago/Turabian StyleKrampa, Francis D., Yaw Aniweh, Gordon A. Awandare, and Prosper Kanyong. 2017. "A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples" Sensors 17, no. 8: 1716. https://doi.org/10.3390/s17081716





