A Novel Approach to Measuring Muscle Mechanics in Vehicle Collision Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Data Processing
2.2. Calculations
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meijer, R.; Broos, J.; Elrofai, H.; de Bruijn, E.; Forbes, P.; Happee, R. Modelling of bracing in a multi-body active human model. In Proceedings of the IRCOBI Conference 2013, Gothenburg, Sweden, 11–13 September 2013; pp. 576–587. [Google Scholar]
- Beeman, S.M.; Kemper, A.R.; Madigan, M.L.; Duma, S.M. Effects of bracing on human kinematics in low-speed frontal sled tests. Ann. Biomed. Eng. 2011, 39, 2998–3010. [Google Scholar] [CrossRef] [PubMed]
- Beeman, S.M.; Kemper, A.R.; Madigan, M.L.; Franck, C.T.; Loftus, S.C. Occupant kinematics in low-speed frontal sled tests: Human volunteers, Hybrid III ATD, and PMHS. Accid. Anal. Prev. 2012, 47, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Beeman, S.M.; Kemper, A.R.; Duma, S.M. Neck forces and moments of human volunteers and post mortem human surrogates in low-speed frontal sled tests. Traffic Inj. Prev. 2016, 17, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Dibb, A.T.; Cox, C.A.; Nightingale, R.W.; Luck, J.F.; Cutcliffe, H.C.; Myers, B.S.; Arbogast, K.B.; Seacrist, T.; Bass, C.R. Importance of Muscle Activations for Biofidelic Pediatric Neck Response in Computational Models. Traffic Inj. Prev. 2013, 14, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdes, F.J.; Lau, A.; Lamp, J.; Riley, P.; Lessley, D.J.; Damon, A.; Kindig, M.; Kent, R.; Balasubramanian, S.; Seacrist, T.; et al. Analysis of spinal motion and loads during frontal impacts. Comparison between PMHS and ATD. Ann. Adv. Automot. Med. 2010, 54, 61–78. [Google Scholar] [PubMed]
- Iwamoto, M.; Nakahira, Y.; Sugiyama, T. Investigation of pre-impact bracing effects for injury outcome using an active human FE model with 3D geometry of muscles. In Proceedings of the 22nd International Technical Conference on the Enhanced Safety of Vehicles (ESV), Washington, DC, USA, 13–16 June 2011. [Google Scholar]
- Van der Horst, M.J.; Thunnissen, J.G.M.; Happee, R.; van Haaster, R.M.H.P.; Wismans, J.S.H.M. The influence of muscle activity on head-neck response during impact. In Proceedings of the 41st Stapp Car Crash Conference, Lake Buena Vista, FL, USA, 13–14 November 1997; pp. 487–507. [Google Scholar]
- Van der Horst, M.J. Human Head Neck Response in Frontal, Lateral and Rear End Impact Loading—Modelling and Validation. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2002. [Google Scholar]
