Compact Eucapnic Voluntary Hyperpnoea Apparatus for Exercise-Induced Respiratory Disease Detection
Abstract
:1. Introduction
2. Materials and Methods
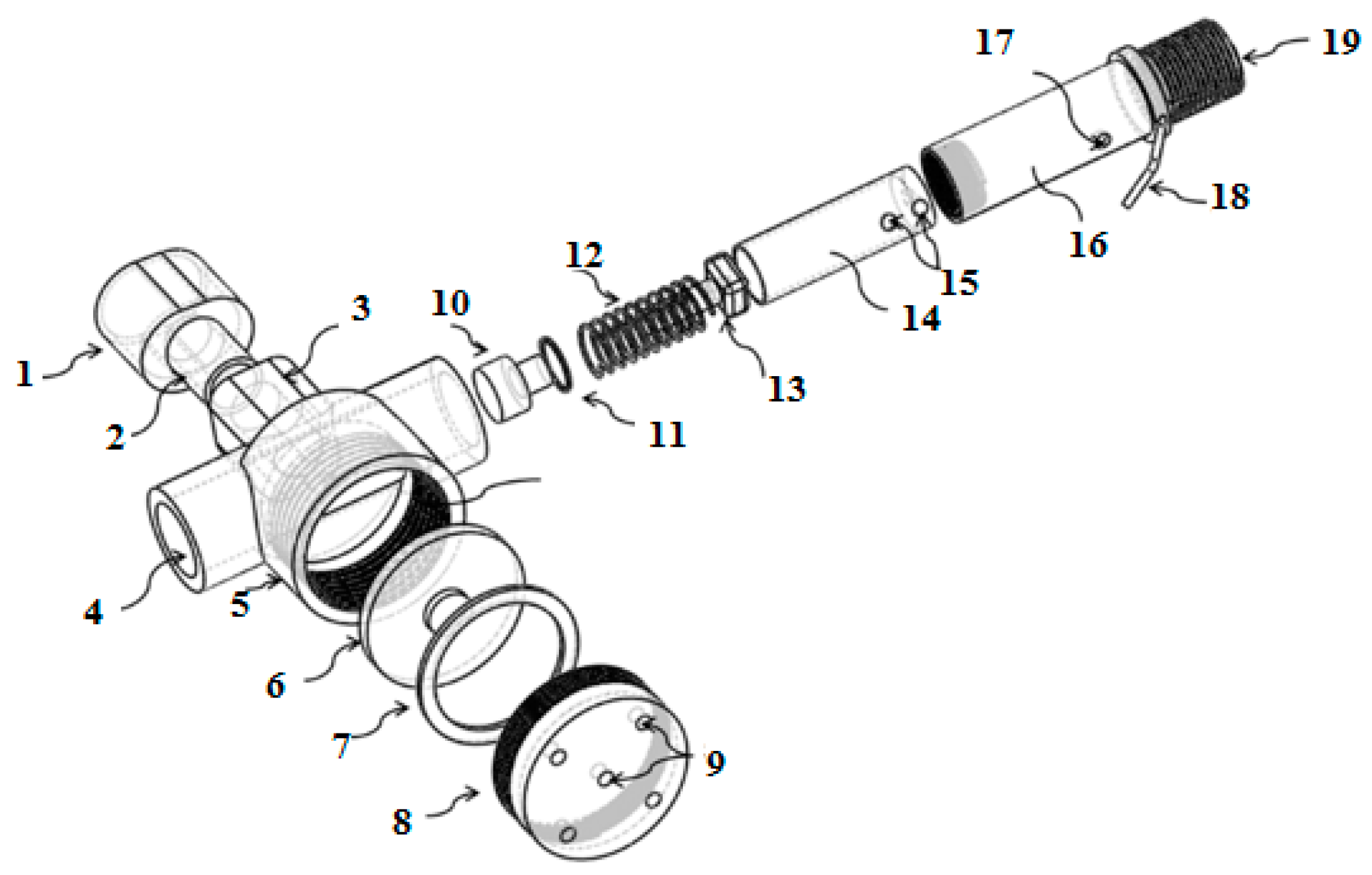
2.1. Breathing Apparatus Design
2.2. Methods
2.2.1. Human Subjects and Study Design
2.2.2. Pre-Challenge
2.2.3. Conventional EVH Challenge
2.2.4. Compact EVH Challenge
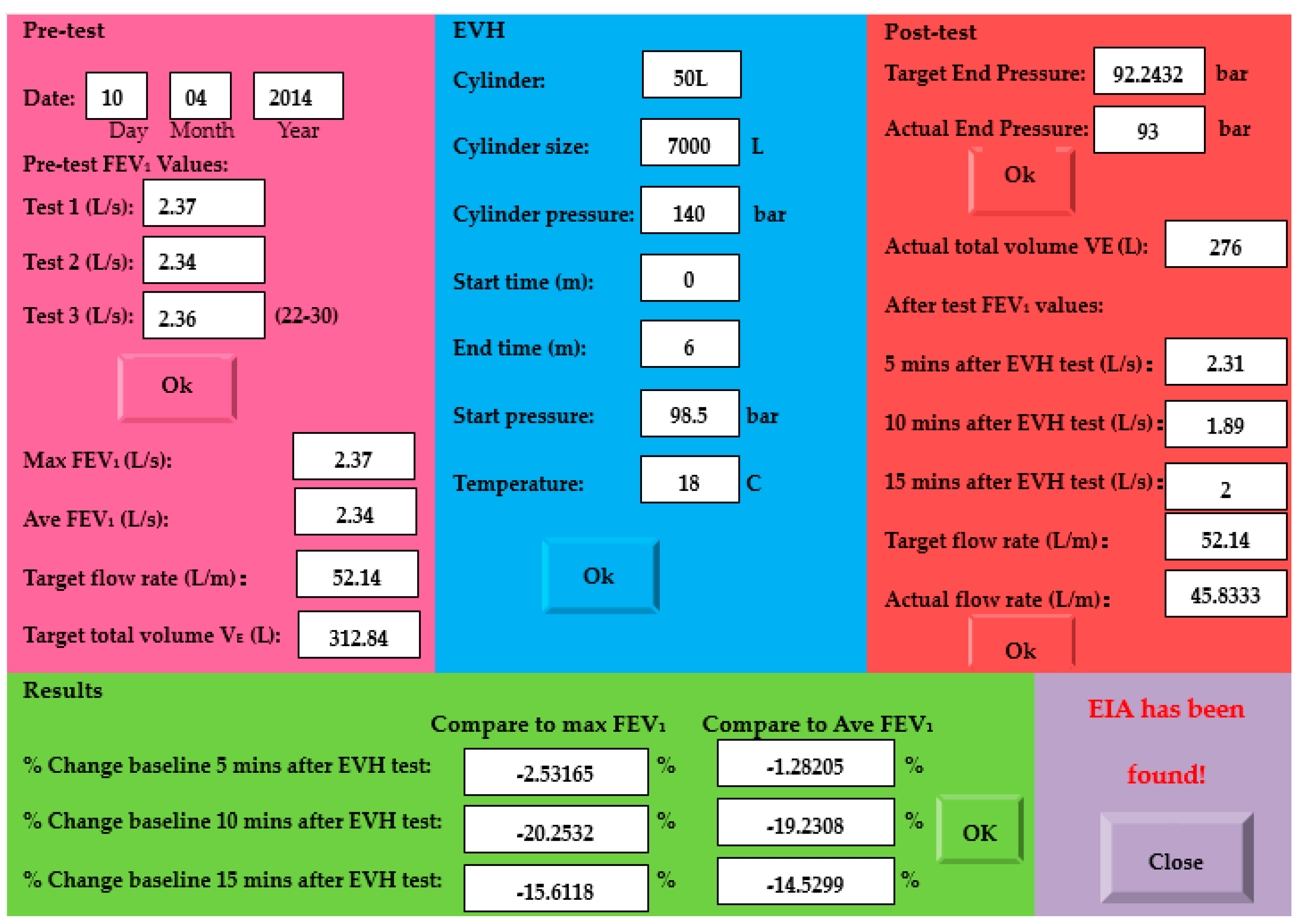
2.3. Measurement of the Airway Response
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johansson, H.; Norlander, K.; Berglund, L.; Janson, C.; Malinovschi, A.; Nordvall, L.; Emtner, M. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2014, 70, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Kippelen, P. Assessment and prevention of exercise-induced bronchoconstriction. Br. J. Sports Med. 2012, 46, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Langdeau, J.B.; Boulet, L.P. Prevalence and mechanisms of development of asthma and airway hyperresponsiveness in athletes. Sports Med. 2001, 31, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Fitch, K.; Perry, C.P.; Sue-Chu, M.; Crapo, R.; McKenzie, D.; Magnussen, H. Responses to Bronchial Challenge Submitted for Approval to Use Inhaled β2-Agonists before an Event at the 2002 Winter Olympics. J. Allergy Clin. Immunol. 