Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges
Abstract
:1. Introduction
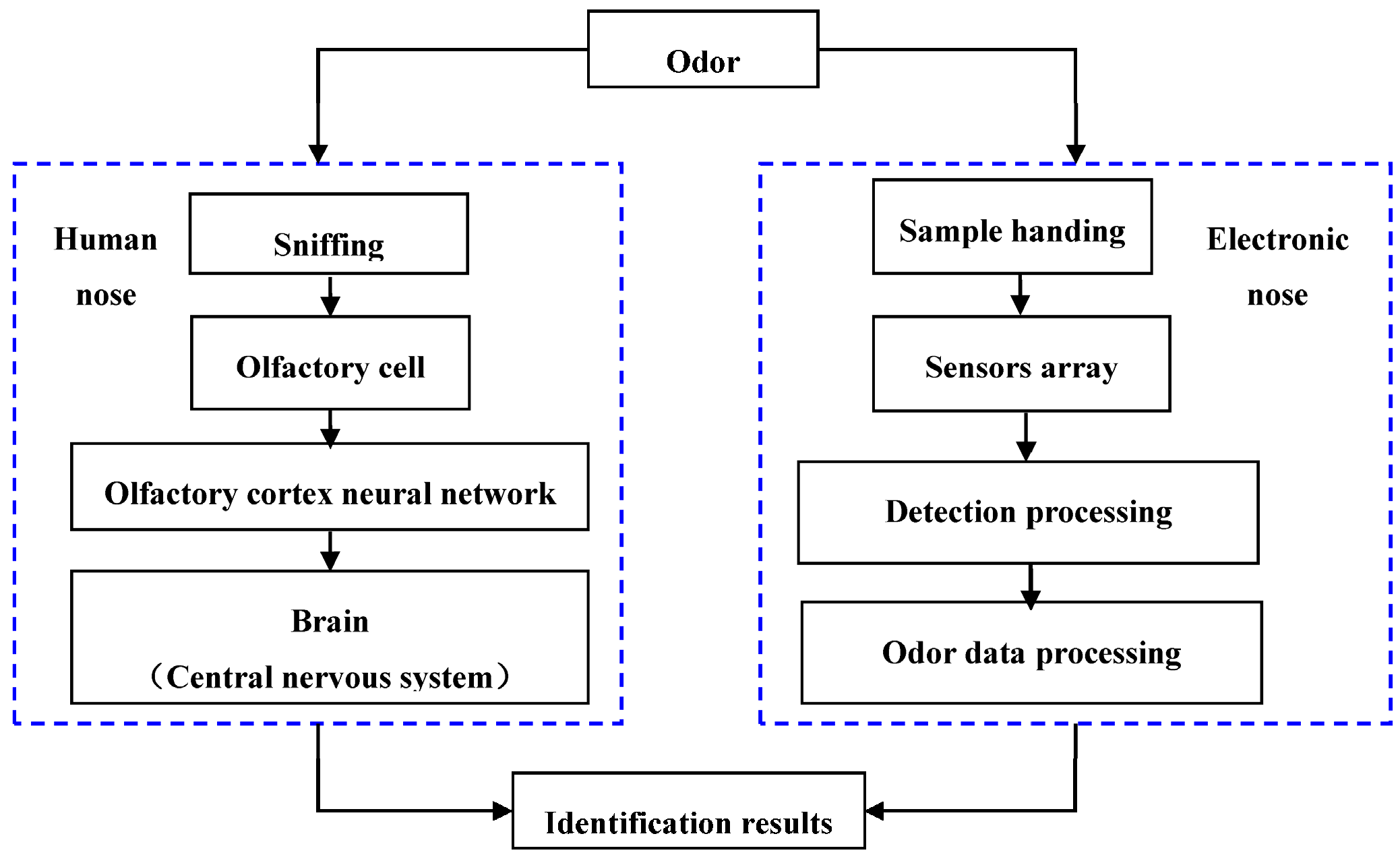
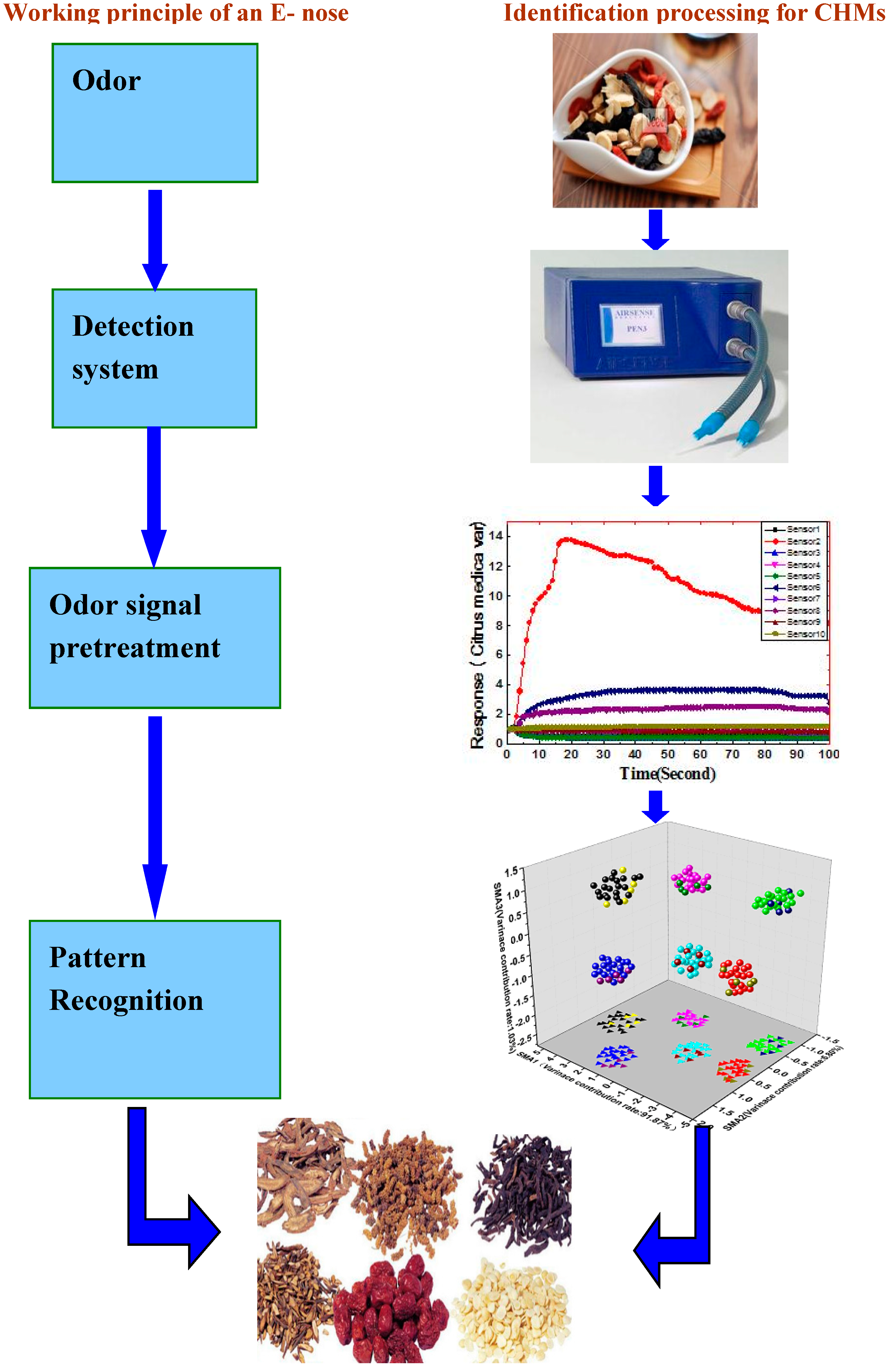
2. Design of the Study
3. Electronic Nose (E-Nose)
3.1. Sample Handling System
3.2. Detection System—Gas Sensor Array
3.3. Data Processing System
3.3.1. Odor Signal Pretreatment
3.3.2. Feature Extraction
3.3.3. Pattern Recognition
4. E-Nose Applications in Identification of CHMs
4.1. Species Identification of CHMs
4.2. Identification of Processed CHM Products
4.3. Regional Identification of CHMs
4.4. Storage Time Identification of CHMs
5. Discussion: Challenges and Future Perspectives
5.1. Challenges
5.1.1. Qualitative Analysis of VOCs in CHMs
5.1.2. Development of New Sensor Materials
5.1.3. Investigation of Appropriate Pattern Recognition Methods
5.2. Future Perspectives
5.2.1. Development of New Drugs
5.2.2. Odor Standardization for CHMs
5.2.3. Odor Remote Reproduction and Remote Diagnosis in Medicine
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, M.A. Current situation and development trend of traditional Chinese Medicine. Lishizhen Med. Mater. Med. Res. 2016, 8, 1956–1960. [Google Scholar]
- Fleischer, T.; Su, Y.C.; Lin, S.J. How do government regulations influence the ability to practice Chinese herbal medicine in western countries. J. Ethnopharmacol. 2017, 196, 104–109. [Google Scholar] [CrossRef] [PubMed]
- How Many Kinds of Chineses Herbal Medicines? Available online: http://zhidao.baidu.com/question/529093904.html (accessed on 8 October 2016).
- Sun, Y.P.; Zhang, T.J.; Cao, H.; Xu, J.; Gong, S.X.; Chen, C.Q. Expression of pungent-taste herbs and their applications in clinical compatibility. Chin. Tradit. Herb. Drugs 2015, 46, 785–790. [Google Scholar]
- Liu, H.Z. Research on the development of Chinese medicine in Africa and its communication strategy. Sci. Technol. Vis. 2016, 4, 146. [Google Scholar]
- Yang, S.; Wu, N.; Yuan, X.; Liu, Y.; Zhong, R.; Wu, C. Current situation and thinking on “odor and taste” identification of traditional chinese medicine. World Sci. Technol. Mod. Tradit. Chin. Med. 2014, 9, 1876–1879. [Google Scholar]
- Zou, H.Q. Methodological Study on the Quality Evaluation of Traditional Chinese Medicine Based on Bionic System; Beijing University of Chinese Medicine: Beijing, China, 2013. [Google Scholar]
- Xu, S.J.; Yang, L.; Xie, P.S. Present situation and Prospect of research on odor identification of traditional Chinese Medicine. Tradit. Chin. Drug Res. Pharmacol. 2011, 22, 228–230. [Google Scholar]
- Zhang, B.; Li, Y.; Zhang, Y.; Li, Z.; Bi, T.; He, Y. Initial transcription process-based identification method of bioactive components in traditional chinese medicine formula. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Zhou, X.Y.; Zhou, S.Y. Identification of Panacis Quinquefolii Radix, Ginseng Radix and its counterfeit and inferior products. J. Mod. Med. Health 2013, 29, 1651–1652. [Google Scholar]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, J.; Wang, Y. A novel framework for analyzing MOS E-nose data based on voting theory: Application to evaluate the internal quality of Chinese pecans. Sens. Actuators B Chem. 2017, 242, 511–521. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, W.; Su, J. Nondestructively sensing of total viable count (TVC) in chicken using an artificial olfaction system based colorimetric sensor array. J. Food Eng. 2015, 168, 259–266. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. TrAC Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Mohtasebi, S.S.; Ghasemi-Varnamkhasti, M. Application of MOS based electronic nose for the prediction of banana quality properties. Measurement 2016, 82, 105–114. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Liu, L. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lei, T.; Zhang, S. A novel headspace integrated E-nose and its application in discrimination of Chinese medical herbs. Sens. Actuators B Chem. 2015, 221, 556–563. [Google Scholar] [CrossRef]
- Qiu, S.; Gao, L.; Wang, J. Classification and regression of ELM, LVQ and SVM for E-nose data of strawberry juice. J. Food Eng. 2015, 144, 77–85. [Google Scholar] [CrossRef]