- Iwamoto, M.; Nakahira, Y.; Kimpara, H. Development and Validation of the Total Human Model for Safety (THUMS) Toward Further Understanding of Occupant Injury Mechanisms in Precrash and During Crash. Traffic Inj. Prev. 2015, 16, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Östh, J.; Brolin, K.; Bråse, D. A human body model with active muscles for simulation of pretensioned restraints in autonomous braking interventions. Traffic Inj. Prev. 2015, 16, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Pick, A.; Cole, D. Neuromuscular dynamics and the vehicle steering task. In Proceedings of the 18th IAVSD Symposium, Kanagawa, Japan, 24–30 August 2003; pp. 182–191. [Google Scholar]
- Brook, S.; Freeman, R.; Rosala, G.; Campean, F.; Dixon, N. Ergonomic Data Measuring System for Driver-Pedals Interaction. SAE Int. J. Passeng. Cars Mech. Syst. 2009, 2, 1071–1078. [Google Scholar] [CrossRef]
- Zajac, F. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Crit. Rev. Biomed. Eng. 1989, 17, 359–410. [Google Scholar] [PubMed]
- Östh, J.; Brolin, K.; Ólafsdóttir, J.M.; Davidsson, J.; Pipkorn, B.; Jakobsson, L.; Törnvall, F.; Lindkvist, M. Muscle activation strategies in human body models for the development of integrated safety. In Proceedings of the 24th International Techical Conference on the Enhanced Safety of Vehicles (ESV), Gothenburg, Sweden, 8–11 June 2015. [Google Scholar]
- de Bruijn, E.; van der Helm, F.C.T.; Happee, R. Analysis of isometric cervical strength with a nonlinear musculoskeletal model with 48 degrees of freedom. Multibody Syst. Dyn. 2016, 36, 339–369. [Google Scholar] [CrossRef]
- Happee, R. Inverse dynamic optimization including muscular dynamics, a new simulation method applied to goal directed movements. J. Biomech. 1994, 27, 953–960. [Google Scholar] [CrossRef]
- Erdemir, A.; McLean, S.; Herzog, W.; van den Bogert, A.J. Model-based estimation of muscle forces exerted during movements. Clin. Biomech. 2007, 22, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.G.; Besier, T.F. An EMG-Driven Musculoskeletal Model to Estimate Muscle Forces and Knee Joint Moments In Vivo. J. Biomech. 2003, 26, 765–776. [Google Scholar] [CrossRef]
