The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season
Abstract
:1. Introduction
- (1)
- How does the measurement setup (a single leaf or a leaf stack) influence a reflectance curve in selected spectral ranges (VIS, NIR and SWIR)?
- (2)
- Does the difference between the reflectances measured on a single leaf and a leaf stack in the above selected spectral ranges differ during the season?
- (3)
- Are the water- and pigment-related indices affected by the contact probe measurement setup (a single leaf or a leaf stack) during the season?
2. Materials and Methods
2.1. Study Site
2.2. Leaf Spectra Measurements
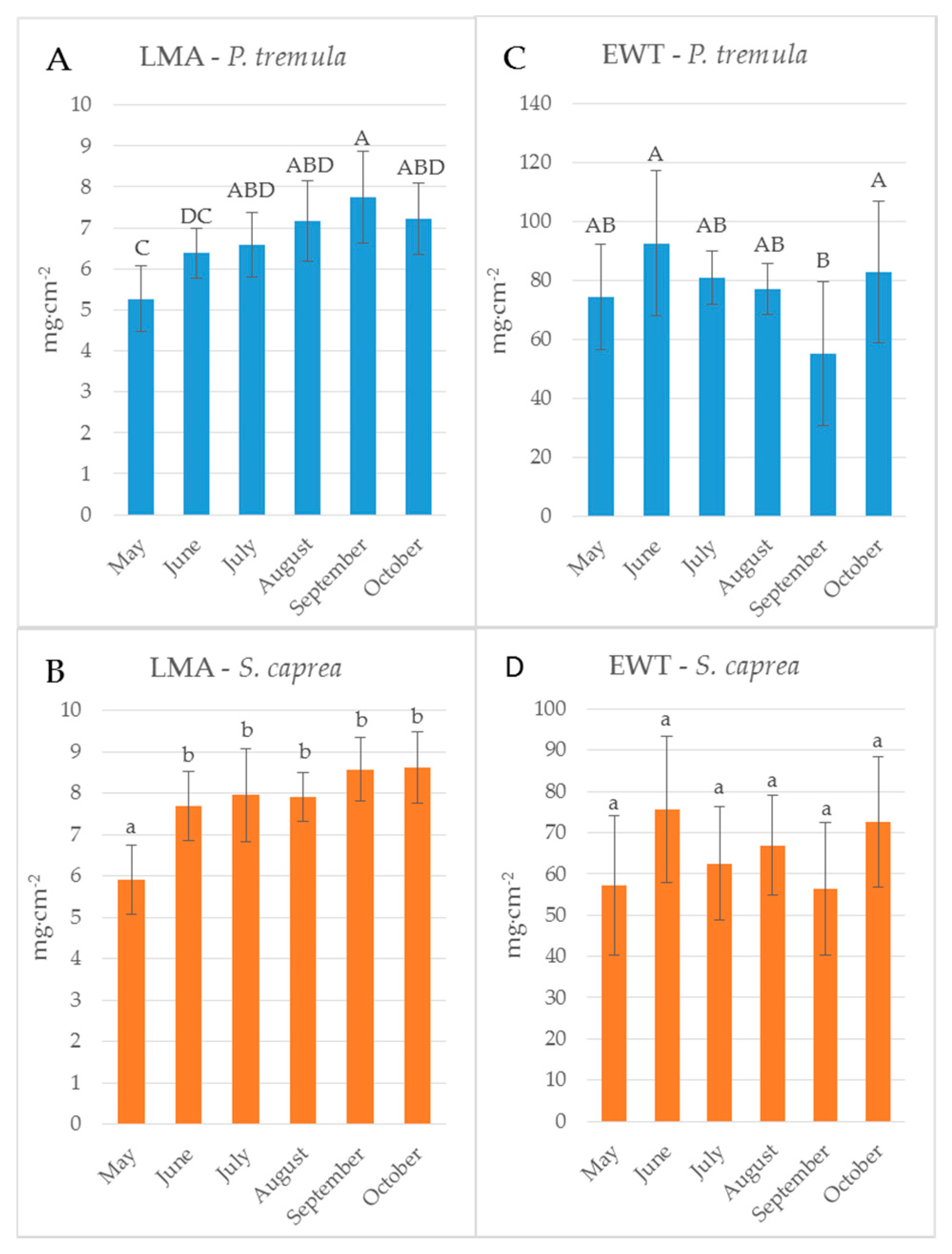
2.3. Assessment of Biophysical Leaf Traits
2.4. Spectral Ranges and Vegetation Indices
2.5. Statistical Analyses
3. Results and Discussion
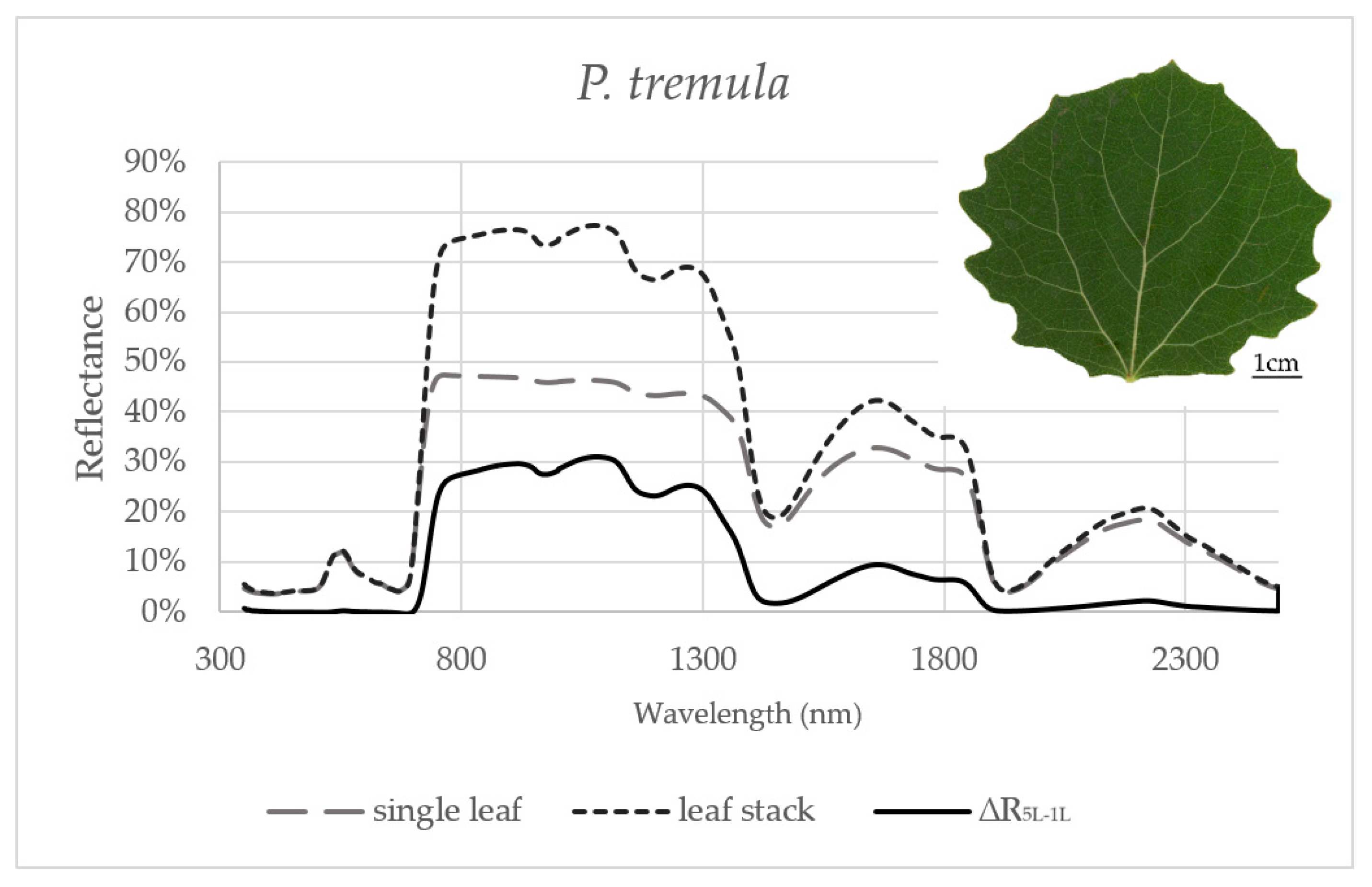
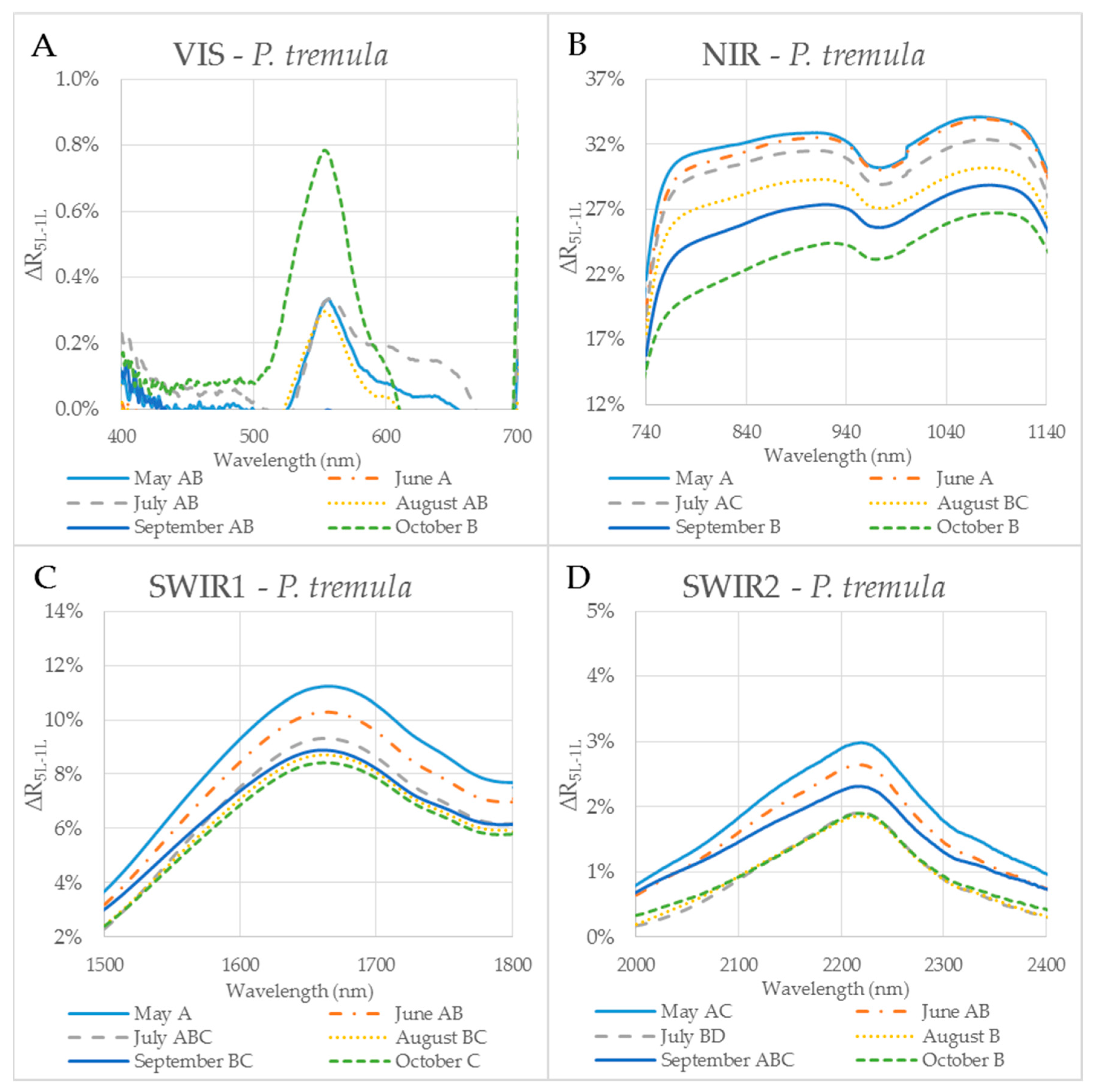
3.1. Effect of the Measurement Setup on the Reflectance Curve in Selected Spectral Ranges
3.2. Seasonal Dynamics of the Difference between Reflectances of a Leaf Stack and a Single Leaf
3.3. The Effect of the Measurement Setup on Vegetation Indices during the Season
3.4. Leaf Stack as an Intermediate Step towards the Canopy Reflectance
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campbell, P.K.E.; Rock, B.N.; Martin, M.E.; Neefus, C.D.; Irons, J.R.; Middleton, E.M.; Albrechtova, J. Detection of initial damage in Norway spruce canopies using hyperspectral airborne data. Int. J. Remote Sens. 2004, 25, 5557–5584. [Google Scholar] [CrossRef]
- Mišurec, J.; Kopačková, V.; Lhotáková, Z.; Hanuš, J.; Weyermann, J.; Entcheva-Campbell, P.; Albrechtová, J. Utilization of hyperspectral image optical indices to assess the Norway spruce forest health status. J. Appl. Remote Sens. 2012, 6, 063545. [Google Scholar]
- Kopačková, V.; Lhotáková, Z.; Oulehle, F.; Albrechtová, J. Assessing forest health via linking the geochemical properties of a soil profile with the biochemical parameters of vegetation. Int. J. Environ. Sci. Technol. 2015, 12, 1987–2002. [Google Scholar] [CrossRef]
- Rapaport, T.; Hochberg, U.; Rachmilevitch, S.; Karnieli, A. The Effect of Differential Growth Rates across Plants on Spectral Predictions of Physiological Parameters. PLoS ONE 2014, 9, e88930. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Zhang, Y. The applicability of empirical vegetation indices for determining leaf chlorophyll content over different leaf and canopy structures. Ecol. Complex. 2014, 17, 119–130. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Mottus, M.; Sulev, M.; Hallik, L. Seasonal Course of the Spectral Properties of Alder and Birch Leaves. