A Cuffless Blood Pressure Measurement Based on the Impedance Plethysmography Technique
Abstract
:1. Introduction
2. Materials and Methods
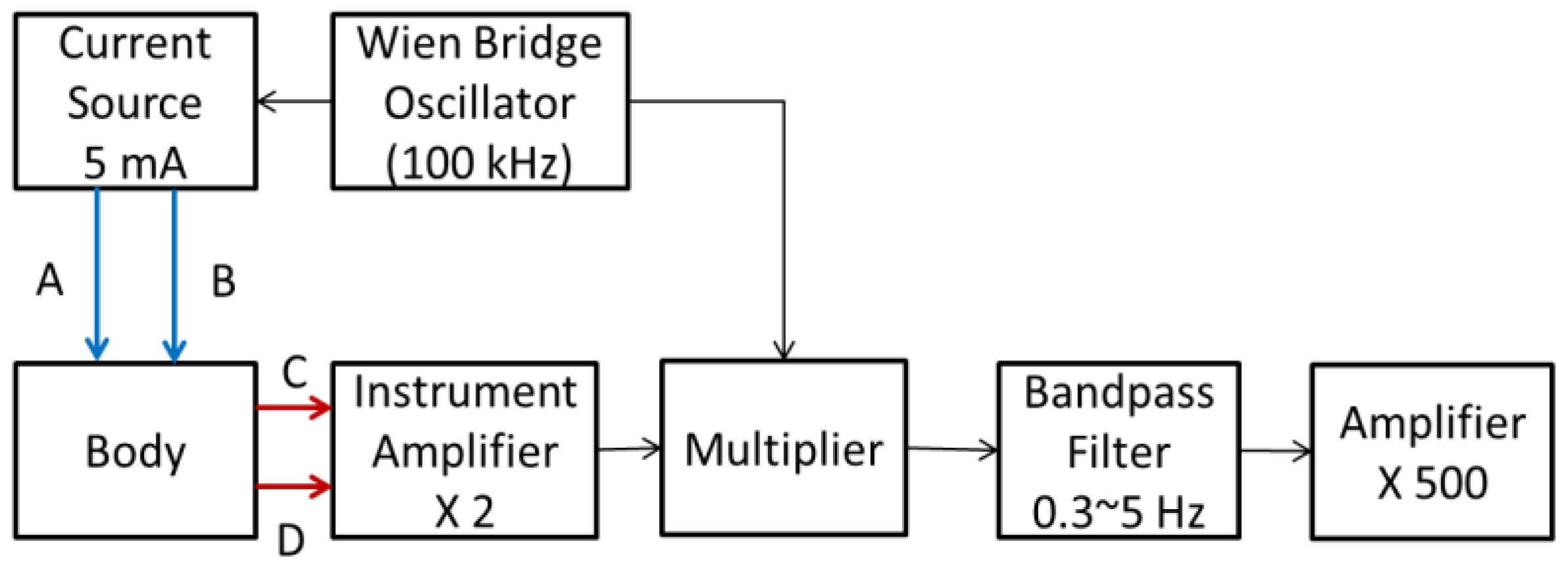
2.1. Impedance Plethysmography Technique
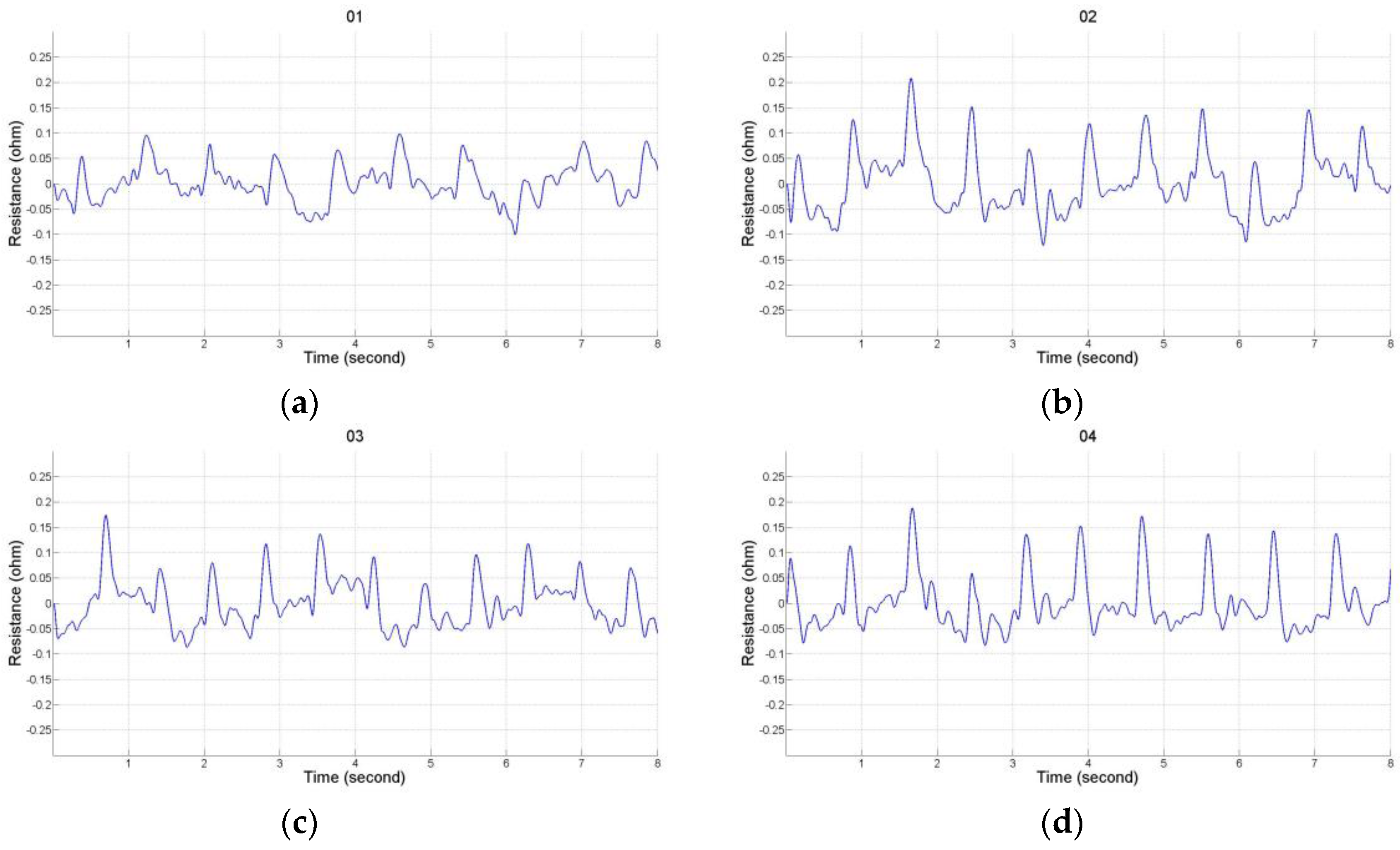
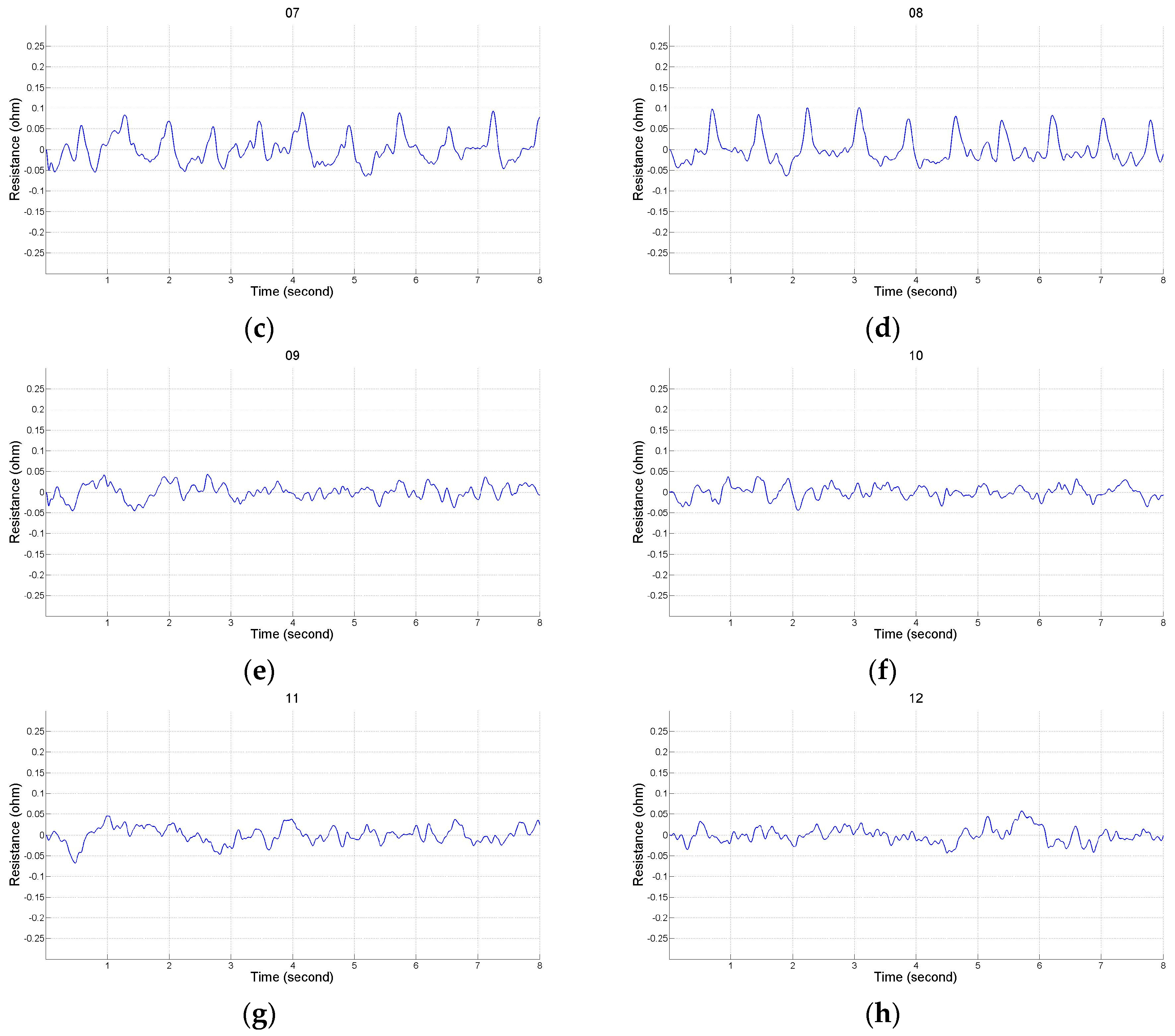
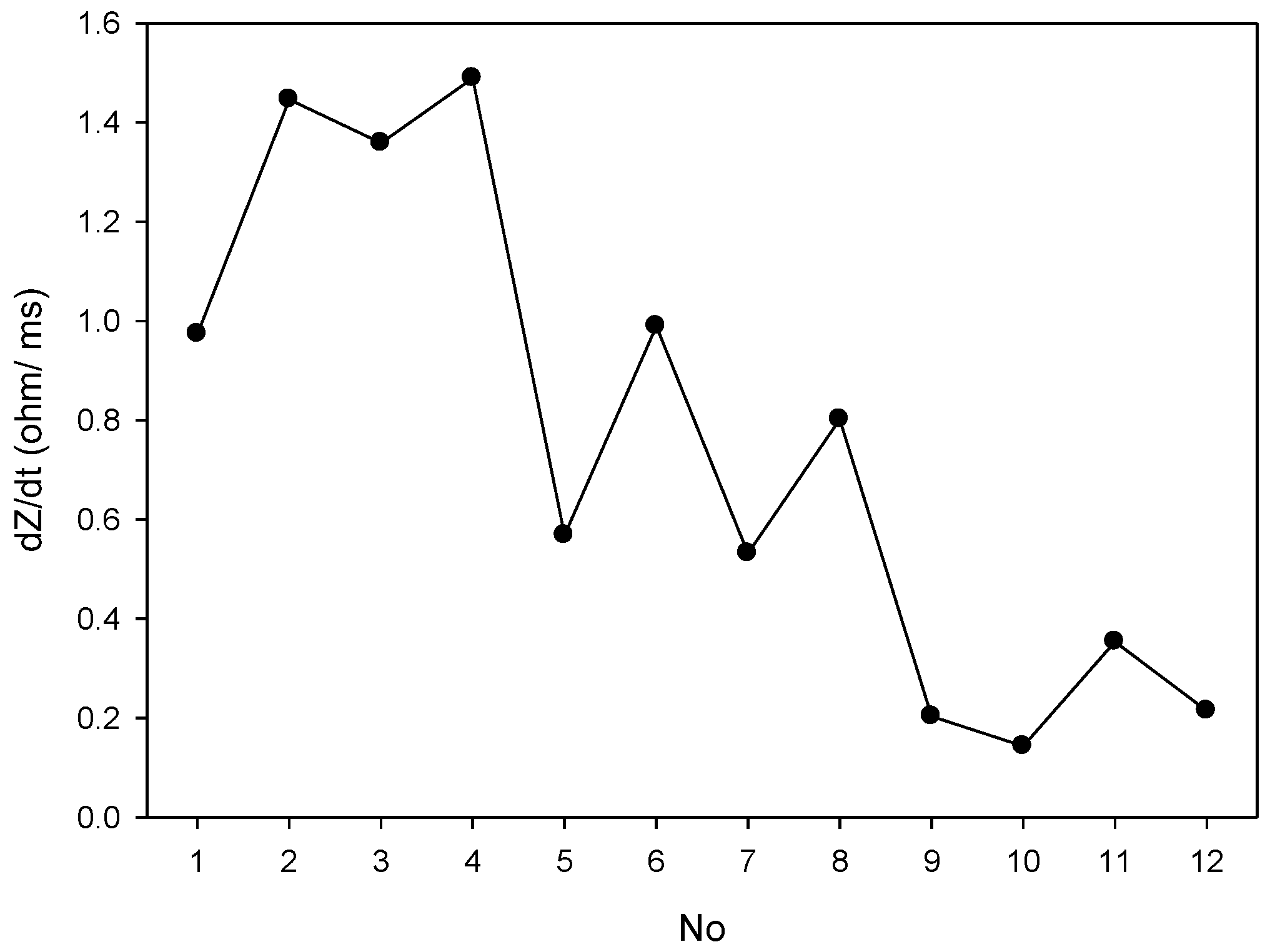
2.2. Impedance Plethysmography Signal
3. Cuffless Blood Pressure Measurement
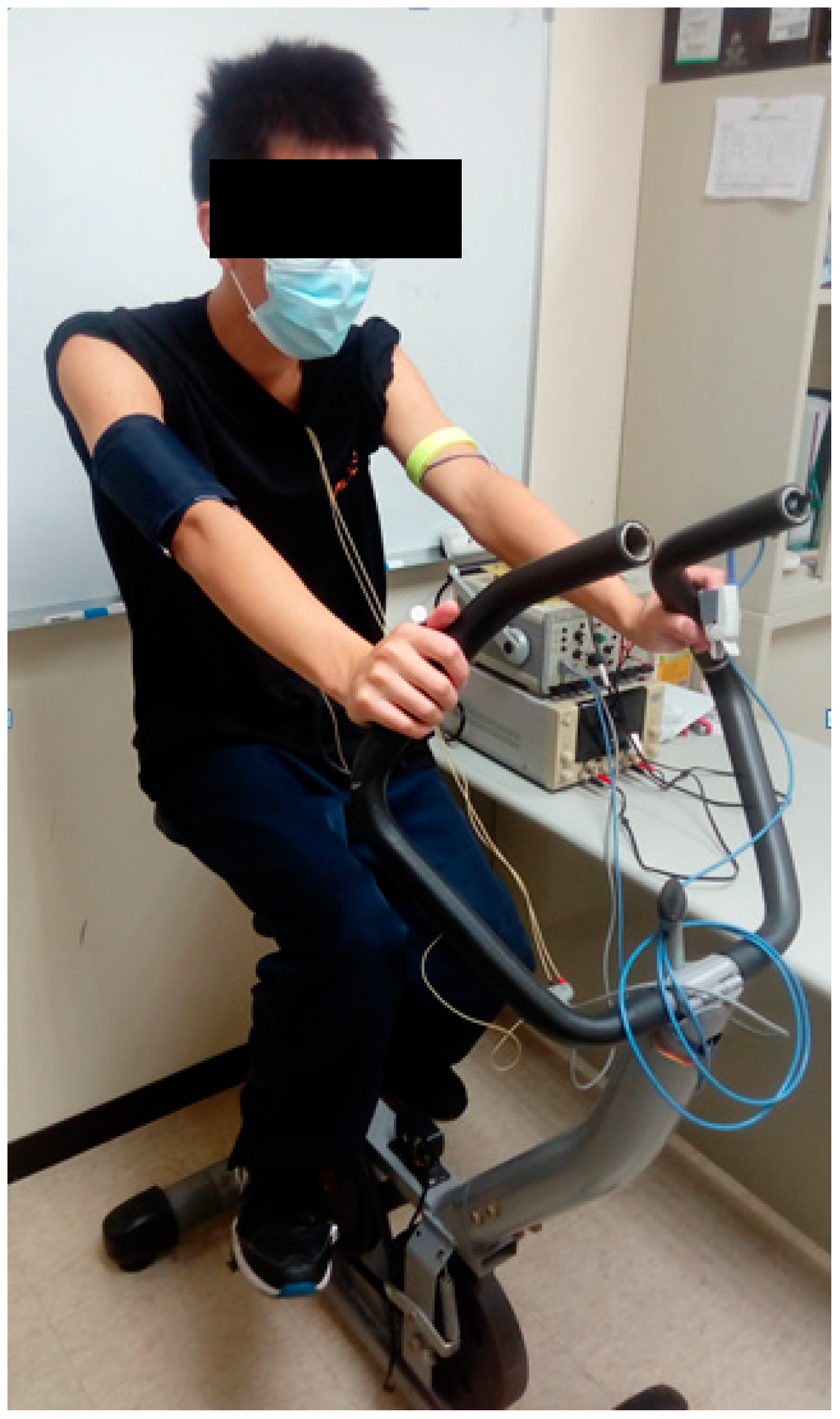
3.1. Experimental Protocol
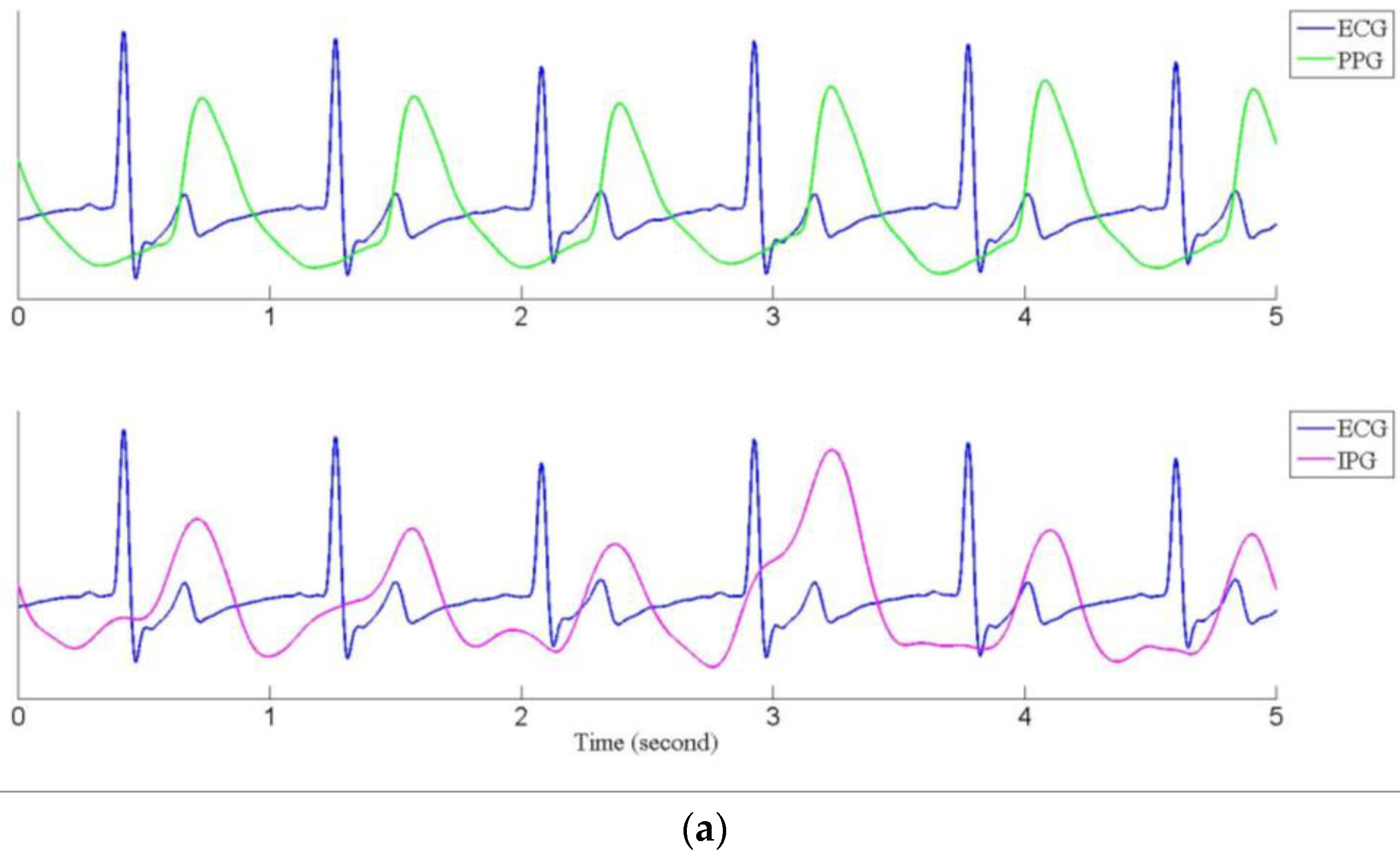
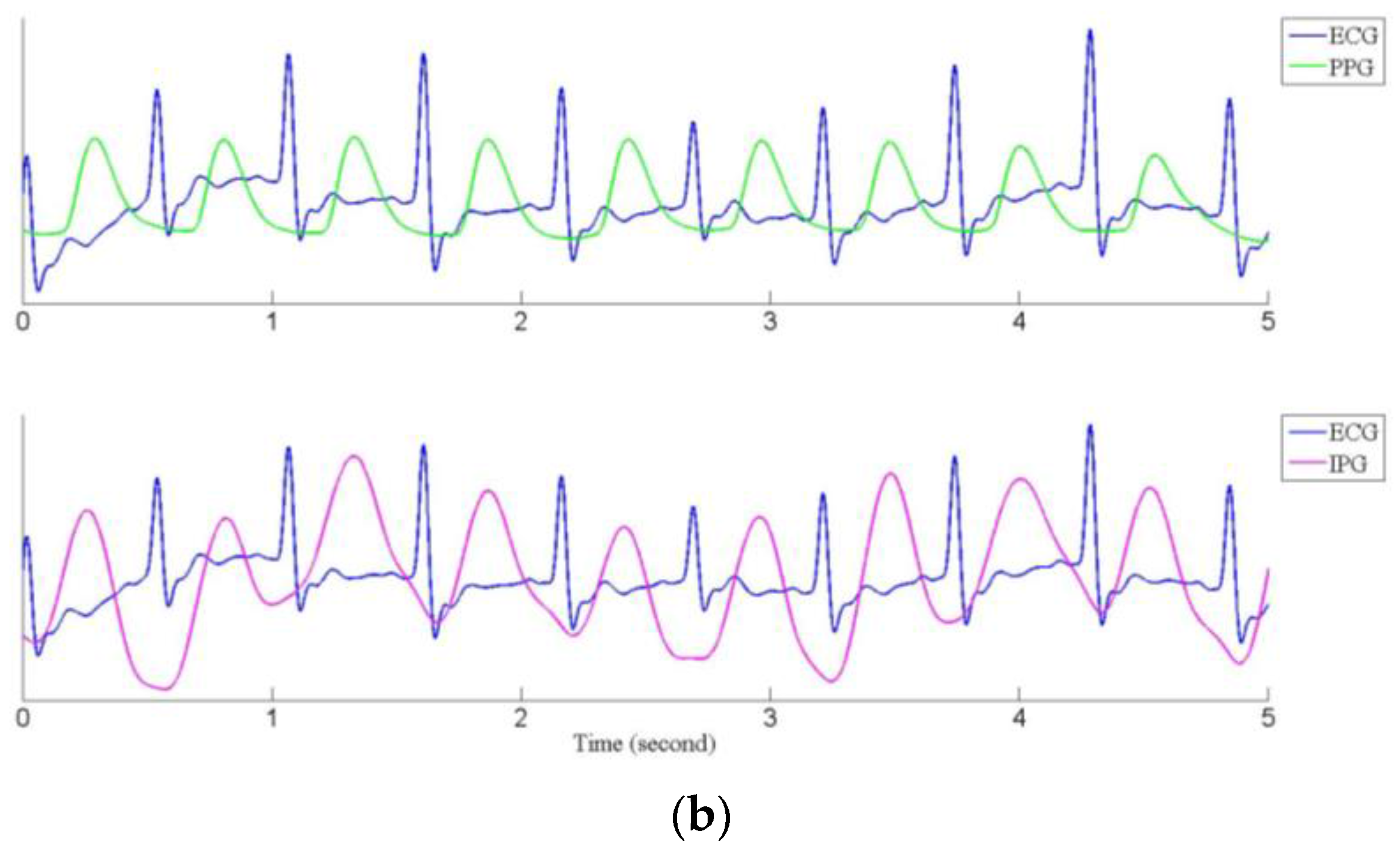
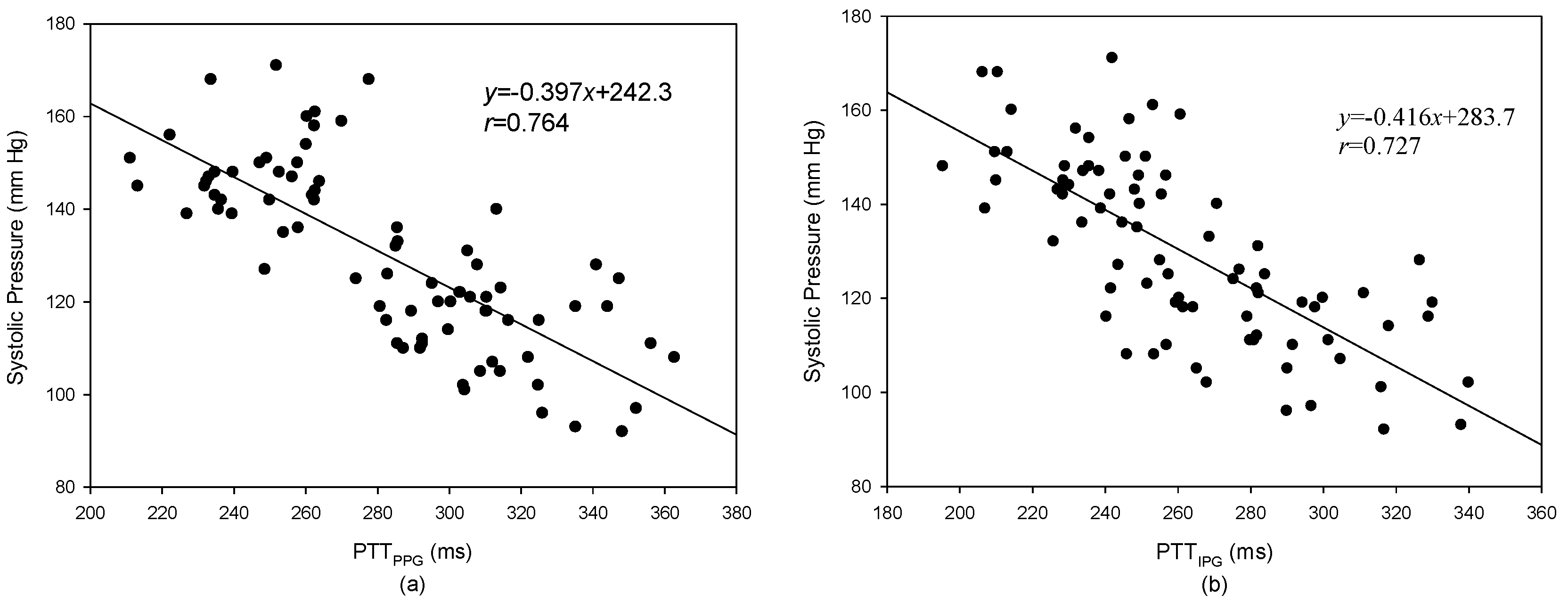
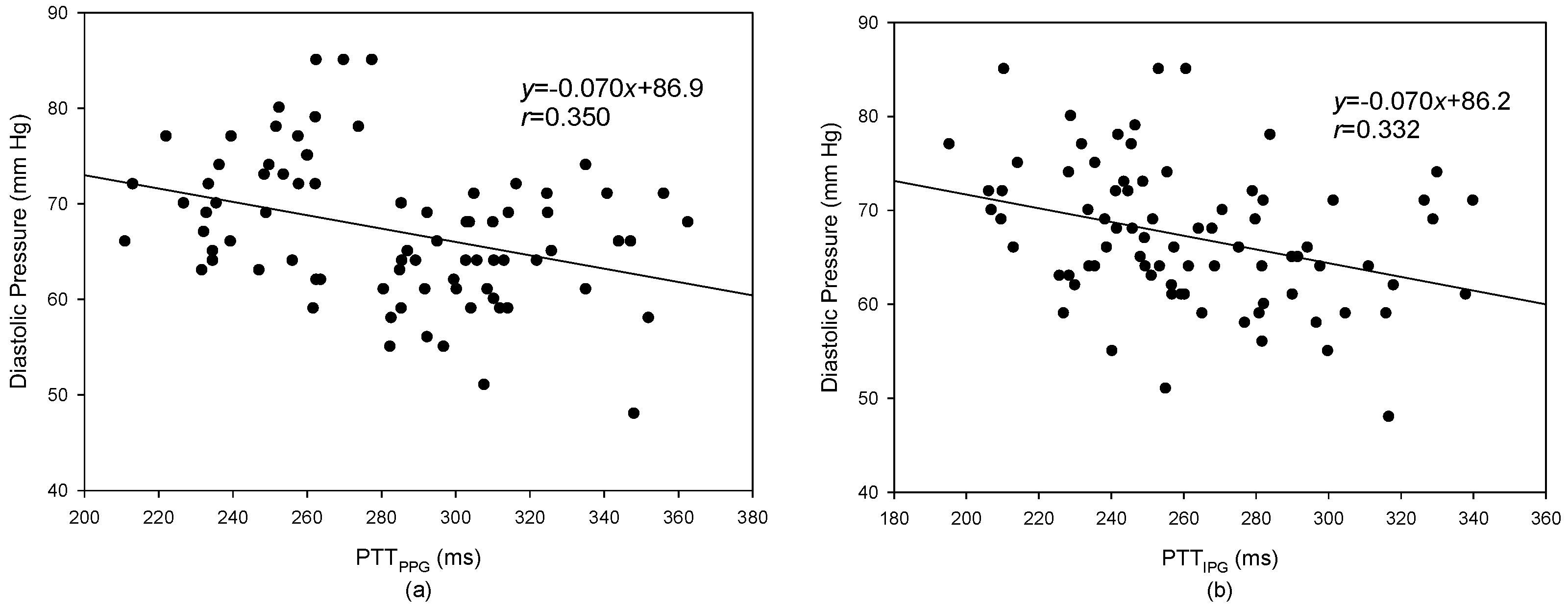
3.2. Pulse Transmission Time
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Tremper, K.K.; Barker, S.J. Pulse oximetry. Anesthesiology 1989, 70, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Medtronic Cardiac Diagnostics & Monitoring for Healthcare Professionals, SEEQ MCT System. Available online: http://www.medtronicdiagnostics.com/us/cardiac-monitors/seeq-mct-system/index.htm (accessed on 8 April 2017).