- Zatsiorsky, V.; Prilutsky, B. Biomechanics of Skeletal Muscles, 1st ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Buchanan, T.S.; Lloyd, D.G.; Manal, K.; Besier, T.F. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. J. Appl. Biomech. 2004, 20, 367–395. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Reggiani, M.; Farina, D.; Lloyd, D.G. EMG-driven forward-dynamic estimation of muscle force and joint moment about multiple degrees of freedom in the human lower extremity. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Amarantini, D.; Martin, L. A method to combine numerical optimization and EMG data for the estimation of joint moments under dynamic conditions. J. Biomech. 2004, 37, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Hwang, S.; Kim, Y. A hybrid static optimisation method to estimate muscle forces during muscle co-activation. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Higginson, J.S.; Ramsay, J.W.; Buchanan, T.S. Hybrid models of the neuromusculoskeletal system improve subject-specificity. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hedenstierna, S.; Halldin, P.; Siegmund, G.P. Neck muscle load distribution in lateral, frontal and rear-end impacts. A three dimensional finite element analysis. Spine 2009, 34, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. The ABC of EMG. A Practical Introduction to Kinesiological Electromyography, version 1.4; Noraxon USA Inc.: Scottsdale, AR, USA, 2006. [Google Scholar]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- De Luca, G. Fundamental Concepts in EMG Signal Acquisition; DelSys Inc.: Boston, MA, USA, 2003. [Google Scholar]
- Bose, D.; Crandall, J.R. Influence of Active Muscle Contribution on the Injury Response of Restrained Car Occupants. Ann. Adv. Automot. Med. 2008, 52, 61–72. [Google Scholar] [PubMed]
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieën, J.H. Methodological aspects of SEMG recordings for force estimation—A tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Disselhorst-Klug, C.; Schmitz-Rode, T.; Rau, G. Surface electromyography and muscle force: Limits in sEMG-force relationship and new approaches for applications. Clin. Biomech. 2009, 24, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, G.P.; Blouin, J.; Brault, J.R.; Hedenstierna, S.; Inglis, J.T. Electromyography of Superficial and Deep Neck Muscles During Isometric, Voluntary, and Reflex Contractions. J. Biomech. Eng. 2006, 129, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Anderst, W.J.; Donaldson, W.F., III; Lee, J.Y.; Kang, J.D. Subject-Specific Inverse Dynamics of the Head and Cervical Spice during In Vivo Dynamic Flexion-Extension. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.; Cormier, J.; Bain, C.; Guzman, H.; Bonugli, E. Validation and Application of a Methodology to Calculate Head Accelerations and Neck Loading in Soccer Ball Impacts. In Proceedings of the 2009 SAE World Congress & Exhibition, Detroit, MI, USA, 20–23 April 2009; SAE Technical Paper 2009-01-0251. Society of Automotive Engineers: Warrendale, PA, USA, 2009. [Google Scholar] [CrossRef]