- Adiguzel, Y.; Kulah, H. Breath sensors for lung cancer diagnosis. Biosens. Bioelectron. 2015, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, A.; Blachman-Braun, R.; Galnares-Olalde, J.A. The possibility of inventing new technologies in the detection of cancer by applying elements of the canine olfactory apparatus. Med. Hypotheses 2015, 85, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Annema, J.T.; Schot, R. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Varnamkhasti, M.; Aghbashlo, M. Electronic nose and electronic mucosa as innovative instruments for real-time monitoring of food dryers. Trends Food Sci. Technol. 2014, 38, 158–166. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic nose as a tool for monitoring the authenticity of food. A review. Food Anal. Method 2016, 10, 1800–1816. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef] [PubMed]
- Giungato, P.; Laiola, E.; Nicolardi, V. Evaluation of industrial roasting degree of coffee beans by using an electronic nose and a stepwise backward selection of predictors. Food Anal. Method 2017, 1–10. [Google Scholar] [CrossRef]
- Xu, K.; Wang, J.; Wei, Z. An Optimization of the MOS electronic nose sensor array for the detection of Chinese pecan quality. J. Food Eng. 2017, 203, 25–31. [Google Scholar] [CrossRef]
- Qin, O.; Zhao, J.; Chen, Q. Instrumental intelligent test of food sensory quality as mimic of human panel test combining multiple cross-perception sensors and data fusion. Anal. Chim. Acta 2014, 841, 68–76. [Google Scholar]
- Yener, S.; Sánchezlópez, J.A.; Granitto, P.M. Rapid and direct volatile compound profiling of black and green teas (Camellia sinensis) from different countries with PTR-ToF-MS. Talanta 2016, 152, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, J.; Gao, L. Qualification and quantisation of processed strawberry juice based on electronic nose and tongue. LWT Food Sci. Technol. 2015, 60, 115–123. [Google Scholar] [CrossRef]
- Xiaolong, S.; Hui, L.; Nan, W.; Qiang, Z. Comparison of different classification methods for analyzing electronic nose data to characterize sesame oils and blends. Sensors 2015, 15, 26726–26742. [Google Scholar]
- Ambeth, K.V.D. Human security from death defying gases using an intelligent sensor system. Sens. Biosens. Res. 2016, 7, 107–114. [Google Scholar]
- Qiu, S.; Wang, J. The prediction of food additives in the fruit juice based on electronic nose with chemometrics. Food Chem. 2017, 230, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.Z.; Trowell, S.; Clifford, D.; Cynkar, W.; Cozzolino, D. Geographical origin of Sauvignon Blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal. Chim. Acta 2009, 648, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Measurement 2017, 103, 227–234. [Google Scholar] [CrossRef]
- Tian, F.; Liang, Z.; Zhang, L. A novel pattern mismatch based interference elimination technique in E-nose. Sens. Actuators B Chem. 2016, 234, 703–712. [Google Scholar] [CrossRef]
- Son, M.; Lee, J.Y.; Ko, H.J. Bioelectronic nose: An emerging tool for odor standardization. Trends Biotechnol. 2017, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Adzhri, R.; Arshad, M.K.M.; Gopinath, S.C.B. High-performance integrated field-effect transistor-based sensors. Anal. Chim. Acta 2016, 917, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qiu, S.; Zhou, C. Printed electronics: materials, technologies and applications. In Printed Electronics; Higher Education Press: Beijing, China, 2016; pp. 54–105. [Google Scholar]
- Subramanian, V.; Chang, J.; Liao, F. Printed organic chemical sensors and sensor systems. In Applications of Organic and Printed Electronics; Springer: New York, NY, USA, 2013; pp. 157–177. [Google Scholar]
- Ayadi, Y.; Rahhal, L.; Vilquin, B. Novel concept of gas sensitivity characterization of materials suited for implementation in FET-based gas sensors. Nanoscale Res. Lett. 2016, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Theunis, J.; Stevens, M.; Botteldooren, D. Sensing the environment. In Participatory Sensing, Opinions and Collective Awareness; Springer International Publishing: Basel, Switzerland, 2017; pp. 21–46. [Google Scholar]
- Lin, C.G.; Hu, J.; Song, Y.F. Polyoxometalate-functionalized nanocarbon materials for energy conversion, energy storage, and sensor systems. Adv. Inorg. Chem. 2017, 8, 776–789. [Google Scholar]
- Zhen, Q.; Qi, D.; Liang, H.U.; Huang, L.Q.; Jimmy, H.; Ping, W. Recent advances in bioinspired olfaction/gustation sensors and their applications. Chin. J. Biomed. Eng. 2014, 33, 609–619. [Google Scholar]
- Kim, T.H.; Sang, H.L.; Lee, J. Single-Carbon-Atomic-Resolution Detection of odorant molecules using a human olfactory receptor-based bioelectronic nose. Adv. Mater. 2009, 21, 91–94. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Li, H.; Zhang, J.; Zhuang, S.; Zhang, F. Impedance sensing and molecular modeling of an olfactory biosensor based on chemosensory proteins of honeybee. Biosens. Bioelectron. 2013, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.P. Bioelectronic Nose. News Inf. Chem. Eng. 2014, 32, 536. [Google Scholar]