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2496–2505. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Gessler, P.E.; Smith, A.M.S.; Robberecht, R. Suitability of existing and novel spectral indices to remotely detect water stress in Populus spp. For. Ecol. Manag. 2006, 229, 170–182. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Meireles, J.E.; Couture, J.J.; Kaproth, M.A.; Kingdon, C.C.; Singh, A.; Serbin, S.P.; Center, A.; Zuniga, E.; Pilz, G.; et al. Associations of Leaf Spectra with Genetic and Phylogenetic Variation in Oaks: Prospects for Remote Detection of Biodiversity. Remote Sens. 2016, 8, 221. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Lhotakova, Z.; Brodsky, L.; Kupkova, L.; Kopackova, V.; Potuckova, M.; Misurec, J.; Klement, A.; Kovarova, M.; Albrechtova, J. Detection of multiple stresses in Scots pine growing at post-mining sites using visible to near-infrared spectroscopy. Environ. Sci. Process. Impacts 2013, 15, 2004–2015. [Google Scholar]
- Einzmann, K.; Ng, W.-T.; Immitzer, M.; Pinnel, N.; Atzberger, C. Method Analysis for Collecting and Processing in-situ Hyperspectral Needle Reflectance Data for Monitoring Norway Spruce; Methodenanalyse zur Erfassung und Prozessierung hyperspektraler in-situ Nadelreflexionsdaten zum Monitoring von Fichten. Photogramm. Fernerkund. Geoinform. 2014, 2014, 423–434. [Google Scholar] [CrossRef]
- Lu, X.; Peng, H. Predicting Cherry Leaf Chlorophyll Concentrations Based on Foliar Reflectance Spectra Variables. J. Indian Soc. Remote Sens. 2015, 43, 109–120. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between Spectral Reflectance and Pigment Concentrations in Stacks of Deciduous Broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Dian, Y.; Le, Y.; Fang, S.; Xu, Y.; Yao, C.; Liu, G. Influence of Spectral Bandwidth and Position on Chlorophyll Content Retrieval at Leaf and Canopy Levels. J. Indian Soc. Remote Sens. 2016, 44, 583–593. [Google Scholar] [CrossRef]
- Gastellu-Etchegorry, J.P.; Martin, E.; Gascon, F. DART: A 3D model for simulating satellite images and studying surface radiation budget. Int. J. Remote Sens. 2004, 25, 73–96. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrow-band indices and PROSPECT + DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Huang, N.; Niu, Z.; Zhan, Y.; Xu, S.; Tappert, M.C.; Wu, C.; Huang, W.; Gao, S.; Hou, X.; Cai, D. Relationships between soil respiration and photosynthesis-related spectral vegetation indices in two cropland ecosystems. Agric. For. Meteorol. 2012, 160, 80–89. [Google Scholar] [CrossRef]