- Seacrist, T.; Arbogast, K.B.; Maltese, M.R.; Garcıa-Espana, J.F.; Lopez-Valdes, F.J.; Kent, R.W.; Tanji, H.; Higuchi, K.; Balasubramanian, S. Kinetics of the cervical spine in pediatric and adult volunteers during low speed frontal impacts. J. Biomech. 2012, 45, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ólafsdóttir, J.M.; Brolin, K.; Blouin, J.-S.; Siegmund, G.P. Dynamic Spatial Tuning of Cervical Muscle Reflexes to Multidirectional Seated Perturbations. Spine 2015, 40, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Stančin, S.; Meglič, A.; Milutinović, V.; Tomažič, S. MC Sensor—A Novel Method for Measurement of Muscle Tension. Sensors 2011, 11, 9411–9425. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Tomažič, S.; Narici, M.; Pišot, R.; Meglič, A. In Vivo Measurement of Muscle Tension: Dynamic Properties of the MC Sensor During Isometric Muscle Contraction. Sensors 2014, 14, 17848–17863. [Google Scholar] [CrossRef] [PubMed]
- Ejima, S.; Ono, K.; Holcombe, S.; Kaneoka, K.; Fukushima, M. A study on occupant kinematics behaviour and muscle activities during pre-impact braking based on volunteer tests. In Proceedings of the IRCOBI 2007, Maastricht, The Netherlands, 19–21 September 2007; pp. 31–45. [Google Scholar]
- Dehner, C.; Schick, S.; Kraus, M.; Scola, A.; Hell, W.; Kramer, M. Muscle activity influence on the kinematics of the cervical spine in frontal tests. Traffic Inj. Prev. 2013, 14, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narayan, Y.; Amell, T. Analysis of low velocity frontal impacts. Clin. Biomech. 2003, 18, 694–703. [Google Scholar] [CrossRef]
- Challis, J.H. A procedure for determining rigid body transformation. J. Biomech. 1995, 28, 733–737. [Google Scholar] [CrossRef]
- Yoganandan, N.; Pintar, F.A.; Zhang, J.; Baisden, J.L. Physical properties of the human head: Mass, center of gravity and moment of inertia. J. Biomech. 2009, 42, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Beier, G.; Schuller, E.; Schuck, M.; Ewing, C.L.; Becker, E.D.; Thomas, D.J. Center of gravity and moments of inertia of human heads. In Proceedings of the IRCOBI 1980, Birmingham, UK, 9–11 September 1980; pp. 218–228. [Google Scholar]
