A Miniature Aerosol Sensor for Detecting Polydisperse Airborne Ultrafine Particles
Abstract
:1. Introduction
2. System for Miniature Aerosol Sensor
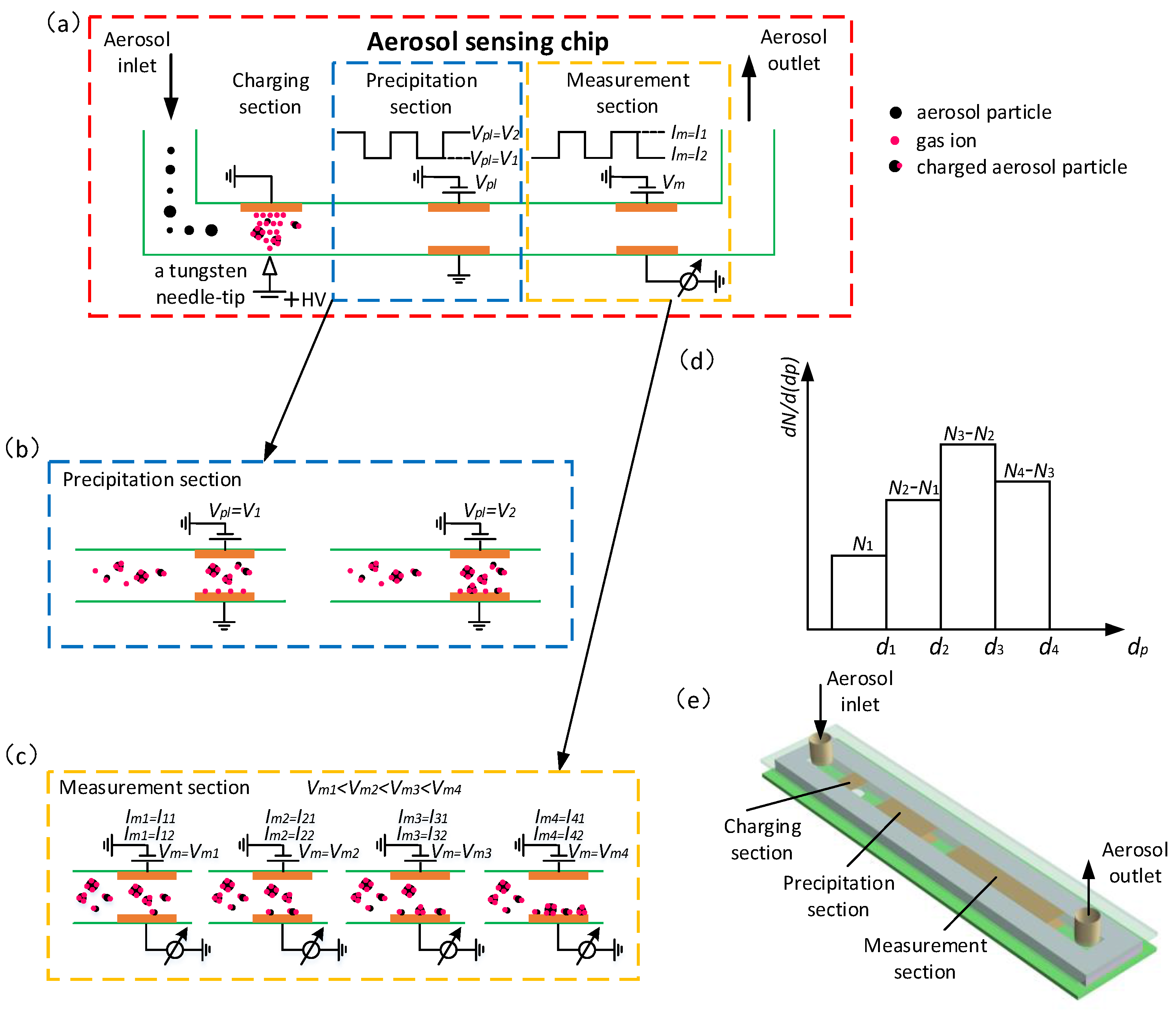
2.1. Working Principle of the Aerosol Sensor