- Clearbridge VitalSigns, About CardioLeaf ULTRA. Available online: http://www.clearbridgevitalsigns. com/ultra.html (accessed on 8 April 2017).
- Buller, M.J.; Tharion, W.J.; Hoyt, R.W.; Jenkins, O.C. Estimation of human internal temperature from wearable physiological sensors. In Proceedings of the 22nd Conference on Innovative Applications of Artificial Intelligence, Atlanta, GA, USA, 11–15 July 2010; Volume 3, pp. 1763–1768. [Google Scholar]
- Liu, S.H.; Cheng, W.C. Fall detection with the support vector machine during scripted and continuous unscripted activities. Sensors 2012, 12, 12301–12316. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, Y.J. Using accelerometers for physical actions recognition by a neural fuzzy network. Telemed. J. E Health 2009, 15, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Cheng, D.C.; Lin, C.M. Arrhythmia identification with two-lead electrocardiograms using artificial neural networks and support vector machines for a portable ECG monitor system. Sensors 2013, 13, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, D.G.; Park, J.; Youm, S.K. Wearable sensing of in-ear pressure for heart rate monitoring with a piezoelectric sensor. Sensors 2015, 15, 23402–23417. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. J. E Health 2011, 17, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dols, S.; Oetomo, S.B.; Feijs, L. Monitoring body temperature of newborn infants at neonatal intensive care units using wearable sensors. In Proceedings of the 5th International Conference on Body Area Networks, Corfu, Greece, 10–12 September 2010; pp. 188–194. [Google Scholar]
- Guo, D.; Tay, F.E.; Xu, L.; Yu, L.; Nyan, M.; Chong, F.; Yap, K.; Xu, B. A long-term wearable vital signs monitoring system using BSN. In Proceedings of the 11th Euromicro Conference on Digital System Design Architectures, Methods and Tools, Parma, Italy, 3–5 September 2008; pp. 825–830. [Google Scholar]