- Plaga, J.A.; Albery, C.; Boehmer, M.; Goodyear, C.; Thomas, G. Design and Development of Anthropometrically Correct Head Forms for Joint Strike Fighter Ejection Seat Testing; Wright-Patterson AFB: Dayton, OH, USA, 2005; p. 56. [Google Scholar]
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing, 4th ed.; Academic Press: London, UK, 2016. [Google Scholar]
- Yoganandan, N.; Pintar, F.A.; Moore, J.; Rinaldi, J.; Schlick, M.; Maiman, D.J. Upper and Lower Neck Loads in Belted Human Surrogates in Frontal Impacts. Ann. Adv. Automot. Med. 2012, 56, 125–136. [Google Scholar] [PubMed]
- Cox, C.A.; Dibb, A.T.; Myers, B.S.; Nightingale, R.W.; Bass, C.R. Adult head and neck dynamics: A sensitivity analysis study during frontal impact. In Proceedings of the IRCOBI Asia 2016, Seoul, Korea, 16–18 May 2016; pp. 24–25. [Google Scholar]
- Trajkovski, A.; Omerović, S.; Krašna, S.; Prebil, I. Loading rate effect on mechanical properties of cervical spine ligaments. Acta Bioeng. Biomech. 2014, 16, 2014. [Google Scholar]
- Panjabi, M.M.; Crisco, J.J.; Vasavada, A.; Oda, T.; Cholewicki, J.; Nibu, K.; Shin, E. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine 2001, 26, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; Choy, H.-Y.; Lee, J.-H.; Han, D.-C. Development of finite element human neck model for vehicle safety simulation. Int. J. Automot. Technol. 2004, 5, 33–46. [Google Scholar]
- Vasavada, A.N.; Li, S.; Delp, S.L. Influence of Muscle Morphometry and Moment Arms on the Moment-Generating Capacity of Human Neck Muscles. Spine 1998, 23, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.V.; Buchanan, T.S.; Delp, S.L. How muscle architecture and moment arms affect wrist flexion-extension moments. J. Biomech. 1997, 30, 705–712. [Google Scholar] [CrossRef]
- Ackland, D.C.; Merritt, J.S.; Pandy, M.G. Moment arms of the human neck muscles in flexion, bending and rotation. J. Biomech. 2011, 44, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.A.; Dibb, A.T.; Cutcliffe, H.C.; Nightingale, R.W.; Myers, B.S.; Vasavada, A.N.; Suderman, B.L.; Bass, C.R. The influence of muscle modeling methods and paths on head and neck response. In Proceedings of the 11th World Congress on Computational Mechanics, 5th European Conference on Computational Mechanics and 6th European Conference on Computational Fluid Dynamics, Barcelona, Spain, 20–25 July 2014; pp. 811–822. [Google Scholar]