- Yi, Q.; Jiapaer, G.; Chen, J.; Bao, A.; Wang, F. Different units of measurement of carotenoids estimation in cotton using hyperspectral indices and partial least square regression. ISPRS J. Photogramm. Remote Sens. 2014, 91, 72–84. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.-F.; Van Devender, A.; Lin, G.-Z.; Peng, C.-L.; Pan, X.-P.; Chen, S.-W.; Gu, Q. Spectral reflectance indices and pigment functions during leaf ontogenesis in six subtropical landscape plants. Plant Growth Regul. 2009, 58, 73–84. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Castro, K.L.; Sanchez-Azofeifa, G.A. Changes in spectral properties, chlorophyll content and internal mesophyll structure of senescing Populus balsamifera and Populus tremuloides leaves. Sensors 2008, 8, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of Red Edge Position and Chlorophyll Content by Reflectance Measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Le Maire, G.; François, C.; Soudani, K.; Berveiller, D.; Pontailler, J.-Y.; Bréda, N.; Genet, H.; Davi, H.; Dufrêne, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- González-Fernández, A.B.; Rodríguez-Pérez, J.R.; Marcelo, V.; Valenciano, J.B. Using field spectrometry and a plant probe accessory to determine leaf water content in commercial vineyards. Agric. Water Manag. 2015, 156, 43–50. [Google Scholar] [CrossRef]
- Baránková, B.; Lazár, D.; Nauš, J. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens. Environ. 2016, 174, 181–196. [Google Scholar] [CrossRef]
- Nakaji, T.; Kosugi, Y.; Takanashi, S.; Niiyama, K.; Noguchi, S.; Tani, M.; Oguma, H.; Nik, A.R.; Kassim, A.R. Estimation of light-use efficiency through a combinational use of the photochemical reflectance index and vapor pressure deficit in an evergreen tropical rainforest at Pasoh, Peninsular Malaysia. Remote Sens. Environ. 2014, 150, 82–92. [Google Scholar] [CrossRef]
- Dillen, S.Y.; de Beeck, M.O.; Hufkens, K.; Buonanduci, M.; Phillips, N.G. Seasonal patterns of foliar reflectance in relation to photosynthetic capacity and color index in two co-occurring tree species, Quercus rubra and Betula papyrifera. Agric. For. Meteorol. 2012, 160, 60–68. [Google Scholar] [CrossRef]
- Fréchette, E.; Wong, C.Y.S.; Junker, L.V.; Chang, C.Y.-Y.; Ensminger, I. Zeaxanthin-independent energy quenching and alternative electron sinks cause a decoupling of the relationship between the photochemical reflectance index (PRI) and photosynthesis in an evergreen conifer during spring. J. Exp. Bot. 2015, 66, 7309–7323. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Ollinger, S.V.; Hollinger, D.Y.; Wicklein, H.F.; Richardson, A.D. Canopy-scale relationships between foliar nitrogen and albedo are not observed in leaf reflectance and transmittance within temperate deciduous tree species. Botany 2011, 89, 491–497. [Google Scholar] [CrossRef]