- Carr, J.J.; Brown, J.M. Introduction to Biomedical Equipment Technology, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; ISBN 0-13-010492-2. [Google Scholar]
- Liu, S.H.; Lin, C.T. A model-based fuzzy logic controller with Kalman filtering for tracking mean arterial pressure. IEEE Trans. Syst. Man Cybern. Part A Human Syst. 2001, 31, 676–686. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar]
- Ahlstrom, C.; Johansson, A.; Uhlin, F.; Länne, T.; Ask, P. Noninvasive investigation of blood pressure changes using the pulse wave transit time: a novel approach in the monitoring of hemodialysis patients. J. Artif. Organs 2005, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sharwood-Smith, G.; Bruce, J.; Drummond, G. Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia. Br. J. Anaesth. 2006, 96, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.Y.A.; Lim, C.S.; Wang, P. Evaluation of blood pressure changes using vascular transit time. Physiol. Meas. 2006, 27, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Dantu, R.; Jonnada, S.; Thiyagaraja, S.; Subbu, K.P. Cuffless differential blood pressure estimation using smart phones. IEEE Trans. Biomed. Eng. 2013, 60, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.C.; Yan, W.R.; Zhang, N.L.; Lin, W.H.; Zhou, X.L.; Zhang, Y.T. Cuffless and continuous blood pressure estimation from the heart sound signals. Sensors 2015, 15, 23653–23666. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kang, H.; Huh, Y.; Kim, K.C. Cuffless and noninvasive measurement of systolic blood pressure, diastolic blood pressure, mean arterial pressure and pulse pressure using radial artery tonometry pressure sensor with concept of Korean traditional medicine. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 3597–3600. [Google Scholar]
- Nyboer, J. Electrical impedance plethysmography: A physical and physiologic approach to peripheral vascular study. Circulation 1950, 11, 811–821. [Google Scholar] [CrossRef]
- Yamakoshi, K.I.; Shimazu, H.; Togawa, T.; Fukuoka, M.; Ito, H. Noninvasive measurement of hematocrit by electrical admittance plethysmography technique. IEEE Trans. Biomed. Eng. 1980, 27, 159–161. [Google Scholar]
- Sherwood, A.; Allen, M.T.; Fahrenberg, J.; Kelsey, R.M.; Lovallo, W.R.; Doornen, L.J.P. Methodological guideline for impedance cardiography. Psychophysiology 1990, 27, 1–23. [Google Scholar] [PubMed]
- Kubicek, W.G.; Karngis, J.N.; Patterson, R.P.; Witsoe, D.A.; Mattson, R.H. Development and evaluation of an impedance cardiograph system. Aerosp. Med. 1966, 37, 1208–1212. [Google Scholar] [PubMed]
- Bernstein, D.P. A new stroke volume equation for thoracic electrical bioimpedance: Theory and rationale. Crit. Care Med. 1986, 14, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhang, Y.; Webster, J.G.; Tompkins, W.J. Motion artifact from spot and band electrodes during impedance cardiography. IEEE Trans. Biomed. Eng. 1986, 3, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Garmin Vivofit 3 Review. Available online: https://www.wareable.com/garmin/garmin-vivofit-3-review (accessed on 8 April 2017).