- Gao, Z.; Lic, C.; Hu, H.; Zhao, H.; Chen, C.; Yu, H. Study of cervical muscle response and injury of driver during a frontal vehicle collision. Biomed. Mater. Eng. 2015, 26, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, G.P.; Danderson, D.J.; Myers, B.S.; Inglis, J.T. Awareness affects the response of human subjects exposed to a single whiplash-like perturbation. Spine 2003, 28, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.S.; Li, L.; Scott, B.W.; Frankland, P.W. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci. Biobehav. Rev. 2002, 26, 1–11. [Google Scholar] [CrossRef]
- Pellettiere, J.A.; Sanders, M.A.; Doczy, E. Neck muscle activation levels during frontal impacts. In Proceedings of the 42nd Annual SAFE Association Symposium, Salt Lake City, UT, USA, 27–29 September 2004; pp. 177–185. [Google Scholar]
- McGehee, D.V.; Youland, R.; Boer, E.R.; Anton, D.C.; Meyers, A.; Manser, M.M. The use of EMG and video to decompose driver crash avoidance and bracing response. In Proceedings of the 4th Driving Simulation Conference—North America (DSC-NA 2007), Iowa City, IA, USA, 12–14 September 2007; pp. 1–10. [Google Scholar]
- Winters, J.M.; Stark, L. Estimated mechanical properties of synergistic muscles involved in movements of a variety of human joints. J. Biomech. 1998, 21, 1027–1041. [Google Scholar] [CrossRef]
- Brolin, K.; Halldin, P.; Leijonhufvud, I. The effect of muscle activation on neck response. Traffic Inj. Prev. 2005, 6, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kallemeyn, N.; Gandhi, A.; Kode, S.; Shivanna, K.; Smucker, J.; Grosland, N. Validation of a C2–C7 cervical spine finite element model using specimen-specific flexibility data. Med. Eng. Phys. 2010, 32, 482–489. [Google Scholar] [CrossRef] [PubMed]
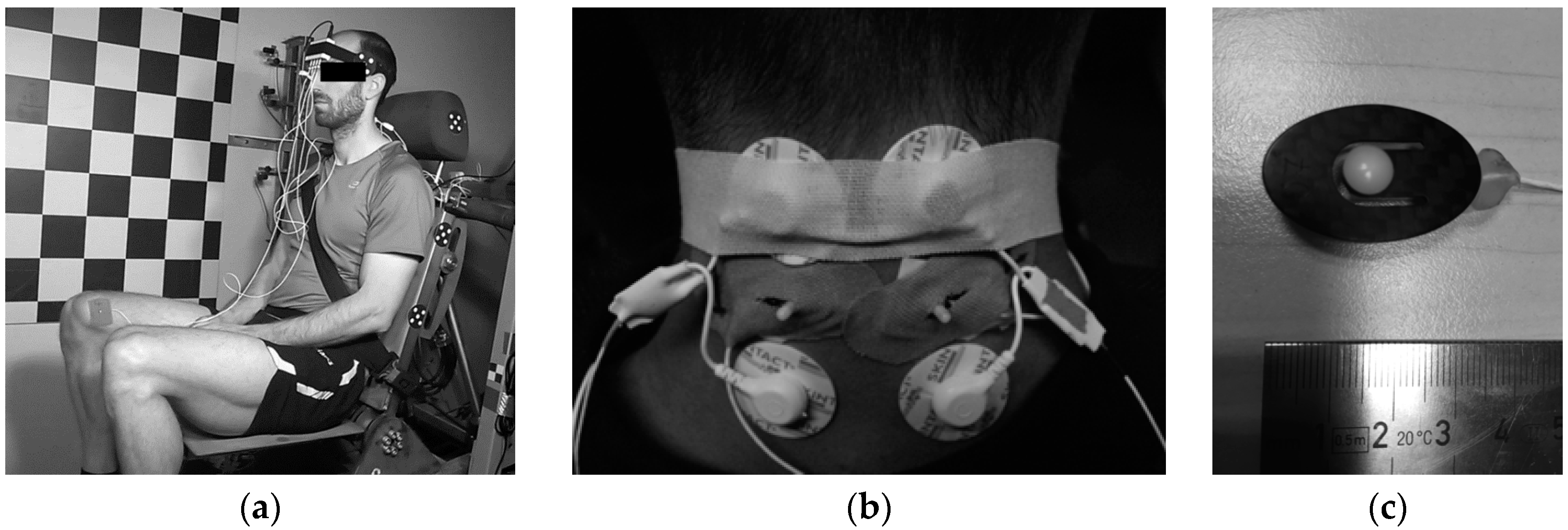


| Low | Medium | High | |
|---|---|---|---|
| impact velocity (km/h) | 7.6 ± 0.39 | 9.5 ± 0.50 | 11.5 ± 0.38 |
| max. deceleration (g) | 1.7 ± 0.05 | 2.6 ± 0.07 | 3.8 ± 0.11 |
| Test Subject | Center of Gravity Location (cm) | Occipital Condyle Location (cm) | Mass | Moment of Inertia |
|---|---|---|---|---|
| Males: 1, 2, 4, 5, 7, 9, 10 | 4.323 | 226.05 | ||
| Females: 3, 6, 8 | 4.125 | 198.5 |
| Low | Medium | High | |
|---|---|---|---|
| head position x (cm) | 1.9 ± 2.5 | 0.8 ± 2.0 | 0.2 ± 2.4 |
| Frankfurt plane (°) | 2.2 ± 5.3 | −2.5 ± 5.7 | −3.0 ± 6.9 |
| Low | Medium | High | (EMG) | p-Value (EMG) | (MC) | p-Value (MC) | |
|---|---|---|---|---|---|---|---|
| Peak values | |||||||
| head excursion (mm) | 141.1 ± 39.9 | 168.1 ± 34.9 | 178.5 ± 26.5 | 0.285 | 0.0271 | 0.444 | 0.0003 |