- Frouz, J.; Keplin, B.; Pižl, V.; Tajovský, K.; Starý, J.; Lukešová, A.; Nováková, A.; Balı́k, V.; Háněl, L.; Materna, J.; et al. Soil biota and upper soil layer development in two contrasting post-mining chronosequences. Ecol. Eng. 2001, 17, 275–284. [Google Scholar] [CrossRef]
- Mobasheri, M.R.; Fatemi, S.B. Leaf Equivalent Water Thickness assessment using reflectance at optimum wavelengths. Theor. Exp. Plant Physiol. 2013, 25, 196–202. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Liu, J.G.; Moore, J.M. Hue image RGB colour composition. A simple technique to suppress shadow and enhance spectral signature. Int. J. Remote Sens. 1990, 11, 1521–1530. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Le Maire, G.; François, C.; Dufrêne, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Smith, R.C.G.; Adams, J.; Stephens, D.J.; Hick, P.T.; Smith, R.C.G.; Adams, J.; Stephens, D.J.; Hick, P.T. Forecasting wheat yield in a Mediterranean-type environment from the NOAA satellite. Crop Pasture Sci. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Maccioni, A.; Agati, G.; Mazzinghi, P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J. Photochem. Photobiol. B 2001, 61, 52–61. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- McMurtrey, J.E.; Chappelle, E.W.; Kim, M.S.; Meisinger, J.J.; Corp, L.A. Distinguishing nitrogen fertilization levels in field corn (Zea mays L.) with actively induced fluorescence and passive reflectance measurements. Remote Sens. Environ. 1994, 47, 36–44. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using Near- and Middle-Infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Serrano, L.; Gamon, J.A.; Berry, J. Estimation of leaf area with an integrating sphere. Tree Physiol. 1997, 17, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; NASA/GSFC Type III Final Report; NASA: Greenbelt, MD, USA, 1974; p. 371. [Google Scholar]
- Stimson, H.C.; Breshears, D.D.; Ustin, S.L.; Kefauver, S.C. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote Sens. Environ. 2005, 96, 108–118. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.; Jackson, T.J. Vegetation water content estimation for corn and soybeans using spectral indices derived from MODIS near- and short-wave infrared bands. Remote Sens. Environ. 2005, 98, 225–236. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Bravo, C.; Moshou, D.; Oberti, R.; West, J.; McCartney, A.; Bodria, L.; Ramon, H. Foliar Disease Detection in the Field Using Optical Sensor Fusion. J. Sci. Res. Dev. 2004, 6, 1–14. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Suárez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Garrity, S.R.; Eitel, J.U.H.; Vierling, L.A. Disentangling the relationships between plant pigments and the photochemical reflectance index reveals a new approach for remote estimation of carotenoid content. Remote Sens. Environ. 2011, 115, 628–635. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilisation de la Haute Resolution Spectrale pour Suivre L’etat des Couverts Vegetaux. In Proceedings of the Spectral Signature of Objects in Remote Sensing, Aussois, France, 18–22 January 1988; p. 279. [Google Scholar]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I.; Lloret, P.; Muñoz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R. Land cover mapping at BOREAS using red edge spectral parameters from CASI imagery. J. Geophys. Res. 1999, 104, 27921–27933. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Rueda, C.; Ustin, S. Water content estimation in vegetation with MODIS reflectance data and model inversion methods. Remote Sens. Environ. 2003, 85, 109–124. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Buschmann, C.; Lenk, S.; Lichtenthaler, H.K. Reflectance spectra and images of green leaves with different tissue structure and chlorophyll content. Isr. J. Plant Sci. 2012, 60, 49–64. [Google Scholar] [CrossRef]
- Slaton, M.R.; Hunt, E.R.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf Form and Photosynthesis. BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Cordón, G.B.; Lagorio, M.G. Optical properties of the adaxial and abaxial faces of leaves. Chlorophyll fluorescence, absorption and scattering coefficients. Photochem. Photobiol. Sci. 2007, 6, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Stuckens, J.; Verstraeten, W.W.; Delalieux, S.; Swennen, R.; Coppin, P. A dorsiventral leaf radiative transfer model: Development, validation and improved model inversion techniques. Remote Sens. Environ. 2009, 113, 2560–2573. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, A.G.; Féret, J.-B.; Jacquemoud, S.; Ustin, S.L. Deriving leaf mass per area (LMA) from foliar reflectance across a variety of plant species using continuous wavelet analysis. ISPRS J. Photogramm. Remote Sens. 2014, 87, 28–38. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef]