- Jin, H.J.; Sang, H.L.; Kim, T.H. Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens. Bioelectron. 2012, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Pan, Y.; Chi, L. Gas Sensors Based on Polymer Field-Effect Transistors. Sensors 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, D.; Zhou, J. Porous Organic Field-Effect Transistors for Enhanced Chemical Sensing Performances. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.A.; Del, N.S.M.; Crisolino Pozas, Á.P. Headspace-programmed temperature vaporizer-mass spectrometry and pattern recognition techniques for the analysis of volatiles in saliva samples. Talanta 2016, 160, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ryman, S.K.; Bruce, N.D.B.; Freund, M.S. Temporal responses of chemically diverse sensor arrays for machine olfaction using artificial intelligence. Sens. Actuators B Chem. 2016, 231, 666–674. [Google Scholar] [CrossRef]
- Burlachenko, J.; Kruglenko, I.; Snopok, B.; Persaud, K. Sample handling for electronic nose technology: State of the art and future trends. TrAC Trends Anal. Chem. 2016, 82, 222–236. [Google Scholar] [CrossRef]
- Chiu, S.W.; Tang, K.T. Towards a chemiresistive sensor-integrated electronic nose: A review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.M.; Agudo, J.E.; García, M.A.; García Orellana, C.J.; González Velasco, H.M.; Gallardo, C.R. A compact and low cost electronic nose for aroma detection. Sensors 2013, 13, 5528–5541. [Google Scholar] [CrossRef] [PubMed]
- Reisert, J.; Restrepo, D. Molecular tuning of odorant receptors and its implication for odor signal processing. Chem. Sens. 2009, 34, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Ruan, S.; Denœux, T. An evidential classifier based on feature selection and two-step classification strategy. Pattern Recognit. 2015, 48, 2318–2327. [Google Scholar]
- Akbar, M.A.; Ali, A.A.S.; Amira, A. An empirical study for PCA- and LDA-based feature reduction for gas identification. IEEE Sens. J. 2016, 16, 5734–5746. [Google Scholar] [CrossRef]
- Faleh, R.; Othman, M.; Gomri, S. A transient signal extraction method of WO3, gas sensors array to identify polluant gases. IEEE Sens. J. 2016, 16, 3123–3130. [Google Scholar]
- Yan, J.; Duan, S.; Huang, T. Hybrid feature matrix construction and feature selection optimization-based multi-objective QPSO for electronic nose in wound infection detection. Sens. Rev. 2016, 36, 23–33. [Google Scholar] [CrossRef]
- Min, H.K.; Hou, Y.; Park, S. A computationally efficient scheme for feature extraction with kernel discriminant analysis. Pattern Recognit. 2016, 50, 45–55. [Google Scholar] [CrossRef]
- Yan, J.; Guo, X.; Duan, S.; Jia, P.; Wang, L.; Peng, C. Electronic nose feature extraction methods: A review. Sensors 2015, 15, 27804–27831. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, A.; Jamoussi, S.; Benayed, Y. A multi-objective genetic algorithm for simultaneous model and feature selection for support vector machines. Artif. Intell. Rev. 2017, 1–21. [Google Scholar] [CrossRef]
- Wan, C.; Freitas, A.A. An empirical evaluation of hierarchical feature selection methods for classification in bioinformatics datasets with gene ontology-based features. Artif. Intell. Rev. 2017, 22–40. [Google Scholar] [CrossRef]
- Fujioka, K.; Tomizawa, Y.; Shimizu, N.; Ikeda, K.; Manome, Y. Improving the performance of an electronic nose by wine aroma training to distinguish between drip coffee and canned coffee. Sensors 2015, 15, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.W. SCLS: Multi-label feature selection based on scalable criterion for large label set. Pattern Recognit. 2017, 66, 342–352. [Google Scholar] [CrossRef]
- Puggini, L.; Mcloone, S. Forward selection component analysis: Algorithms and applications. IEEE Trans. Pattern Anal. Mach. Intell. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Miao, D.; Pedrycz, W. Granular multi-label feature selection based on mutual information. Pattern Recognit. 2017, 67, 410–423. [Google Scholar] [CrossRef]
- Berkhout, D.J.; Benninga, M.A.; van Stein, R.M.; Brinkman, P.; Niemarkt, H.J.; Boer, N.K. Effects of sampling conditions and environmental factors on fecal volatile organic compound analysis by an electronic nose device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y. S.; Jung, S. C.; Oh, S. Diagnosis of bovine tuberculosis using a metal oxide-based electronic nose. Lett. Appl. Microbiol. 2015, 60, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lü, E.; Lu, H.; Zhou, Z.; Yu, W.; Jing, Y. Quality detection of litchi stored in different environments using an electronic nose. Sensors 2016, 16, 852. [Google Scholar] [CrossRef] [PubMed]