2003, 111, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Spangler, M.; Hawley, H.; Barnes, N.; Saxena, S. A review of guidelines and pharmacologic options for asthma treatment, with a focus on exercise-induced bronchoconstriction. Phys. Sportsmed. 2013, 41, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.W.; Wilber, R.L.; Szmedra, L.; Jenkinson, D.M.; Mayers, L.B.; Im, J. Exercise-Induced Asthma Screening of Elite Athletes: Field Versus Laboratory Exercise Challenge. Med. Sci. Sports Exerc. 2000, 32, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.W.; Anderson, S.D.; Spiering, B.A.; Judelson, D.A. Field Exercise vs Laboratory Eucapnic Voluntary Hyperventilation to Identify Airway Hyperresponsiveness in Elite Cold Weather Athletes. Chest 2004, 125, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kippelen, P.; Tufvesson, E.; Ali, L.; Bjermer, L.; Anderson, S.D. Urinary CC16 after challenge with dry air hyperpnoea and mannitol in recreational summer athletes. Respir. Med. 2013, 107, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, K.M.; Argyros, G.J.; Roach, J.M.; Eliasson, A.H.; Phillips, Y.Y. Interpretation of eucapnic voluntary hyperventilation in the diagnosis of asthma. Chest 1995, 108, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.H.; Les, A.; Price, O.J.; Dickinson, J.W.; Matteo, B. Eucapnic Voluntary Hyperpnea: Gold Standard for Diagnosing Exercise-Induced Bronchoconstriction in Athletes? Sports Med. 2016, 46, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.W.; Slee, J.B. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J. Allergy Clin. Immunol. 2008, 122, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.R. Simplified eucapnic voluntary hyperventilation challenge. J. Allergy Clin. Immunol. 1984, 73, 676–679. [Google Scholar] [CrossRef]
- Anderson, S.D.; Argyros, G.J.; Magnussen, H.; Holzer, K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br. J. Sports Med. 2001, 35, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.H.; Engh, G.; Mørk, M. Exercise-induced bronchoconstriction depends on exercise load. Respir. Med. 2000, 94, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.H.; Engh, G.; Mørk, M.; Schrøder, E. Cold air inhalation and exercise-induced bronchoconstriction in relationship to metacholine bronchial responsiveness: Different patterns in asthmatic children and children with other chronic lung diseases. Respir. Med. 1998, 92, 308–315. [Google Scholar] [CrossRef]