- Potůčková, M.; Červená, L.; Kupková, L.; Lhotáková, Z.; Lukeš, P.; Hanuš, J.; Novotný, J.; Albrechtová, J. Comparison of Reflectance Measurements Acquired with a Contact Probe and an Integration Sphere: Implications for the Spectral Properties of Vegetation at a Leaf Level. Sensors 2016, 16, 1801. [Google Scholar] [CrossRef] [PubMed]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Guanter, L.; Estellés, V.; Moreno, J. Spectral calibration and atmospheric correction of ultra-fine spectral and spatial resolution remote sensing data. Application to CASI-1500 data. Remote Sens. Environ. 2007, 109, 54–65. [Google Scholar] [CrossRef]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]




| Populus tremula | Salix caprea | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Formula | R2 | May | June | Jul | Aug | Sep | Oct | R2 | May | June | Jul | Aug | Sep | Oct | |||
| 1L | 5L | 1L | 5L | |||||||||||||||
| BGI | =R450/R550 | 0.42 | 0.33 | 0.20 | 0.16 | [25] | ||||||||||||
| BI | =(R800+R670+R550)/sqrt(3) | 0.01 | 0.10 | 0.00 | 0.00 | [43] | ||||||||||||
| Carter4 | =R710/R760 | 0.52 | 0.42 | 0.40 | 0.33 | [44] | ||||||||||||
| CI | =(R800-R550)/R800 | 0.42 | 0.34 | 0.31 | 0.30 | [43] | ||||||||||||
| CRI550 | =(1/R515)-(1/R550) | [45] | ||||||||||||||||
| CRI700 | =(1/R515)-(1/R700) | [45] | ||||||||||||||||
| CTR | =R695/R760 | 0.40 | 0.29 | 0.36 | 0.22 | [44] | ||||||||||||
| CUR | =(R675·R690)/R6833 | 0.02 | 0.01 | 0.00 | 0.00 | [46] | ||||||||||||
| Datt | =(R850-R710)/(R850-R680) | 0.51 | 0.44 | 0.35 | 0.35 | [47] | ||||||||||||
| Datt2 | =R850/R710 | 0.50 | 0.41 | 0.37 | 0.38 | [47] | ||||||||||||
| DD | =(R749-R720)-(R701-R672) | 0.53 | 0.42 | 0.39 | 0.32 | [48] | ||||||||||||
| DVI | =R800-R670 | 0.31 | 0.21 | 0.09 | 0.06 | [49] | ||||||||||||
| G | =R554/R677 | 0.21 | 0.15 | 0.12 | 0.10 | [25] | ||||||||||||
| Gitelson2 | =(R750-R800/R695-R740)-1 | 0.39 | 0.24 | 0.41 | 0.31 | [45] | ||||||||||||
| GM94a | =R750/R700 | 0.47 | 0.32 | 0.43 | 0.35 | [50] | ||||||||||||
| gNDVI780 | =(R780-R550)/(R780+R550) | 0.42 | 0.34 | 0.32 | 0.31 | [51] | ||||||||||||
| GRg | =(R800/R550)-1 | 0.40 | 0.29 | 0.30 | 0.34 | [45] | ||||||||||||
| Macc01 | =(R780-R710)/(R780-R680) | 0.52 | 0.45 | 0.38 | 0.34 | [52] | ||||||||||||
| MCARI | =((R700-R670)-0.2·(R700-R550))·(R700/R670) | 0.43 | 0.33 | 0.30 | 0.27 | [7] | ||||||||||||
| MCARI1 | =1.2·(2.5·(R800-R670)-1.3·(R800-R550)) | 0.02 | 0.09 | 0.00 | 0.01 | [53] | ||||||||||||
| MCARI2 | =((R750-R705)-0.2·(R750-R550))·(R750/R705) | 0.54 | 0.36 | 0.44 | 0.31 | [54] | ||||||||||||
| MCARI2/OSAV2 | 0.55 | 0.36 | 0.43 | 0.28 | [54] | |||||||||||||
| MCARI/OSAVI | 0.46 | 0.35 | 0.31 | 0.28 | [7] | |||||||||||||
| McM94 | =R700/R670 | 0.37 | 0.28 | 0.28 | 0.23 | [55] | ||||||||||||
| MND | =(R750-R445)/(R750+R705-2·R445) | 0.53 | 0.42 | 0.42 | 0.34 | [15] | ||||||||||||
| mND705 | =(R750-R705)/(R750+R705-2·R445) | 0.53 | 0.42 | 0.42 | 0.34 | [15] | ||||||||||||
| MNDVI1 | =(R755-R745)/(R755+R745) | 0.43 | 0.44 | 0.27 | 0.30 | [56] | ||||||||||||