- Guz, Ł.; Łagód, G.; Jaromin-Gleń, K.; Suchorab, Z.; Sobczuk, H.; Bieganowski, A. Application of gas sensor arrays in assessment of wastewater purification effects. Sensors 2015, 15, 1–21. [Google Scholar]
- Lippolis, V.; Ferrara, M.; Cervellieri, S. Rapid prediction of ochratoxin A-producing strains of Penicillium on dry-cured meat by MOS-based electronic nose. Int. J. Food Microbiol. 2015, 218, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, M.; Shu, D. Monitoring wheat straw fermentation process using electronic nose with pattern recognition methods. Anal. Methods UK 2015, 7, 6006–6011. [Google Scholar] [CrossRef]
- Ciptohadijoyo, R.S.; Litananda, W.S.; Rivai, M.; Purnomo, M.H. Electronic nose based on partition column integrated with gas sensor for fruit identification and classification. Comput. Electron. Agric. 2016, 121, 429–435. [Google Scholar]
- Fu, J.; Li, G.; Qin, Y.; Freeman, W.J. A pattern recognition method for electronic noses based on an olfactory neural network. Sens. Actuators B Chem. 2007, 125, 489–497. [Google Scholar] [CrossRef]
- Feng, B.; Chen, B.; Liu, H. Radar HRRP target recognition with deep networks. Pattern Recognit. 2017, 61, 379–393. [Google Scholar] [CrossRef]
- Yin, B.C.; Wang, W.T.; Wang, L.C. Review of deep learning. J. B. Univ. Technol. 2015, 41, 48–59. [Google Scholar]
- Choi, J.K.; Hwang, I.S.; Kim, S.J. Design of selective gas sensors using electrospun Pd-doped SnO2, hollow nanofibers. Sens. Actuators B Chem. 2010, 150, 191–199. [Google Scholar] [CrossRef]
- Zou, H.Q.; Li, S.; Huang, Y.H. Rapid identification of asteraceae plants with improved RBF-ANN classification models based on MOS sensor E-nose. Evid.-Based Complement. Altern. Med. 2014, 2014, 425341. [Google Scholar] [CrossRef] [PubMed]
- Chilo, J.; Pelegri-Sebastia, J.; Cupane, M.; Sogorb, T. E-nose application to food industry production. IEEE Instrum. Meas. Mag. 2016, 19, 27–33. [Google Scholar] [CrossRef]
- Luo, D.H.; Chen, H.Q. A novel approach for classification of Chinese herbal medicines using diffusion maps. Int. J. Pattern Recognit. 2015, 1, 104–112. [Google Scholar] [CrossRef]
- Röck, F.; Nicolae Barsan, A.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2016, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, C.; Wu, H.; Yang, Y.; Wang, X.; Zhang, Y. Detection method and pattern recognition of ginseng and american ginseng pieces by electronic nose. China J. Chin. Mater. Med. 2012, 37, 1165–1168. [Google Scholar]
- Liu, H.X.; Luo, D.H.; Ji, S.G. Identification and classification of Chinese medicinal materials based on electronic nose. Jilin J. Tradit. Chin. Med. 2011, 31, 580–583. [Google Scholar]
- Wu, L.L.; Zheng, D.; Zheng, B.Z. Study of Zanthoxylum bungeanum Maxim. Varieties discriminating method by electronic nose technology. Chin. J. Sens. Actuators 2013, 11, 1473–1477. [Google Scholar]
- Lin, H.; Yan, Y.; Zhao, T.; Peng, L.; Zou, H.; Li, J. Rapid discrimination of apiaceae plants by electronic nose coupled with multivariate statistical analyses. J. Pharm. Biomed. Anal. 2013, 84, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Sheng-Guo, J.I.; Zhuang, J.J.; Wei-Dong, L.I. To differentiate varieties of Chinese herbal medicine based on bionic olfaction. J. Guangdong Pharm. Univ. 2009, 25, 356–358. [Google Scholar]
- Luo, D.; Fan, D.; Yu, H.; Li, Z. A new processing technique for the identification of Chinese herbal medicine. In Proceedings of the 2013 5th International Conference on Computational and Information Sciences (ICCIS), Shiyan, China, 21–23 June 2013; pp. 474–477. [Google Scholar]
- Zou, H.; Han, Y.; Xing, S.; Lin, Q.; Wei, Z.; Xiong, Y. Electronic nose and its application in chinese materia medica. World Sci. Technol. 2012, 14, 2120–2125. [Google Scholar] [CrossRef]
- Shen, G. Study on the chemical constituents of volatile oil of atractylodes macrocephala koidz. produced in Zhejiang province. Anhui Agric. Sci. Bull. 2008, 14, 128–133. [Google Scholar]
- Xu, M.; Yang, S.L.; Zhang, C.; Wan, J.; Wu, N.; Li, X.Y. Discrimination of Coptis chinensis Franch. and its processed products by odor objectify. China J. Chin. Mater. Med. 2015, 40, 89–93. [Google Scholar]