- Koskela, H.O.; Räsänen, S.H.; Tukiainen, H.O. The diagnostic value of cold air hyperventilation in adults with suspected asthma. Respir. Med. 1997, 91, 470–478. [Google Scholar] [CrossRef]
- Phillips, Y.Y.; Jaeger, J.J.; Laube, B.L.; Rosenthal, R.R. Eucapnic voluntary hyperventilation of compressed gas mixture. A simple system for bronchial challenge by respiratory heat loss. Am. Rev. Respir. Dis. 1985, 131, 31–35. [Google Scholar] [PubMed]
- Eliasson, A.H.; Phillips, Y.Y.; Rajagopal, K.R.; Howard, R.S. Sensitivity and specificity of bronchial provocation testing: An evaluation of four techniques in exercise-induced bronchospasm. Chest 1992, 102, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Argyros, G.J.; Roach, J.M.; Hurwitz, K.M.; Eliasson, A.H.; Phillips, Y.Y. Eucapnic voluntary hyperventilation as a bronchoprovocation technique: Development of a standarized dosing schedule in asthmatics. Chest 1996, 109, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Couillard, S.; Bougault, V.; Turmel, J.; Boulet, L.P. Perception of bronchoconstriction following methacholine and eucapnic voluntary hyperpnea challenges in elite athletes. Chest 2014, 145, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Bood, J.R.; Anderson, S.D.; Romer, L.M.; Dahlén, B.; Dahlén, S.E.; Kippelen, P. A standard, single dose of inhaled terbutaline attenuates hyperpnoea-induced bronchoconstriction and mast cell activation in athletes. J. Appl. Physiol. 