Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots
Abstract
:1. Introduction
2. Material and Methods
2.1. Living Nanorobots Preparation
2.2. Chemotaxis Assay on Soft Agar
2.3. Chemotaxis Assay on Solid Agar
2.4. Capillary Chemotaxis Assays
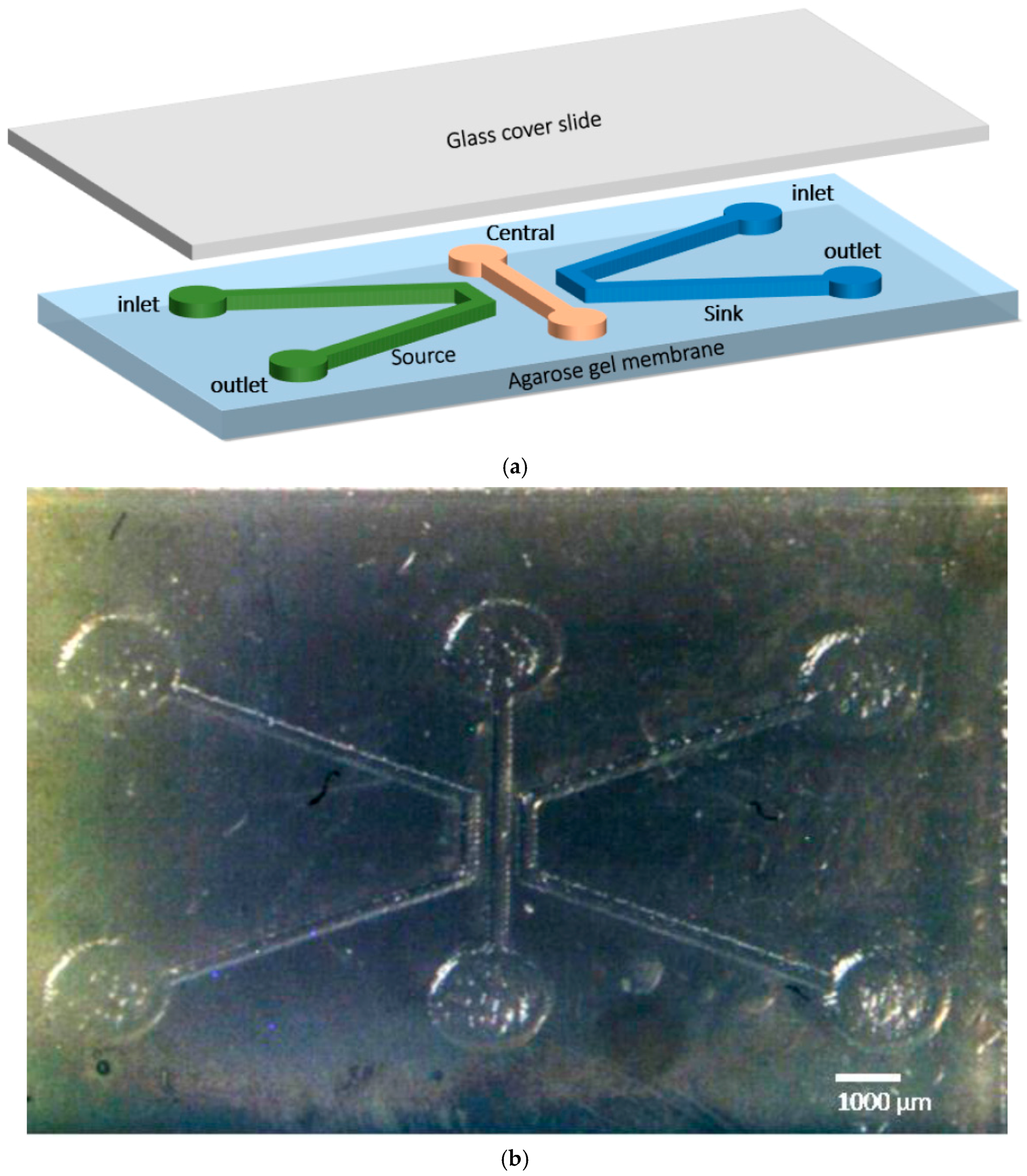
2.5. Chemotaxis Assay Using Fabricated Microfluidic Chip
3. Results and Discussion
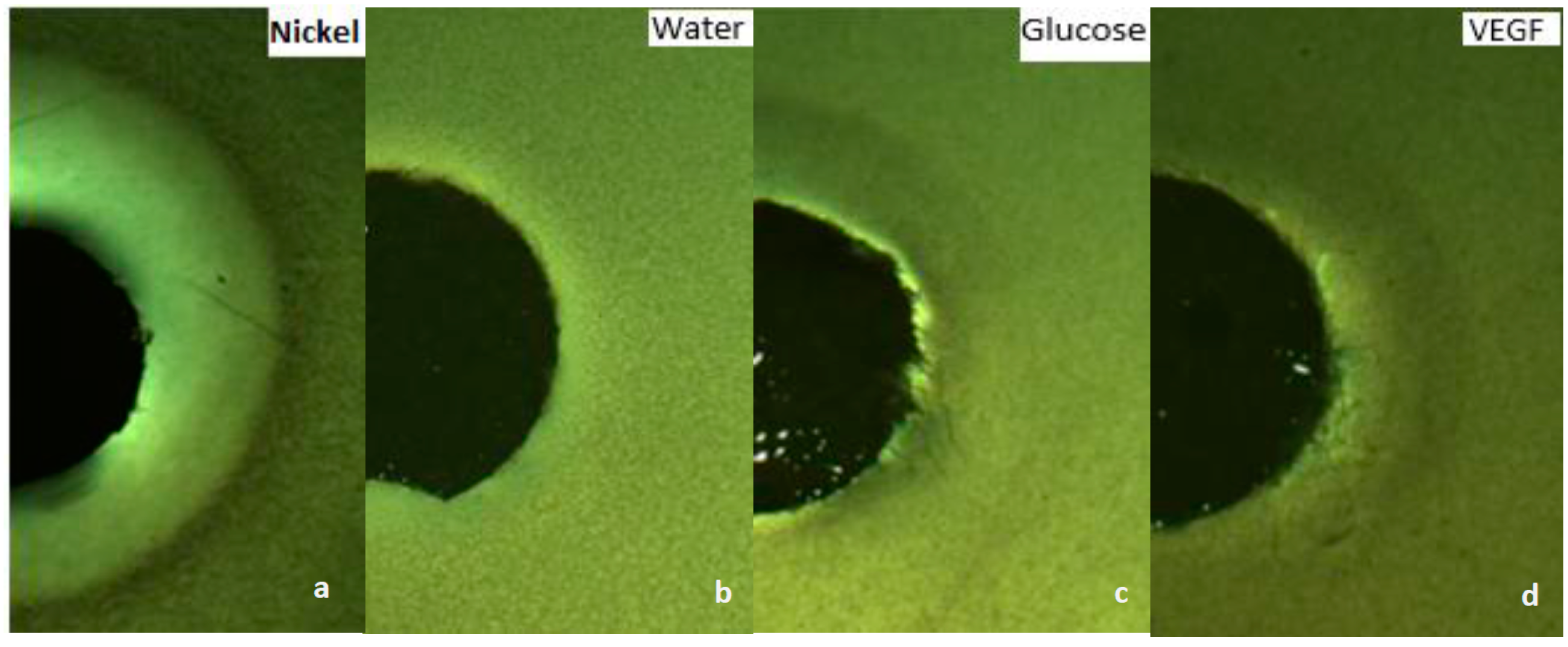
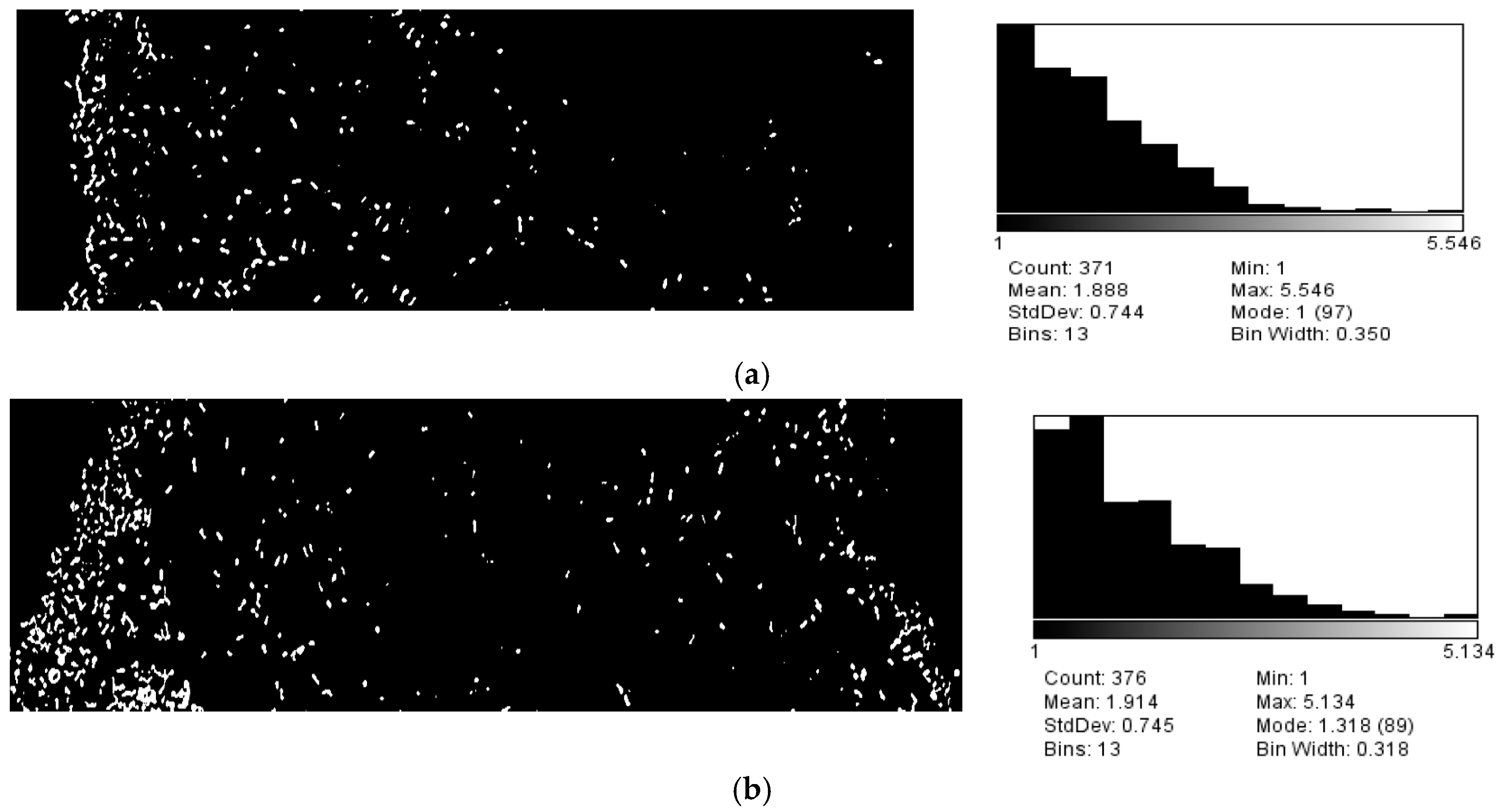
3.1. Growth VEGF Chemotaxis
3.2. Motility VEGF Chemotaxis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en (accessed on 8 March 2017).
- Types of Cancer Treatment—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 4 April 2017).