| head rotation (°) | 17.8 ± 9.9 | 26.6 ± 12.5 | 31.7 ± 12.3 | 0.153 | 0.2423 | 0.397 | 0.0016 |
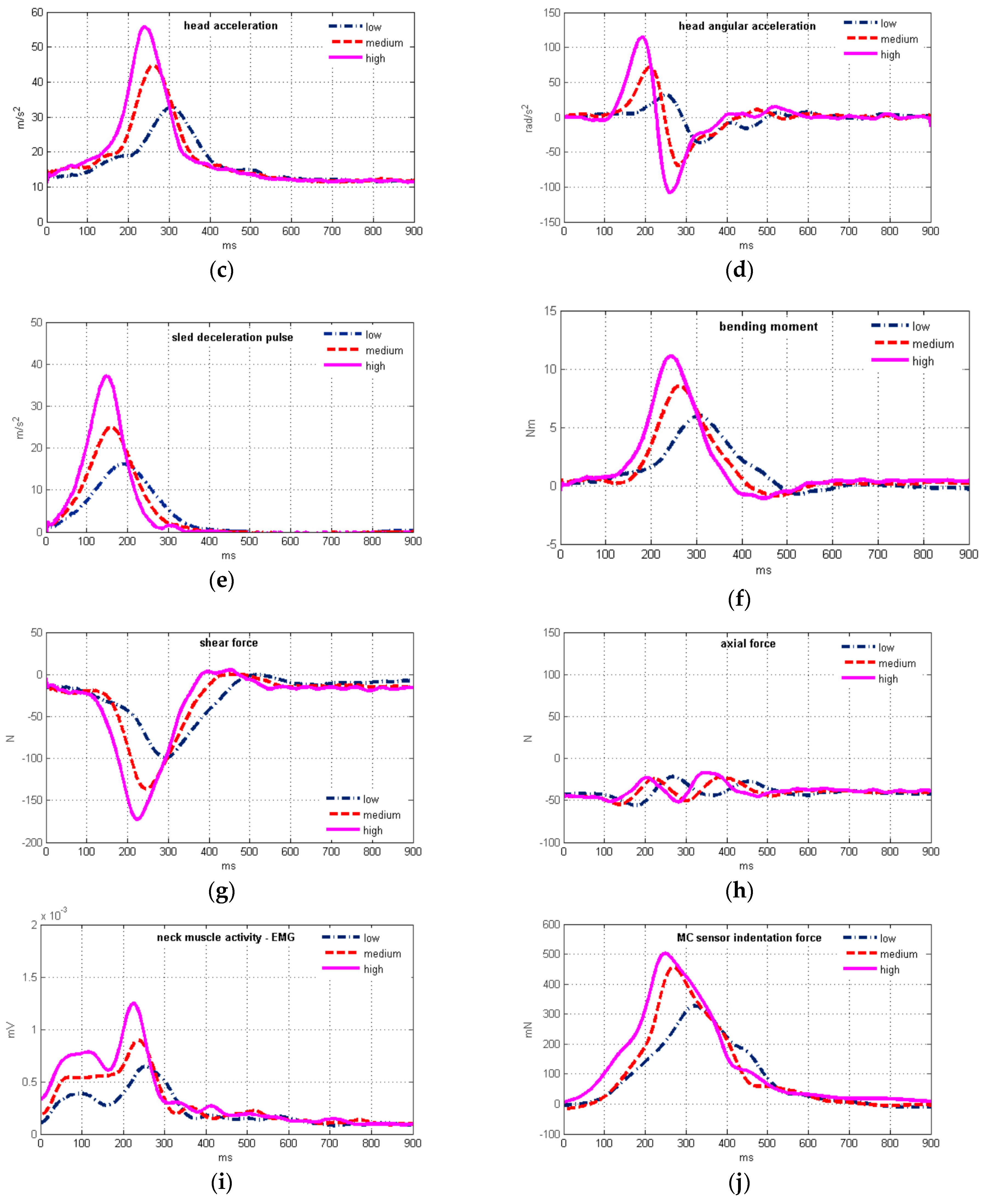
| head acceleration (m/s2) | 32.7 ± 2.9 | 41.7 ± 3.8 | 50.7 ± 4.7 | 0.715 | <0.0001 | 0.500 | <0.0001 |
| angular acceleration (rad/s2) | −52.1 ± 23.9 | −97.0 ± 30.0 | −122.6 ± 36.7 | −0.504 | <0.0001 | −0.553 | <0.0001 |
| axial force (N) | 14.4 ± 12.0 | 7.5 ± 16.7 | 3.3 ± 16.3 | −0.057 | 0.6643 | −0.363 | 0.0043 |
| shear force (N) | 103.9 ± 12.8 | 139.7 ± 19.3 | 174.7 ± 26.4 | 0.670 | <0.0001 | 0.510 | <0.0001 |
| bending moment (Nm) | −6.28 ± 1.03 | −8.98 ± 2.09 | −11.42 ± 2.56 | −0.567 | <0.0001 | −0.515 | <0.0001 |
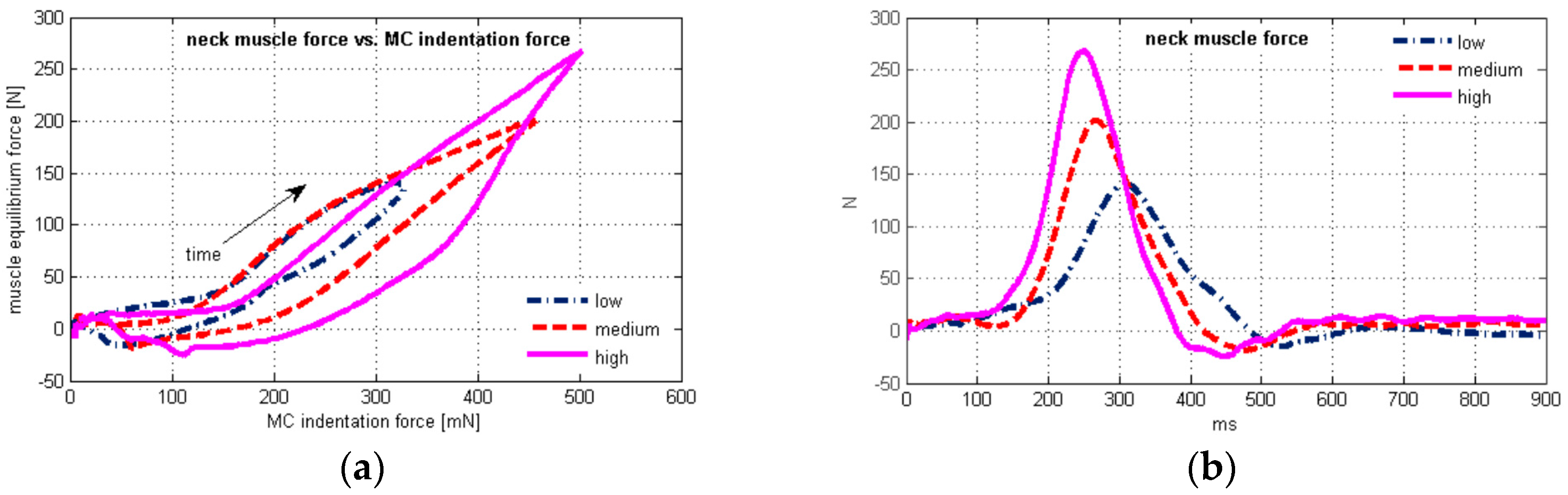
| neck muscle force (N) | 146.1 ± 28.4 | 213.6 ± 61.4 | 277.9 ± 77.9 | 0.513 | <0.0001 | 0.575 | <0.0001 |
| EMG (µV) | 63.0 ± 13.2 | 86.6 ± 17.3 | 108.2 ± 23.2 | ||||
| MC sensor (mN) | 381.2 ± 181.3 | 481.5 ± 174.8 | 572.4 ± 214.3 | ||||
| Timing of peak values | |||||||
| head excursion (ms) | 332.0 ± 27.7 | 289.5 ± 26.1 | 264.8 ± 17.9 | 0.411 | 0.0011 | 0.542 | <0.0001 |
| head rotation (ms) | 387.5 ± 41.5 | 341.9 ± 41.3 | 318.4 ± 45.1 | 0.154 | 0.2386 | 0.329 | 0.0100 |
| head acceleration (ms) | 289.1 ± 16.2 | 239.6 ± 13.4 | 220.9 ± 10.6 | 0.594 | <0.0001 | 0.753 | <0.0001 |
| angular acceleration (ms) | 328.2 ± 16.3 | 290.4 ± 28.7 | 264.7 ± 22.2 | 0.349 | 0.0062 | 0.563 | <0.0001 |
| axial force (ms) | 327.9 ± 85.0 | 309.9 ± 96.9 | 290.9 ± 85.9 | 0.047 | 0.7222 | 0.158 | 0.2258 |
| shear force (ms) | 293.1 ± 18.1 | 243.9 ± 11.9 | 223.6 ± 9.4 | 0.591 | <0.0001 | 0.742 | <0.0001 |
| bending moment (ms) | 308.5 ± 17.9 | 264.9 ± 17.4 | 246.1 ± 11.3 | 0.557 | <0.0001 | 0.638 | <0.0001 |
| neck muscle force (ms) | 311.4 ± 17.3 | 268.5 ± 19.8 | 250.8 ± 14.4 | 0.513 | <0.0001 | 0.600 | <0.0001 |
| EMG (ms) | 244.0 ± 42.4 | 205.3 ± 34.9 | 196.0 ± 28.9 | ||||
| MC sensor (ms) | 315.9 ± 25.4 | 267.3 ± 22.1 | 246.6 ± 21.0 |
| Delay (ms) | Low | Medium | High | |||
|---|---|---|---|---|---|---|
| EMG | MC | EMG | MC | EMG | MC | |
| head excursion | 87.9 ± 41.9 | 16.0 ± 41.8 | 84.2 ± 46.2 | 22.1 ± 37.9 | 68.7 ± 36.8 | 18.0 ± 31.3 |
| head rotation | 143.5 ± 54.1 | 71.6 ± 56.0 | 136.6 ± 86.0 | 74.5 ± 51.5 | 122.4 ± 61.7 | 71.7 ± 54.1 |
| head acceleration | 45.0 ± 38.5 | −26.8 ± 25.1 | 34.2 ± 33.9 | −27.8 ± 21.0 | 24.8 ± 29.1 | −25.8 ± 23.6 |
| angular acceleration | 84.2 ± 50.5 | 12.3 ± 35.5 | 80.3 ± 48.9 | 18.2 ± 34.1 | 65.0 ± 35.6 | 14.4 ± 29.5 |