- Huang, X.S.; Li, W.M.; Zhang, X.L.; Jia, J.; Zhang, H.H.; Wu, C.J. Discriminating processed Areca catechu L. degree of stir-frying and quantizing empirical index based on color difference meter and electronic nose fingerprint analyzer. China J. Chin. Mater. Med. 2009, 34, 1786–1791. [Google Scholar]
- Kong, F.Y.; Shao, L.; Hu, H.H. Application of electronic nose method in the identification of Siegesbeckia orientalis L. from different producing areas. J. China Pharm. 2014, 19, 1793–1795. [Google Scholar]
- Zhong, L.; Wang, Y.W.; Liu, Y.J. Research on identification of Leonurus japonicus Houtt. from Sichuan by electronic nose. World Sci. Technol. Mod. Tradit. Chin. Med. 2014, 6, 1384–1390. [Google Scholar]
- Wu, N.; Yang, S.L.; Yan, D. Advances in consideration and application of identification methods for powered Chinese material medica. China Tradit. Herb. Drugs 2015, 46, 1413–1419. [Google Scholar]
- Li, S.; Li, X.R.; Wang, G.L.; Nie, L.X.; Yang, Y.J.; Wu, H.Z. Rapid discrimination of Chinese Panax ginseng C.A. Mey. and Korean Panax ginseng C.A. Mey. an electronic nose coupled with chemometrics. J. Pharm. Biomed. Anal. 2012, 70, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.P.; Chen, H.P.; Peng, C.; Jia, H.F. Electronic nose used in evaluating Ligusticum chuanxiong hort. produced in different regions and of different levels. Pharm. Clin. Chin. Mater. Med. 2013, 4, 7–10. [Google Scholar]
- Han, B.X.; Zhao, Y.Y.; Zhu, Z.X.; Chen, N.F. Identification of the origin of “dabaiju” by electronic nose. Res. Pract. Chin. Med. 2012, 1, 16–18. [Google Scholar]
- Zheng, J.B.; Yang, L.; Chen, J.B.; Wang, Y.M. Study on odor source localization method based on bionic olfaction. Appl. Mech. Mater. 2013, 4, 391–395. [Google Scholar] [CrossRef]
- Zou, H.Q.; Liu, Y.; Tao, O.; Lin, H.; Su, Y.Z.; Lin, X.L. Optimization method of mos sensor array for identification of traditional Chinese medicine based on electronic nose. China J. Chin. Mater. Med. 2013, 38, 161–166. [Google Scholar]
- Lin, H.; Zhao, T.; Zou, H.Q.; Peng, L.; Jia-Hui, L.I.; Ren, Z.Y. Study on identification to different origin of cultivated and wild Oxybaphus Himalaicus Edgew. by electronic nose. Chin. J. Tradit. Chin. Med. Pharm. 2014, 29, 1834–1837. [Google Scholar]
- Wang, W.T. Study on Identification of Chinese Medicine by Electronic Nose; Beijing University of Chinese Medicine: Beijing, China, 2009. [Google Scholar]
- Chen, W.G.; Sheng, J. Infection of the content of total flavonoids in Chrysanthemum morifolium Ramat. from different storage time and storage condition. Lishizhen Med. Mater. Med. Res. 2006, 17, 1483–1484. [Google Scholar]
- Zhang, W.Y.; Zhu, C.H.; Zhou, H.J. Changes in aromatic components in raw Pu-erh tea during storage. Food Sci. 2010, 31, 153–155. [Google Scholar]
- Hao, Y.Q. Influencing factors of the quality of TCM medicine storage. Clin. J. Chin. Med. 2013, 11, 114–115. [Google Scholar]
- Zhang, C.L.; Li, K.; Fu, L.N. Current status of the relationship between the storage and the active components of traditional Chinese medicine. Guangzhou Chem. Ind. 2015, 20, 18–19. [Google Scholar]
- Jiang, S.; Wang, J. Internal quality detection of Chinese pecans (carya cathayensis) during storage using electronic nose responses combined with physicochemical methods. Postharvest Biol. Technol. 2016, 118, 17–25. [Google Scholar] [CrossRef]
- Wu, S.Y.; Luo, D.H.; Deng, B.R. Study of identification for Chinese medicinal herbs in different producing areas and harvest time. J. Sens. Technol. 2011, 24, 10–13. [Google Scholar]
- Zou, H.Q.; Liu, Y.; Yan, Y.H. Research of E-nose technology and application. J. Sens. Technol. 2011, 17, 6–11. [Google Scholar]
- Eric, B.; Christine, L.; Mark, N. Economic Botany Collections: A source of material evidence for exploring historical changes in Chinese medicinal materials. J. Ethnopharmacol. 2017, 200, 209–227. [Google Scholar]
- Jon, C.; Isobel, B.; Flavia, V. Exploring the use of cost-effective membrane materials for Microbial Fuel Cell based sensors. Electrochim. Acta 2017, 231, 319–326. [Google Scholar]
- Zhao, Z.; Tian, F.; Liao, H. A Novel Spectrum Analysis Technique for Odor Sensing in Optical Electronic Nose. Sens. Actuators B Chem. 2015, 222, 769–779. [Google Scholar] [CrossRef]
- Roine, A.; Saviauk, T.; Kumpulainen, P. Rapid and Accurate Detection of Urinary Pathogens by Mobile IMS-Based Electronic Nose: A Proof-of-Principle Study. PLoS ONE 2014, 9, 114279. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Voros, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Samotaev, N.; Golovin, A.; Vasilyev, V. IMS Development at NRNU MEPhI. In Sensors and Microsystems; Springer International Publishing: Basel, Switzerland, 2014; pp. 447–451. [Google Scholar]