2016, 120, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.; Brannan, J.D. Alternatives to exercise challenge for the objective assessment of exercise induced bronchospasm: Eucapnic voluntary hyperpnoea and the osmotic challenge tests. Breathe 2010, 7, 52–63. [Google Scholar] [CrossRef]
- Holzer, K.; Anderson, S.D.; Chan, H.K.; Douglass, J. Mannitol as a Challenge Test to Identify Exercise-induced Bronchoconstriction in Elite Athletes. Am. J. Respir. Crit. Care Med. 2003, 167, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Brannan, J.D. Methods for “Indirect” Challenge Tests Including Exercise, Eucapnic Voluntary Hyperpnea, and Hypertonic Aerosols. Clin. Rev. Allergy Immunol. 2003, 24, 27–54. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Nemadea, H.B.; Bandyopadhyay, D. Nano-enabled paper humidity sensor for mobile based point-of-care lung function monitoring. Biosens. Bioelectron. 2017, 94, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Janik, P.; Janik, M.; Wróbel, Z. Integrated micro power frequency breath detector. Sens. Actuators A Phys. 2016, 239, 79–89. [Google Scholar] [CrossRef]
- Madsen, S.; Baczuk, J.; Thorup, K.; Barton, R.; Patwari, N.; Langell, J.T. A noncontact RF-based respiratory sensor: Results of a clinical trial. J. Surg. Res. 2016, 203, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arlotto, P.; Grimaldi, M.; Naeck, R.; Ginoux, J.M. An Ultrasonic Contactless Sensor for Breathing Monitoring. Sensors 2014, 14, 15371–15386. [Google Scholar] [CrossRef] [PubMed]
- Janik, P.; Janik, M.; Wróbel, M.Z. Micro-condensation sensor for monitoring respiratory rate and breath strength. Sens. Actuators A Phys. 2012, 185, 160–167. [Google Scholar] [CrossRef]
- American Thoracic Society; European Respiratory Society. ATS/ER recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar]
- Ishizuka, T.; Matsuzaki, S.; Aoki, H.; Yatomi, M.; Kamide, Y.; Hisada, T.; Tsuburai, T.; Dobashi, K.; Ohshima, K.; Akiyama, K. Prevalence of asthma symptoms based on the european community respiratory health survey questionnaire and feno in university students: Gender differences in symptoms and feno. Allergy Asthma Clin. Immunol. 2011, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Norlander, K.; Janson, C.; Malinovschi, A.; Nordang, L.; Emtner, M. The relationship between exercise induced bronchial obstruction and health related