- Saini, R.K.; Chouhan, R.; Bagri, L.P.; Bajpai, A.K. Strategies of targeting tumors and cancers. J. Can. Res. Updat. 2012, 1, 129–152. [Google Scholar]
- Farsad, N.; Yilmaz, H.B.; Eckford, A.; Chae, C.-B.; Guo, W. A Comprehensive Survey of Recent Advancements in Molecular Communication. IEEE Commun. Surv. Tutor. 2016, 18, 1887–1919. [Google Scholar] [CrossRef]
- Atakan, B.; Akan, O.B.; Balasubramaniam, S. Body area nanonetworks with molecular communications in nanomedicine. IEEE Commun. Mag. 2012, 50, 28–34. [Google Scholar] [CrossRef]
- Garmory, H.S.; Leary, S.E.C.; Griffin, K.F.; Williamson, E.D.; Brown, K.A.; Titball, R.W. The use of live attenuated bacteria as a delivery system for heterologous antigens. J. Drug Target. 2003, 11, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, S.; Isogawa, Y.; Suda, T.; Moritani, Y.; Sutoh, K. A Design of an Autonomous Molecule Loading/Transporting/Unloading System Using DNA Hybridization and Biomolecu-lar Linear Motors. In Proceedings of the European Nano Systems 2005, Paris, France, December 2005. [Google Scholar]
- Moritani, Y.; Hiyama, S.; Suda, T. Molecular Communication among Nanomachines Using Vesicles. NSTI Nanotechnol. 2006, 2, 705–708. [Google Scholar]
- Baban, C.K.; Cronin, M.; O’Hanlon, D.; O’Sullivan, G.C.; Tangney, M. Bacteria as vectors for gene therapy of cancer. Bioeng. Bugs 2010, 1, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003, 4, 548–556. [Google Scholar] [CrossRef]
- Al-Fandi, M.; Jaradat, M.; Al-Rousan, M.; Al-Ebbini, L.; Jaradat, S. Flagellated Bacteria As Self-Navigator Nano/Bio-Robots. J. Mech. Med. Biol. 2012, 12, 1240003:1–1240003:15. [Google Scholar] [CrossRef]
- Berry, R.M.; Berry, M.R. Bacterial Flagella: Flagellar Motor. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2001; ISBN 9780470015902. [Google Scholar]
- Hoeben, A.N.N.; Landuyt, B.; Highley, M.S.M.; Wildiers, H.; Oosterom, A.T.V.A.N.; Bruijn, E.A.D.E.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: Autocrine signalling by VEGF. In VEGF Cancer; Madame Curie Bioscience Database: Austin, TX, USA, 2004; pp. 133–144. [Google Scholar]
- Hazeleger, W.C.; Wouters, J.A.; Rombouts, F.M.; Abee, T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 1998, 64, 3917–3922. [Google Scholar] [PubMed]
- Adler, J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 1973, 74, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Fandi, M.; Jaradat, M.A.; Al-Rousan, M.; Jaradat, S. A living biological nano robot as self-navigator sensor for diseases. In 2011 1st Middle East Conference on Biomedical Engineering; IEEE: Sharjah, United Arab Emirates, 2011; pp. 176–179. [Google Scholar]
- Comprehensive Cancer Information—National Cancer Institute. Available online: https://www.cancer.gov/ (accessed on 5 April 2017).
- Park, D.; Park, S.J.; Cho, S.; Lee, Y.; Lee, Y.K.; Min, J.J.; Park, B.J.; Ko, S.Y.; Park, J.O.; Park, S. Motility analysis of bacteria-based microrobot (bacteriobot) using chemical gradient microchamber. Biotechnol. Bioeng. 2014, 111, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bren, A.; Eisenbach, M. How signals are heard during bacterial chemotaxis: Protein-protein interactions in sensory signal propagation. J. Bacteriol. 2000, 182, 6865–6873. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C. The Rotary Motor of Bacterial Flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Gibbons, R.J. Chemotactic response to formate by Campylobacter concisus and its potential role in gingival colonization. Infect. Immun. 1986, 52, 378–383. [Google Scholar] [PubMed]
- Khanna, M.; Bhavsar, S.; Kapadnis, B. Effect of temperature on growth and chemotactic behaviour of Campylobacter jejuni. Lett. Appl. Microbiol. 2006, 43, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Raterman, E.L.; Welch, R.A. Chemoreceptors of Escherichia coli CFT073 Play Redundant Roles in Chemotaxis toward Urine. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Köhn-Luque, A.; de Back, W.; Yamaguchi, Y.; Yoshimura, K.; Herrero, M.A.; Miura, T. Dynamics of VEGF matrix-retention in vascular network patterning. Phys. Biol. 2013, 10, 66007. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fandi, M.; Alshraiedeh, N.; Oweis, R.; Alshdaifat, H.; Al-Mahaseneh, O.; Al-Tall, K.; Alawneh, R. Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots. Sensors 2017, 17, 1580. https://doi.org/10.3390/s17071580
Al-Fandi M, Alshraiedeh N, Oweis R, Alshdaifat H, Al-Mahaseneh O, Al-Tall K, Alawneh R. Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots. Sensors. 2017; 17(7):1580. https://doi.org/10.3390/s17071580
Chicago/Turabian StyleAl-Fandi, Mohamed, Nida Alshraiedeh, Rami Oweis, Hala Alshdaifat, Omamah Al-Mahaseneh, Khadijah Al-Tall, and Rawan Alawneh. 2017. "Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots" Sensors 17, no. 7: 1580. https://doi.org/10.3390/s17071580