| MNDVI8 | =(R755-R730)/(R755+R730) | 0.52 | 0.46 | 0.36 | 0.35 | [56] | ||||||||||||
| MNDVIre | =(R750-R705)/(R750+R705-R445) | 0.52 | 0.41 | 0.42 | 0.34 | [15] | ||||||||||||
| MSAVI | =0.5·(2·R800+1-sqrt((2·R800+1)2-8·(R800-R670))) | 0.28 | 0.22 | 0.06 | 0.06 | [57] | ||||||||||||
| MSI | =R1600/R820 | [58] | ||||||||||||||||
| MSR | =((R800-R670)-1)/sqrt((R800/R670)+1) | 0.08 | 0.09 | 0.11 | 0.06 | [59] | ||||||||||||
| MTCI | =(R754-R709)/(R709-R681) | 0.51 | 0.44 | 0.39 | 0.38 | [60] | ||||||||||||
| N705 | =(R705-R675)/(R750-R670) | 0.51 | 0.42 | 0.39 | 0.33 | [1] | ||||||||||||
| N715 | =(R715-R675)/(R750-R670) | 0.52 | 0.46 | 0.37 | 0.36 | [1] | ||||||||||||
| N725 | =(R725-R675)/(R750-R670) | 0.50 | 0.48 | 0.37 | 0.36 | [1] | ||||||||||||
| NDII2 | =(R820-R1650)/(R820+R1650) | [61] | ||||||||||||||||
| NDVI1 | =(R800-R670)/(R800+R670) | 0.09 | 0.10 | 0.11 | 0.06 | [20] | ||||||||||||
| NDVI800680 | =(R800-R680)/(R800+R680) | 0.10 | 0.10 | 0.11 | 0.06 | [62] | ||||||||||||
| NDVIre | =(R750-R705)/(R750+R705) | 0.51 | 0.39 | 0.42 | 0.33 | [50] | ||||||||||||
| NDWI | =(R858-R1240)/(R858+R1240) | [63] | ||||||||||||||||
| NDWI2130 | =(R858-R2130)/(R858+R2130) | [64] | ||||||||||||||||
| NMDI | =(R860-(R1640-R2130))/(R860+(R1640-R2130)) | [65] | ||||||||||||||||
| NPCI | =(R680-R430)/(R680+R430) | 0.25 | 0.16 | 0.13 | 0.08 | [66] | ||||||||||||
| OSAVI | =(1+0.16)·(R800-R670)/(R800+R670+0.16) | 0.25 | 0.17 | 0.13 | 0.08 | [30] | ||||||||||||
| OSAVI2 | =(1+0.16)·(R750-R705)/(R750+R705+0.16) | 0.52 | 0.39 | 0.42 | 0.32 | [54] | ||||||||||||
| PRI | =(R570-R530)/(R570+R530) | 0.21 | 0.15 | 0.15 | 0.18 | [67] | ||||||||||||
| PRI·CI-H | =(R680-R500)/R750 | [68] | ||||||||||||||||
| PRI·CI-Y | =(R570-R530)/(R570+R530)·(R760/R700-1) | [69] | ||||||||||||||||
| PRIm1 | =(R515-R530)/(R515+R530) | 0.25 | 0.22 | 0.00 | 0.00 | [68] | ||||||||||||
| PSNDb | =(R800-R635)/(R800+R635) | 0.30 | 0.23 | 0.35 | 0.19 | [24] | ||||||||||||
| PSNDc | =(R800-R470)/(R800+R470) | [24] | ||||||||||||||||
| PSSRa | =R800/R680 | 0.08 | 0.05 | 0.13 | 0.05 | [24] | ||||||||||||
| PSSRb | =R800/R635 | 0.27 | 0.16 | 0.40 | 0.24 | [24] | ||||||||||||
| PSSRc | =R800/R470 | [24] | ||||||||||||||||
| RDVI | =(R800-R670)/(sqrt(R800+R670)) | 0.18 | 0.11 | 0.14 | 0.07 | [70] | ||||||||||||
| REIP | =(700+40·((Rre-R700)/(R740-R700)))/100 | 0.51 | 0.43 | 0.34 | 0.27 | [71] | ||||||||||||
| REP | =700+40·((((R670+R780)/2)-R700)/(R740-R700)) | 0.51 | 0.42 | 0.34 | 0.27 | [72] | ||||||||||||
| REP-Li | =700+40·((R670+R780/2)/(R740-R700)) | 0.51 | 0.42 | 0.34 | 0.27 | [71] | ||||||||||||