- Gamota, D.R.; Brazis, P.; Kalyanasundaram, K. Printed organic and molecular electronics. Mater. Today 2004, 7, 53. [Google Scholar]
- Olofsson, J.K.; Gottfried, J.A. The muted sense: Neuro-cognitive limitations of olfactory language. Trends Cogn. Sci. 2015, 19, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.A.; Dolan, R.J. The nose smells what the eye sees: Crossmodal visual facilitation of human olfactory perception. Neuron 2003, 39, 375–386. [Google Scholar] [CrossRef]
- Wu, H.K.; Ko, Y.S.; Lin, Y.S. The correlation between pulse diagnosis and constitution identification in traditional Chinese medicine. Complement. Ther. Med. 2017, 30, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.L.; Clementi, E.; Wong, W.S. Advanced technologies charting a new path for Traditional Chinese Medicine drug discovery. Pharmacol. Res. 2017, 117, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.H.; Fang, Z.H.; Pharmacy, D.O. Cause investigation of drug-induced diseases and its relationship with traditional Chinese medicine. Chin. J. Tradit. Chin. Med. Pharm. 2017, 1, 328–331. [Google Scholar]
- Liu, T.; Huang, M.; Zou, Q. Analysis of Volatile Compounds in Dictyophora indusiata-Fortified Beef Flavor by GC-MS and GC-O. Food Sci. 2016, 37, 92–98. [Google Scholar]
| State | Model | Number of Sensors | Sensor Material | Manufacturer | Country |
|---|---|---|---|---|---|
| Commercial | i-Pen, i-Pen3, PEN3 | 6, 10 | MOS | Airsense Analytics | Germany |
| Artinose | 38 | MOS | Sysca AG | Germany | |
| Air quality module | 2 | MOS | Applied Sensor | Sweden | |
| Aromascan A32S | 32 | CP | Osmetech Plc | USA | |
| Bloodhound ST 214 | 14 | CP | Scensive Technologies | UK | |
| Cyranose 320 | 32 | CP | Sensigent | USA | |
| FOX 3000, 4000 | 12, 18 | MOS | Alpha MOS | France | |
| LibraNose | 8 | Quartz Crystal Microbalance (QCM) | Technobiochip | Italy | |
| iNose, T-nose | 14, 10 | MOS | Isenso | China | |
| Non-commercial | Bioelectronic noses | -- | Olfactory receptors | Ref [42] | -- |
| Molecularly imprinted polymers noses | -- | Molecularly imprinted polymers | Ref [43,44,45] | -- | |
| Optical sensors | -- | Optical material | Ref [42,46,47] | -- | |
| Nano-bioelectronics | -- | Nanomaterials, animal receptors | Ref [48,49] | -- |
| Sensor Type | Working Principle | Advantages | Disadvantages |
|---|---|---|---|
| Electrochemical sensors (EC) | The sensor reacts with the gas and generates an electrical signal proportional to the gas concentration gas. | 1. Low power consumption 2. Good robustness 3. Room temperature operation | 1. It isn’t applicable to aromatic hydrocarbons 2. Low sensitivity 3. Large volume |
| Metal oxide sensors (MOS) | The surface gas and oxide react to generate resistance changes according to the gas concentration. | 1. Fast response, short recovery 2. High sensitivity 3. Long life, High reproducibility, convenient replacement | 1. It is easy to react with sulfur compounds and produce damage to the sensor 2. Work at high temperature, High power consumption |
| Conducting polymer sensor (CP) | The resistance of the sensor is changed by the chemical reaction between the surface gas and the polymer, which forms the electrical signal. | 1. High sensitivity 2. Fast response, short recovery 3. Easy synthesis 4. Room temperature operation 5. Not easy to corrosion by sulfur compounds or weak acids | 1. Sensitive to environmental humidity 2.complex manufacturing process 3. Sensor life is short, generally 9~18 months |
| Surface acoustic wave sensors (SAW) | The surface gas flows through the sensors consisting of piezoelectric material and adsorbing material, which generates surface wave. | 1. Fast response 2. Low cost 3. Miniaturization | 1. High power consumption, high signal to noise ratio 2.Complex manufacturing process 3. Interface circuit complexity |
| Optical sensors (OS) | Measure the modulation of light properties or characteristics, such as changes in light absorbance, color, wave-length (colorimetric), upon exposure to gas analytics. | 1. Low energy consumption 2. High signal-to-noise ratio 3. High sensitivity | 1. Poor adaptability to environment 2. Low accuracy when long distance measurement |
| Biomimetic sensors (BS) | Sensors are composed of a fixed cell, an enzyme or other bioactive substances. | 1. Good performance 2. High sensitivity 3. Suitable for on-site analysis 4. Suitable for more complex applications | 1. Poor repeatability 2. Poor stability 3. Difficult to mass production |