- The Best Apple Watch Apps: 50 Apps Tried and Tested. Available online: https://www.wareable.com/apple-watch/best-apple-watch-apps-832 (accessed on 8 April 2017).



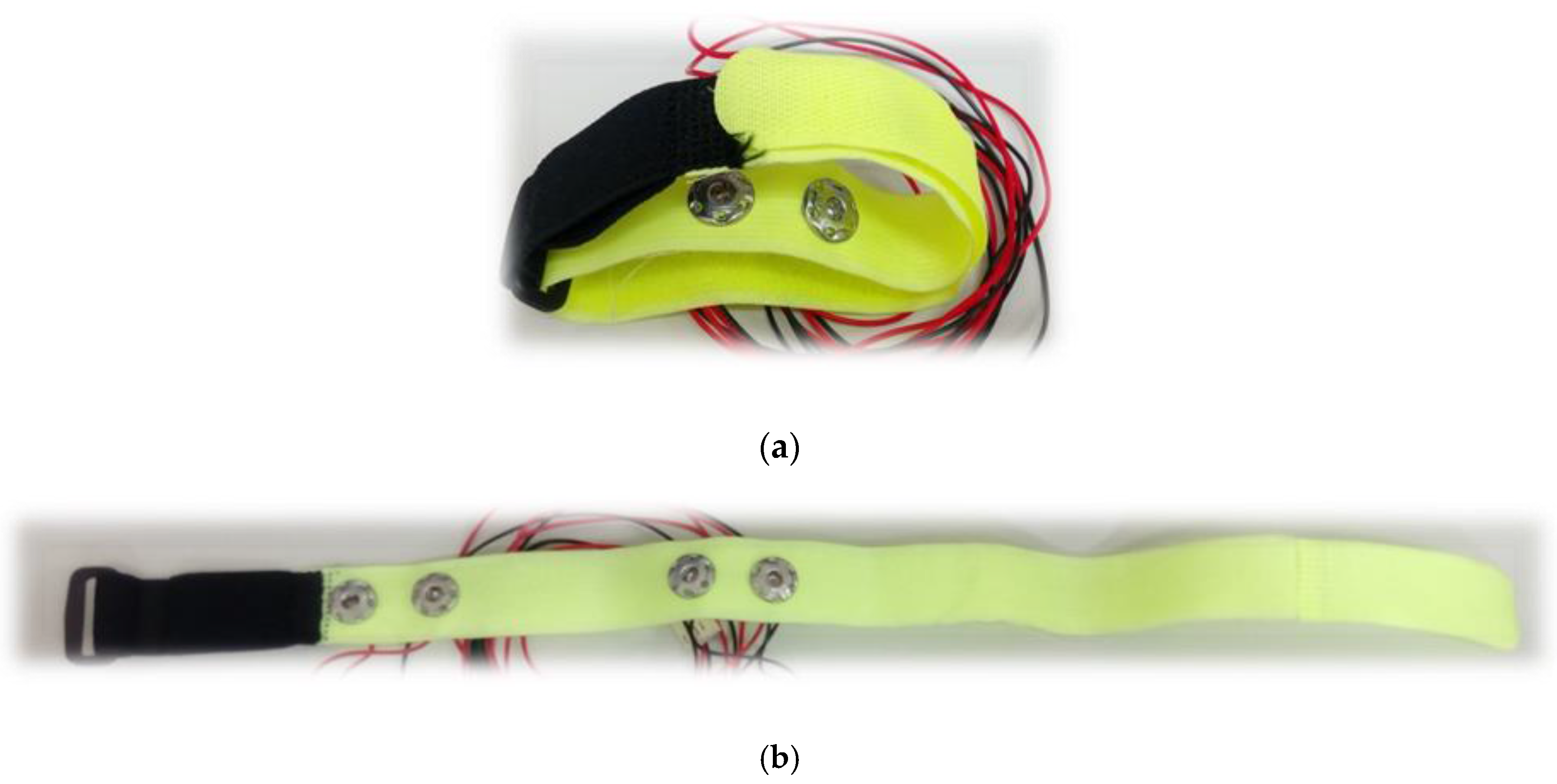


| No. | Electrodes T1, T2 | Electrodes R1, R2 | No. | Electrodes T1, T2 | Electrodes R1, R2 |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| 3 | 4 | ||||
| 5 | 6 | ||||
| 7 | 8 | ||||
| 9 | 10 | ||||
| 11 | 12 |
| Before Exercise | After Exercise | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject No. | Sys (mmHg) | Dia (mmHg) | HR (BPM) | PTTPPG (ms) | PTTIPG (ms) | Sys (mmHg) | Dia (mmHg) | HR (BPM) | PTTPPG (ms) | PTTIPG (ms) |
| 1 | 108 | 61 | 84 | 300 | 318 | 145 | 66 | 109 | 232 | 249 |
| 2 | 121 | 68 | 79 | 314 | 252 | 135 | 69 | 81 | 286 | 234 |
| 3 | 108 | 55 | 74 | 292 | 257 | 126 | 57 | 82 | 285 | 226 |
| 4 | 124 | 60 | 73 | 306 | 311 | 142 | 58 | 112 | 264 | 257 |
| 5 | 108 | 63 | 81 | 312 | 305 | 146 | 78 | 124 | 237 | 256 |
| 6 | 92 | 62 | 67 | 335 | 338 | 137 | 73 | 100 | 258 | 245 |
| 7 | 114 | 71 | 76 | 335 | 330 | 156 | 82 | 110 | 270 | 261 |
| 8 | 105 | 61 | 72 | 325 | 340 | 146 | 67 | 127 | 222 | 232 |
| 9 | 113 | 62 | 107 | 274 | 284 | 135 | 50 | 141 | 211 | 213 |
| 10 | 111 | 61 | 73 | 317 | 279 | 136 | 53 | 111 | 256 | 234 |
| 11 | 108 | 65 | 82 | 356 | 301 | 162 | 79 | 126 | 278 | 210 |
| 12 | 108 | 60 | 94 | 287 | 292 | 142 | 64 | 128 | 233 | 238 |
| 13 | 107 | 65 | 72 | 363 | 246 | 136 | 67 | 131 | 227 | 207 |
| 14 | 124 | 61 | 69 | 283 | 277 | 145 | 77 | 102 | 250 | 228 |
| 15 | 112 | 61 | 87 | 311 | 298 | 155 | 76 | 102 | 262 | 247 |
| 16 | 123 | 65 | 75 | 289 | 262 | 144 | 60 | 122 | 262 | 227 |
| 17 | 112 | 70 | 79 | 344 | 294 | 164 | 79 | 130 | 260 | 214 |
| 18 | 91 | 48 | 64 | 348 | 317 | 140 | 64 | 72 | 313 | 250 |
| 19 | 115 | 62 | 93 | 310 | 282 | 137 | 75 | 107 | 254 | 249 |
| 20 | 113 | 66 | 89 | 309 | 290 | 153 | 69 | 100 | 235 | 236 |
| Mean ± Std | 112.9 ± 9.6 | 64.8 ± 6.7 | 79.5 ± 10.5 | 315.5 ± 25.0 | 293.6 ± 27.4 | 146.6 * ± 9.6 | 63.8 ± 7.3 | 110.8 * ± 18.4 | 254.7 * ± 24.9 | 235.6 * ± 16.1 |