quality of life in female and male adolescents from a general population. BMC Pulm. Med. 2016, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Richard, R.R.; Harvey, J.H. Apparatus and Method for Eucapnic Voluntary Hyperventilation Testing. U.S. Patent 20,120,253,218, 4 October 2012. [Google Scholar]
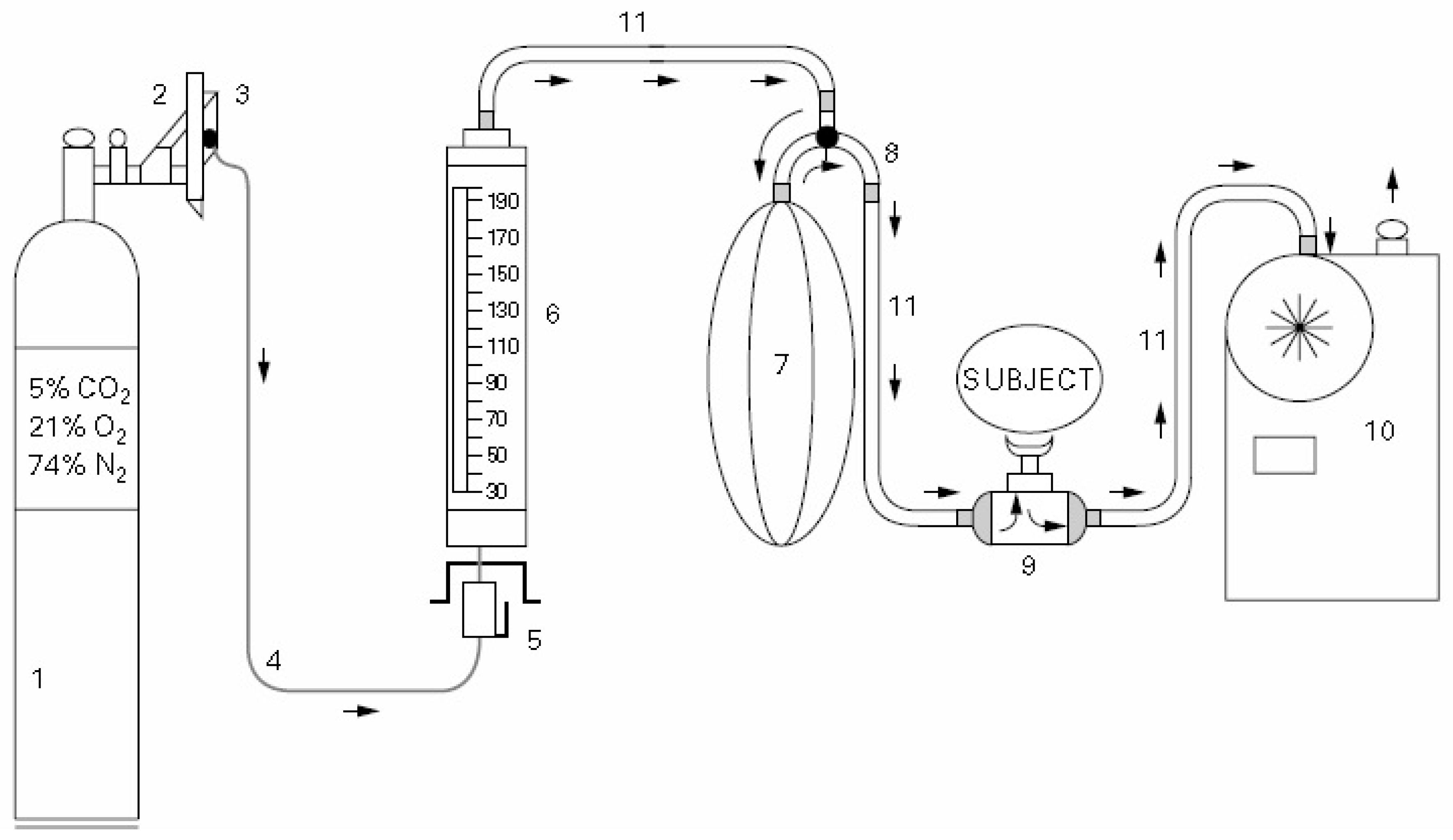
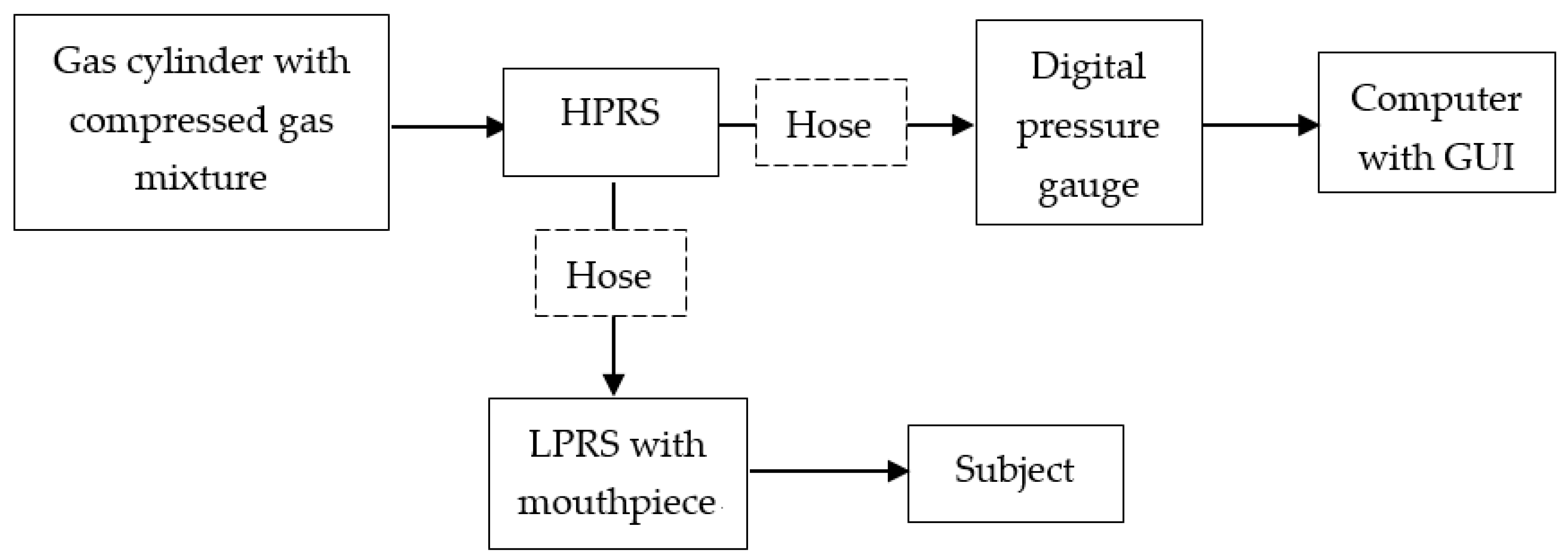
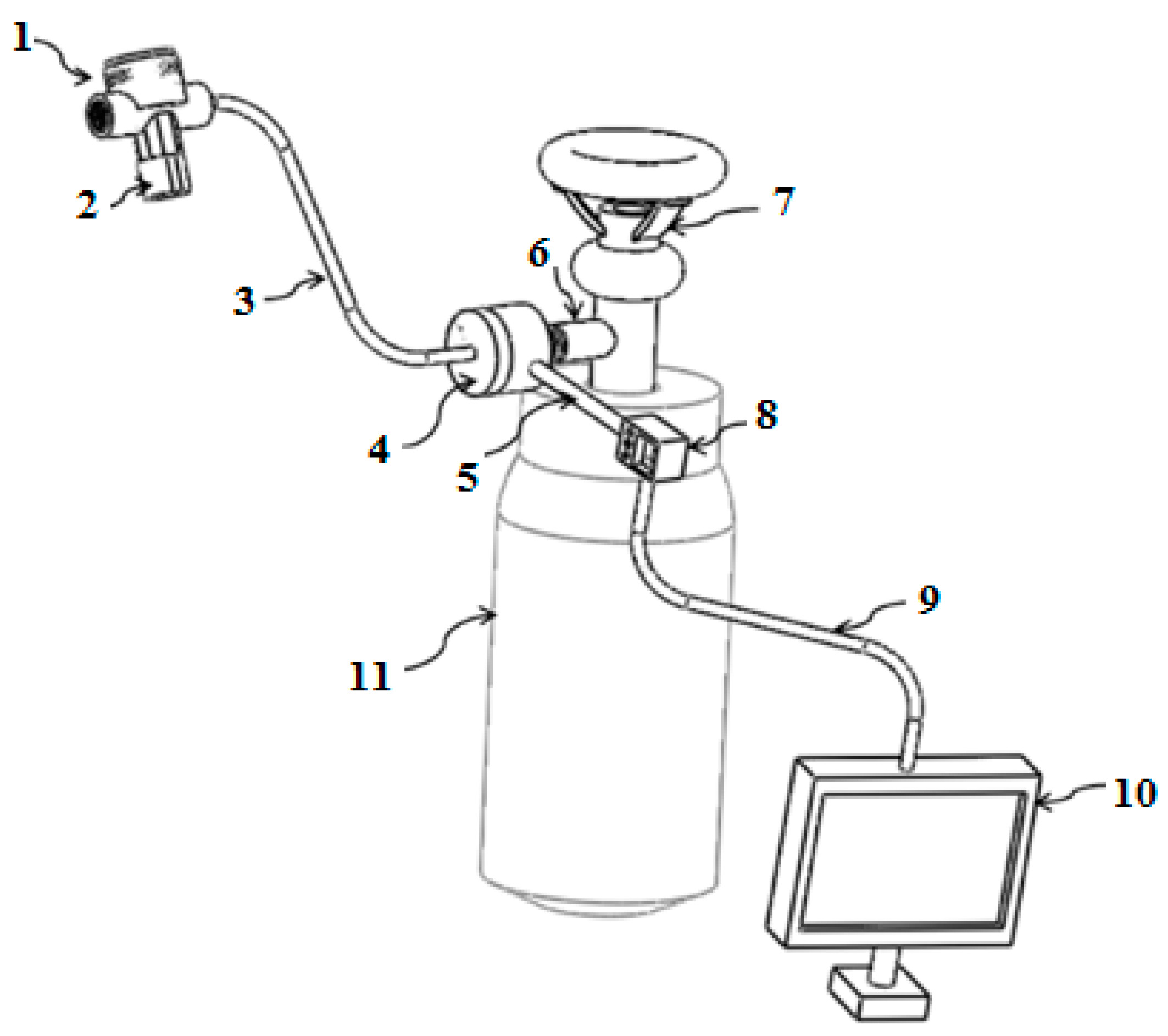
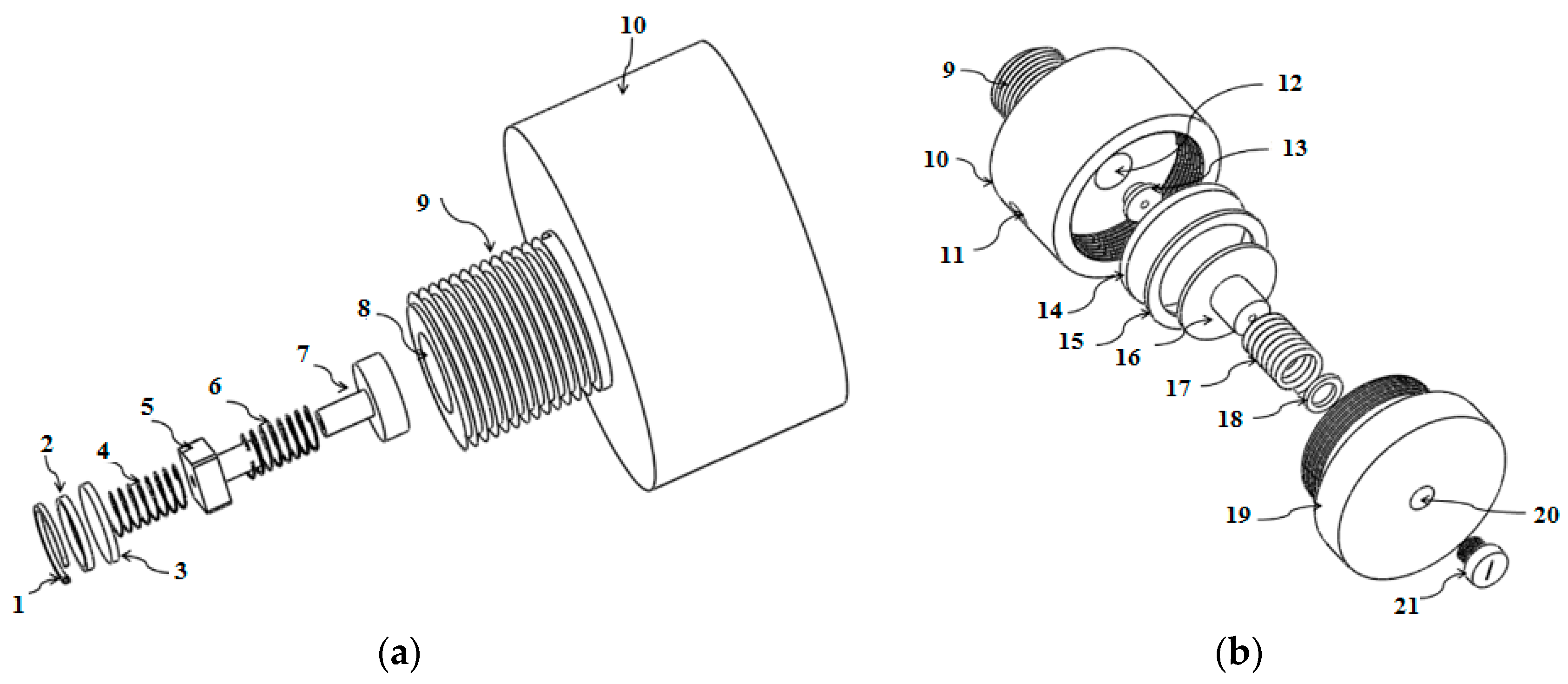
| All Participants | Male Participants | Female Participants | |
|---|---|---|---|
| Number (n) | 22 | 11 | 11 |
| Age (years) | 27.3 3.0 | 28.2 2.54 | 26.3 3.3 |
| Body Mass Index (kg/m2) | 20.4 1.60 | 20.7 0.88 | 20.9 2.09 |
| Smoking (yes) | 2 (9.09%) | 2 (18.2%) | 0 (0%) |
| Shortness of breath | 3 (13.64%) | 1 (9.09%) | 2 (18.2%) |
| Wheezing | 5 (22.73%) | 2 (18.2%) | 3 (13.64%) |
| Cough | 3 (13.64%) | 2 (18.2%) | 3 (13.64%) |
| Night symptoms | 3 (13.64%) | 2 (18.2%) | 1 (9.09%) |
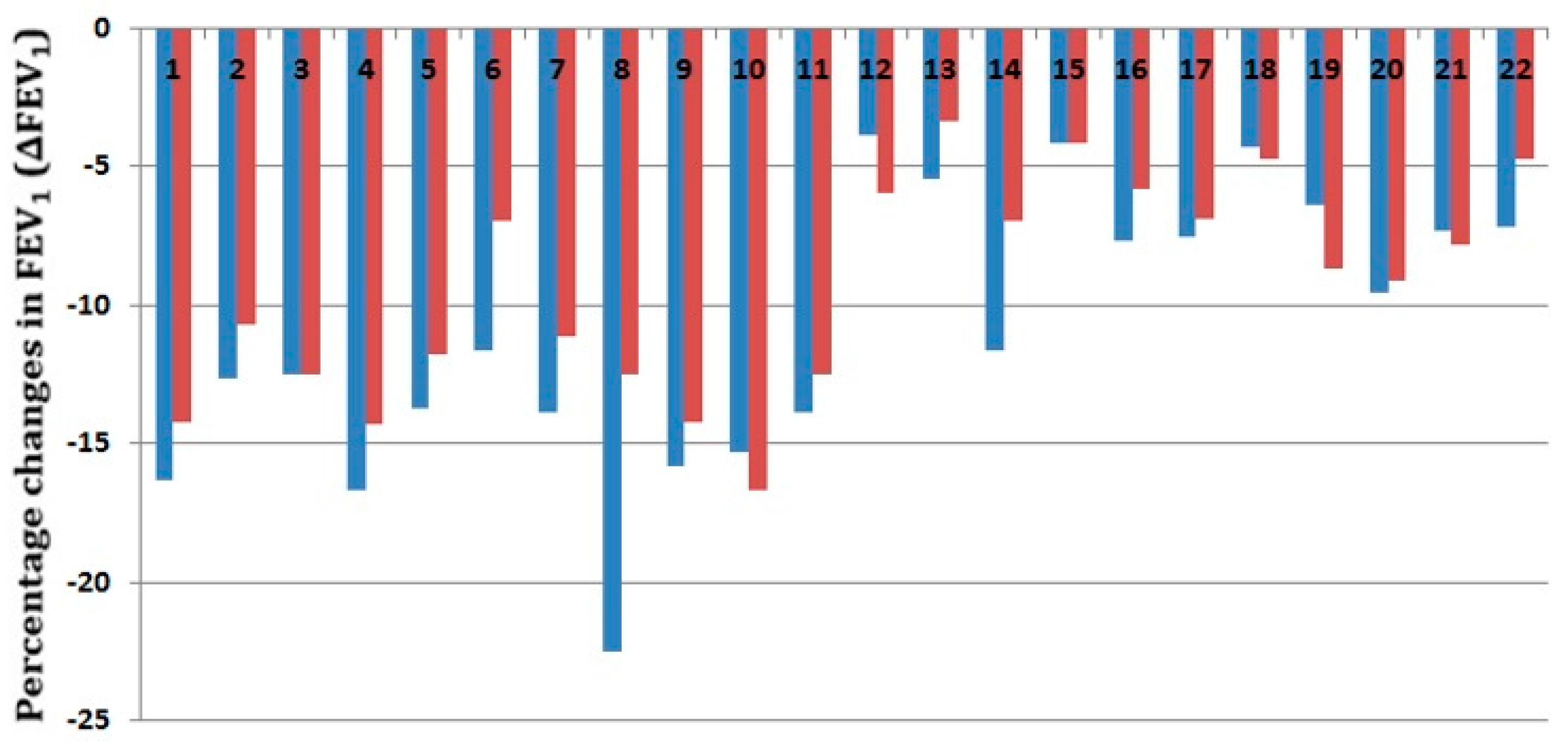
| Subject | Baseline FEV1 (Liters) | Predicted FEV1 | Compact EVH FEV1 (%) | Conventional EVH FEV1 (%) |
|---|---|---|---|---|
| 1 | 4.78 | 103 | −16.32 | −14.23 |
| 2 | 5.15 | 120 | −12.62 | −10.68 |
| 3 | 4.8 | 104 | −12.5 | −12.5 |
| 4 | 4.2 | 97 | −16.67 | −14.29 |