| Methods | Formula |
|---|---|
| Difference | |
| Relative | |
| Fraction | |
| Sensor auto scaling | |
| Array Auto Scaling |
| Methods | Formula |
|---|---|
| Logarithmic | |
| First derivatives | |
| Second derivatives |
| Model | Common Method | Basic Principle | Application Area |
|---|---|---|---|
| Statistical recognition model | Principal component analysis (PCA) | A mathematical statistical analysis method. A set of related variables are converted to another set of linear unrelated variables by orthogonal transformation, and the linear unrelated variables are called principal components. | Medical information classification, population statistics, mathematical analysis. |
| Linear discriminant analysis (LDA) | The high dimensional sample data is projected into a low dimensional vector space, which is conducive to the best classification. So in the new subspace, there is a greater distance between the class and a smaller distance in class. | Face recognition, identification of CHMs. | |
| Support vector machine (SVM) | It is based on statistical learning theory including two basic principles, VC (Vapnik-Chervonenkis) dimension theory and structural risk minimization principle. It shows many unique advantages in solving small samples, nonlinear and high dimensional pattern recognition. | Biological information processing, text classification and handwriting recognition. | |
| K-nearest neighbor (KNN) | It is to determine the classification of the samples according to the nearest one or a few samples. The algorithm is simple and easy to implement, and especially is suitable for multiple classification problems. | Forecast estimate, biological, medical, economic and other fields. | |
| Intelligent recognition model | Artificial neural network (ANN) | By imitating the behavior characteristics of human or animal neural network, a mathematical model is established which is to carry out the distributed information processing. | Pattern recognition, intelligent robot, automatic control, prediction and estimation, biology, medicine, economy, etc. |
| Deep learning (DL) | The feature of the original space is transformed into the feature of the new space, and the hierarchical feature representation is obtained by the multilayer feature transform. | Speech recognition, synthesis and Machine Translation; image classification and recognition, etc. | |
| Fuzzy inference (FIS) | Based on the fuzzy set theory, the method is to simulate the human brain to process the non-accurate or nonlinear data information. | Household electrical appliances, expert system, intelligent control, etc. | |
| Genetic algorithm (GA) | The method is to simulate the process of natural evolution and to search for the optimal solution, which consists of selection operation, exchange operation and mutation operation. | Function optimization; production scheduling problem, automatic control, image recognition, etc. |
| Selected Samples | Experimental Results | E-Nose Model | Data Processing Algorithm | Ref. |
|---|---|---|---|---|
| Pogostemon cablin (Blanco) Benth., Mentha haplocalyx Briq | The correct recognition rates were 100% (LDA model) and 98% (PCA model) | PEN3 (Airsense Analytics, Germany) | PCA, LDA | [91] Liu, H.X. |
| Six kinds of Zanthoxylum bungeanum Maxim | BP-NN analysis was the best among three selected methods, and the initial discriminant rate and cross validation rate in BP-NN analysis were 99% and 96.2% respectively. | E-nose System (made up of eight sensors constructed in Lab) | Back Propagation Neural Network (BP-NN), Probabilistic Neural Network (PNN), SVM | [92] Wu, L.L. |
| Apiaceae plants | The identification rate of ten-folds cross validation was 94.71%. | FOX3000 (Alpha MOS, France) | LDA, PCA, Hierarchical clustering analysis (HCA), ANN | [93] Lin, H. |
| Seven medicines (Illicium verum Hook. f., Amomi Fructus Rotundus, Ligusticum chuanxiong hort., Eugenia caryophyllata Thunb., Schizonepeta tenuifolia Briq., Cinnamomum cassia Presl, Amomum villosum Lour.) | The correct recognition rates were 98% (LDA model) and 96% (PCA model) respectively. | PEN3 | LDA, PCA | [94] Liu, H.X. |
| Amomum villosum Lour., Pogostemon cablin Benth., Leonurus japonicus Houtt., Houttuynia cordata Thunb., Mentha haplocalyx Briq. and Bupleurum chinense DC. | The odor fingerprint of Mentha haplocalyx Briq. had 15 common peaks and the largest average value, while that of Bupleurum chinense DC. had only 11 common peaks and the smallest average value. | PEN3 | LDA, PCA, LDA + PCA | [95] Luo, D. |