| RMSR | =((R750/R705)-1)/sqrt((R750/R705)+1) | 0.50 | 0.38 | 0.42 | 0.35 | [54] | ||||||||||||
| RNIRCRI550 | =((1/R515-1/R550)·R770) | [45] | ||||||||||||||||
| RNIRCRI700 | =((1/R515-1/R700)·R770) | [45] | ||||||||||||||||
| Rre | =(R670+R780)/2 | 0.25 | 0.22 | 0.05 | 0.05 | [71] | ||||||||||||
| SIPI | =(R800-R445)/(R800-R680) | 0.32 | 0.23 | 0.17 | 0.09 | [73] | ||||||||||||
| SIPI680 | =(R800-R455)/(R800-R680) | 0.32 | 0.24 | 0.12 | 0.08 | [73] | ||||||||||||
| SIPI705 | =(R800-R455)/(R800-R705) | 0.50 | 0.41 | 0.35 | 0.26 | [73] | ||||||||||||
| SR | =R800/R670 | 0.07 | 0.06 | 0.13 | 0.06 | [49] | ||||||||||||
| SR2 | =R750/R710 | 0.51 | 0.41 | 0.41 | 0.37 | [74] | ||||||||||||
| SR3 | =R750/R550 | 0.40 | 0.29 | 0.33 | 0.34 | [75] | ||||||||||||
| SRPI | =R430/R680 | 0.25 | 0.15 | 0.12 | 0.08 | [73] | ||||||||||||
| SRWI | =R858/R1240 | [76] | ||||||||||||||||
| TCARI | =3·((R700-R670)-0.2·(R700-R550)·(R700/R670)) | 0.38 | 0.30 | 0.22 | 0.22 | [77] | ||||||||||||
| TCARI/OSAVI | 0.42 | 0.33 | 0.27 | 0.25 | [77] | |||||||||||||
| TCARI2 | =3·((R750-R705)-0.2·(R750-R550)·(R750/R705)) | 0.12 | 0.29 | 0.00 | 0.26 | [54] | ||||||||||||
| TCARI2/OSAVI2 | 0.41 | 0.35 | 0.28 | 0.35 | [54] | |||||||||||||
| TVI | =0.5·(120·(R750-R550)-200·(R670-R550)) | 0.08 | 0.09 | 0.00 | 0.01 | [78] | ||||||||||||
| VI[700] | =(R700-R670)/(R700+R670) | 0.38 | 0.30 | 0.28 | 0.21 | [79] | ||||||||||||
| Vogelmann | =R740/R720 | 0.51 | 0.44 | 0.40 | 0.37 | [80] | ||||||||||||
| Vogelmann2 | =(R734-R747)/(R715+R726) | 0.52 | 0.45 | 0.38 | 0.36 | [80] | ||||||||||||
| WI | =R900/R970 | [81] | ||||||||||||||||
| P. tremula | VIS | NIR | SWIR1 | SWIR2 |
| Variance | 0.0% | 0.2% | 0.1% | 0.0% |
| Max | 3.2% | 43.3% | 14.0% | 4.8% |
| Min | −3.9% | 10.0% | 0.7% | −0.7% |
| Mean | 0.0% | 28.7% | 7.2% | 1.3% |
| SD | 0.6% | 4.2% | 2.4% | 1.1% |
| S. caprea | VIS | NIR | SWIR1 | SWIR2 |
| Variance | 0.0% | 0.2% | 0.1% | 0.0% |
| Max | 10.1% | 37.4% | 14.0% | 6.0% |
| Min | −3.0% | 6.3% | 1.1% | −0.9% |
| Mean | 0.1% | 26.3% | 7.2% | 1.6% |
| SD | 0.9% | 5.0% | 2.4% | 1.2% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuwirthová, E.; Lhotáková, Z.; Albrechtová, J. The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season. Sensors 2017, 17, 1202. https://doi.org/10.3390/s17061202
Neuwirthová E, Lhotáková Z, Albrechtová J. The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season. Sensors. 2017; 17(6):1202. https://doi.org/10.3390/s17061202
Chicago/Turabian StyleNeuwirthová, Eva, Zuzana Lhotáková, and Jana Albrechtová. 2017. "The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season" Sensors 17, no. 6: 1202. https://doi.org/10.3390/s17061202