| Before Exercise | After Exercise | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject No. | Sys (mmHg) | Dia (mmHg) | HR (BPM) | PTTPPG (ms) | PTTIPG (ms) | Sys (mmHg) | Dia (mmHg) | HR (BPM) | PTTPPG (ms) | PTTIPG (ms) |
| 1 | 114 | 54 | 84 | 297 | 300 | 144 | 62 | 118 | 247 | 222 |
| 2 | 106 | 63 | 78 | 322 | 254 | 114 | 54 | 84 | 283 | 210 |
| 3 | 110 | 50 | 70 | 293 | 282 | 131 | 58 | 77 | 286 | 259 |
| 4 | 125 | 60 | 82 | 303 | 282 | 146 | 61 | 129 | 235 | 228 |
| 5 | 102 | 63 | 98 | 304 | 316 | 141 | 74 | 128 | 236 | 258 |
| 6 | 95 | 66 | 73 | 326 | 290 | 141 | 73 | 114 | 262 | 229 |
| 7 | 123 | 68 | 76 | 341 | 327 | 156 | 82 | 116 | 263 | 241 |
| 8 | 105 | 58 | 91 | 304 | 268 | 130 | 63 | 121 | 249 | 227 |
| 9 | 104 | 53 | 128 | 325 | 329 | 133 | 56 | 141 | 213 | 198 |
| 10 | 115 | 50 | 91 | 300 | 260 | 140 | 52 | 129 | 232 | 213 |
| 11 | 115 | 62 | 96 | 310 | 264 | 145 | 74 | 116 | 253 | 204 |
| 12 | 109 | 64 | 103 | 293 | 280 | 152 | 70 | 130 | 260 | 228 |
| 13 | 121 | 65 | 97 | 303 | 242 | 147 | 74 | 125 | 240 | 184 |
| 14 | 117 | 64 | 71 | 281 | 259 | 149 | 72 | 103 | 249 | 199 |
| 15 | 125 | 68 | 81 | 305 | 282 | 165 | 75 | 103 | 252 | 220 |
| 16 | 110 | 60 | 91 | 314 | 265 | 149 | 63 | 136 | 263 | 218 |
| 17 | 118 | 70 | 90 | 347 | 258 | 161 | 76 | 153 | 234 | 184 |
| 18 | 96 | 58 | 63 | 352 | 297 | 127 | 51 | 65 | 308 | 209 |
| 19 | 118 | 68 | 87 | 295 | 275 | 144 | 79 | 112 | 258 | 231 |
| 20 | 119 | 64 | 85 | 286 | 281 | 147 | 71 | 110 | 240 | 226 |
| 114.4 ± 10.2 | 70.6 ± 5.0 | 86.6 ± 14.3 | 310.0 ± 19.9 | 280.5 ± 23.8 | 145.2 * ± 13.2 | 69.5 ± 8.4 | 115.5 * ± 21.4 | 252.9 * ± 21.5 | 219.2 * ± 20.3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Cheng, D.-C.; Su, C.-H. A Cuffless Blood Pressure Measurement Based on the Impedance Plethysmography Technique. Sensors 2017, 17, 1176. https://doi.org/10.3390/s17051176
Liu S-H, Cheng D-C, Su C-H. A Cuffless Blood Pressure Measurement Based on the Impedance Plethysmography Technique. Sensors. 2017; 17(5):1176. https://doi.org/10.3390/s17051176
Chicago/Turabian StyleLiu, Shing-Hong, Da-Chuan Cheng, and Chun-Hung Su. 2017. "A Cuffless Blood Pressure Measurement Based on the Impedance Plethysmography Technique" Sensors 17, no. 5: 1176. https://doi.org/10.3390/s17051176