| Raw Atractylodes macrocephala Koidz. and processed Atractylodes macrocephala Koidz | The RSD of the relative peak area of the common peaks were less than 1.2%, and the relative retention time of each peak was less than 1.1%. | FOX 3000 | PCA | [97] Shen, G. |
| Four different samples of processed Coptis chinensis Franch. | PCA analysis was the best one in the selected four methods, and the initial discriminant rate and cross validation rate in PCA analysis were 100% and 94.4% respectively. | FOX 4000 (Alpha MOS, France) | PCA, LDA, Statistical Quality Control analysis (SQC), Soft Independent Modeling analysis (SIMCA) | [98] Xu, M. |
| Raw Areca catechu L. and processed Areca catechu L. | ANN analysis showed the best performance among three selected methods, and the initial discriminant rate and cross validation rate in ANN model were 100% and 97% respectively. | FOX 4000 | PCA, LDA, ANN | [99] Huang, X.S. |
| Siegesbeckia orientalis L. from different producing areas | The ten-folds cross validation rate was 93.19%. | FOX 3000 | PCA | [100] Kong, F.Y. |
| Leonurus japonicus Houtt. from Sichuan | PCA showed better performance than DFA. | FOX 4000 | PCA, DFA | [101] Zhong, L. |
| Fritillaria cirrhosa D. Don and Fritillaria thunbergii Miq | The initial discriminant rate and cross validation rate were 98% and 95% respectively. | FOX 4000 | PCA | [102] Wu, N. |
| Chinese Panax ginseng C.A. Mey. and Korean Panax ginseng C.A. Mey | The ten-folds cross validation rates of the three models were 96.12%, 97.56%, 92.39% respectively. | FOX 3000 | PCA, Discriminant factorial analysis (DFA), SIMCA (soft independent model of class analogy) | [103] Li, S. |
| Ligusticum chuanxiong hort. samples from different regions | The correct identification rate was 92.1% based an E-nose system. | FOX 4000 | PCA, LDA | [104] Chen, L. |
| Chrysanthemummorifolium RaTnat. in different habitats | The cross validation rates were 94.38% for PCA and 91.46% for DFA. | FOX 4000 | PCA, DFA | [105] Han, B.X. |
| Identification of Mentha haplocalyx Briq. from different regions; The odor fingerprint of Mentha haplocalyx Briq | Samples from Guangdong province had 18 common peaks with the average value of 12.67, while Mentha haplocalyx Briq. from Guangxi province had only 14 common peaks and the average value of 11.81. | PEN3 | PCA, PLS | [106] Zheng, J.B. |
| Amomum villosum Lour. from different regions | The performance of NBN model was the best and the initial discriminant rate and cross validation rate were 98% and 95.2% respectively. | FOX 3000 | PCA, Fisher-LDA, Naive Bayes Net (NBN), Radial Basis Function (RBF), Random Forests (RF) | [107] Zou, H.Q. |
| Oxybaphus himalaicus Edgew. from different regions | The performance of ANN model was the best and the initial discriminant rate and cross validation rate were 100% and 96.8% respectively. | FOX 3000 | DFA, HCA, ANN | [108] Lin, H. |
| Saposhnikovia divaricata (Turcz.) Schischk., Bupleurum Chinense DC. and Angelica sinensis (Oliv.) Diels. | SIMCA had more advantages in identification of Angelica sinensis (Oliv.) Diels than the other two models. The IDR and 10-FCVR were 96.6% and 95.2%; in identification of Bupleurum Chinense DC., IDR and 10-FCVR were 94.8% and 93.9%; in identification of Saposhnikovia divaricata (Turcz.) Schischk., IDR and 10-FCVR were 91.8% and 88.3%. | FOX 3000 | PCA, SIMCA, DFA | [109] Wang, W.T. |
| Amomum villosum Lour. in different storage times | The identification performance of PCA + LDA (R2 = 0.9472, RMSE = 0.7618) was better than PCA (R2 = 0.9262, RMSE = 0.8238) and LDA (R2 = 0.9086, RMSE = 0.8952). | PEN3 | PCA, LDA, PCA + LDA | [115] Wu, S.Y. |
| Panax quinquefolium L. in different storage times | The identification rate 89.76% of ten-folds cross validation showed that the E-nose system could also identify Panax quinquefolium L. samples with different storage time. | FOX 3000 | ANN | [116] Zou, H.Q. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Luo, D.; GholamHosseini, H.; Li, Z.; He, J. Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges. Sensors 2017, 17, 1073. https://doi.org/10.3390/s17051073
Zhou H, Luo D, GholamHosseini H, Li Z, He J. Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges. Sensors. 2017; 17(5):1073. https://doi.org/10.3390/s17051073
Chicago/Turabian StyleZhou, Huaying, Dehan Luo, Hamid GholamHosseini, Zhong Li, and Jiafeng He. 2017. "Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges" Sensors 17, no. 5: 1073. https://doi.org/10.3390/s17051073