| 5 | 5.1 | 120 | −13.73 | −11.76 |
| 6 * | 4.3 | 100 | −11.63 | −6.98 |
| 7 | 3.6 | 79 | −13.89 | −11.11 |
| 8 | 4 | 105 | −22.5 | −12.5 |
| 9 | 3.8 | 86 | −15.79 | −14.21 |
| 10 | 3.78 | 85 | −15.34 | −16.67 |
| 11 | 3.6 | 78 | −13.89 | −12.5 |
| 12 | 4.89 | 105 | −3.89 | −5.93 |
| 13 * | 4.76 | 103 | −5.46 | −3.36 |
| 14 | 4.3 | 100 | −11.63 | −6.98 |
| 15 | 4.8 | 104 | −4.17 | −4.17 |
| 16 | 5.2 | 125 | −7.69 | −5.77 |
| 17 | 5.2 | 116 | −7.5 | −6.9 |
| 18 | 4.8 | 97 | −4.3 | −4.7 |
| 19 | 4.38 | 99 | −6.39 | −8.68 |
| 20 | 4.18 | 97 | −9.57 | −9.09 |
| 21 | 4.1 | 98 | −7.32 | −7.8 |
| 22 | 4.2 | 101 | −7.14 | −4.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Al-Jumaily, A. Compact Eucapnic Voluntary Hyperpnoea Apparatus for Exercise-Induced Respiratory Disease Detection. Sensors 2017, 17, 1139. https://doi.org/10.3390/s17051139
Wang L, Al-Jumaily A. Compact Eucapnic Voluntary Hyperpnoea Apparatus for Exercise-Induced Respiratory Disease Detection. Sensors. 2017; 17(5):1139. https://doi.org/10.3390/s17051139
Chicago/Turabian StyleWang, Lulu, and Ahmed Al-Jumaily. 2017. "Compact Eucapnic Voluntary Hyperpnoea Apparatus for Exercise-Induced Respiratory Disease Detection" Sensors 17, no. 5: 1139. https://doi.org/10.3390/s17051139