| axial force | 83.9 ± 105.2 | 12.0 ± 98.7 | 104.6 ± 105.7 | 42.5 ± 98.5 | 94.8 ± 92.7 | 44.1 ± 87.5 |
| shear force | 49.0 ± 39.4 | −22.8 ± 26.8 | 38.6 ± 33.3 | −23.4 ± 22.0 | 27.6 ± 28.8 | −23.0 ± 24.1 |
| bending moment | 64.5 ± 38.1 | −7.3 ± 31.9 | 59.6 ± 37.7 | −2.4 ± 29.5 | 50.0 ± 29.5 | −0.5 ± 26.8 |
| neck muscle force | 67.3 ± 37.9 | −4.5 ± 31.7 | 63.2 ± 40.9 | 1.2 ± 30.8 | 54.7 ± 33.9 | 4.1 ± 30.1 |
| Low | Medium | High | ||||
|---|---|---|---|---|---|---|
| vs. EMG | vs. MC | vs. EMG | vs. MC | vs. EMG | vs. MC | |
| head excursion | <0.0001 | 0.0635 | <0.0001 | 0.0064 | <0.0001 | 0.0058 |
| head rotation | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| head acceleration | <0.0001 | 0.0001 | 0.0002 | <0.0001 | 0.0012 | 0.0001 |
| angular acceleration | <0.0001 | 0.1373 | <0.0001 | 0.0272 | <0.0001 | 0.0416 |
| axial force | 0.0020 | 0.5921 | 0.0003 | 0.0684 | 0.0002 | 0.0360 |
| shear force | <0.0001 | 0.0012 | <0.0001 | 0.0001 | 0.0004 | 0.0004 |
| bending moment | <0.0001 | 0.3142 | <0.0001 | 0.7203 | <0.0001 | 0.9245 |
| neck muscle force | <0.0001 | 0.5316 | <0.0001 | 0.8637 | <0.0001 | 0.5475 |
| Subject | Low | Medium | High | |||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 1 | Test 2 | Test 1 | |
| 1 | 0.776 | 0.936 | 0.824 | 0.886 | 0.851 | 0.849 |
| 2 | 0.631 | 0.739 | 0.724 | 0.844 | 0.877 | 0.722 |
| 3 | 0.381 | 0.400 | 0.512 | 0.500 | 0.493 | 0.513 |
| 4 | 0.829 | 0.947 | 0.827 | 0.950 | 0.877 | 0.842 |
| 5 | 0.773 | 0.746 | 0.910 | 0.821 | 0.899 | 0.913 |
| 6 | 0.883 | 0.881 | 0.801 | 0.807 | 0.825 | 0.855 |
| 7 | 0.768 | 0.857 | 0.738 | 0.762 | 0.691 | 0.809 |
| 8 | 0.780 | 0.781 | 0.766 | 0.822 | 0.710 | 0.783 |
| 9 | 0.899 | 0.886 | 0.838 | 0.883 | 0.839 | 0.838 |
| 10 | 0.944 | 0.920 | 0.942 | 0.721 | 0.856 | 0.862 |
| * mean | 1.181 | 1.157 | 1.143 | |||
| mean | 0.827 | 0.820 | 0.815 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krašna, S.; Đorđević, S.; Hribernik, M.; Trajkovski, A. A Novel Approach to Measuring Muscle Mechanics in Vehicle Collision Conditions. Sensors 2017, 17, 1389. https://doi.org/10.3390/s17061389
Krašna S, Đorđević S, Hribernik M, Trajkovski A. A Novel Approach to Measuring Muscle Mechanics in Vehicle Collision Conditions. Sensors. 2017; 17(6):1389. https://doi.org/10.3390/s17061389
Chicago/Turabian StyleKrašna, Simon, Srđan Đorđević, Marija Hribernik, and Ana Trajkovski. 2017. "A Novel Approach to Measuring Muscle Mechanics in Vehicle Collision Conditions" Sensors 17, no. 6: 1389. https://doi.org/10.3390/s17061389