2.2. System for the Aerosol Sensor
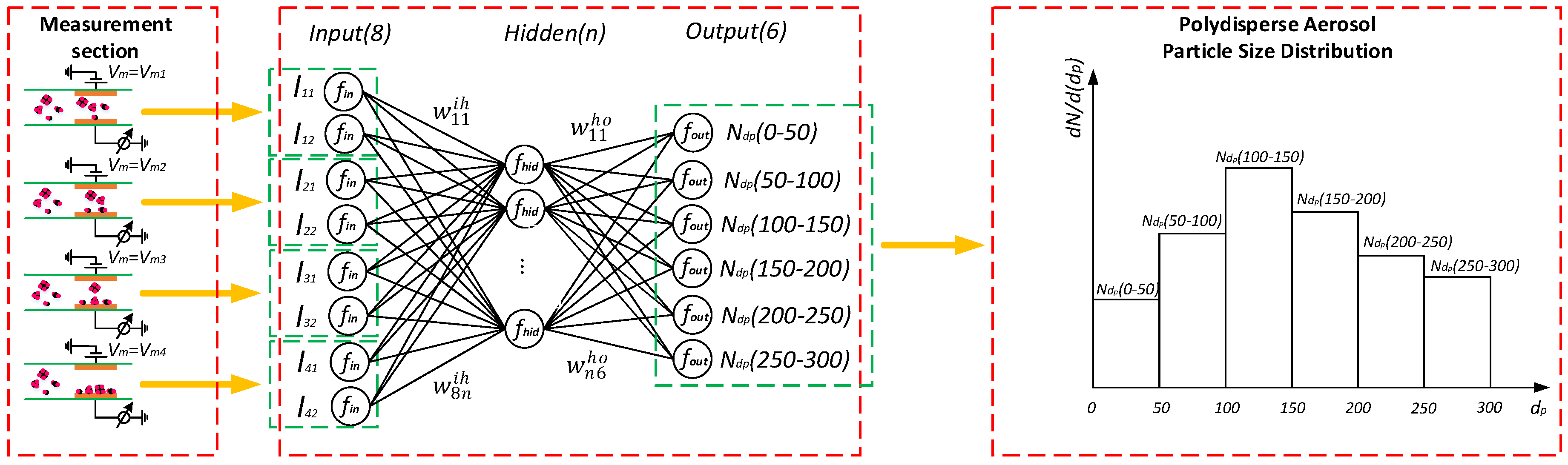
2.3. Data Fusion Algorithm for Identifying Polydisperse Particle Size Distribution
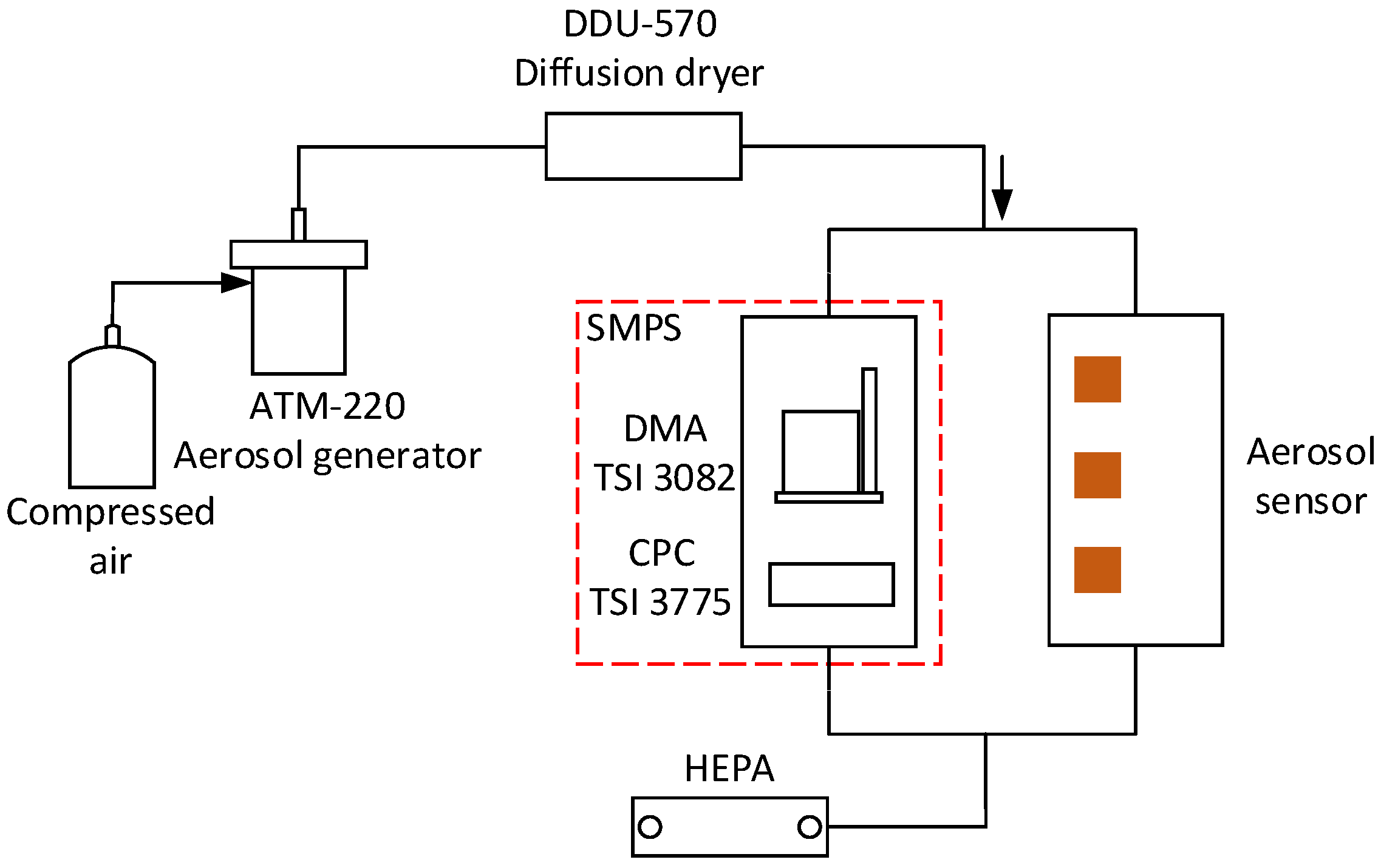
3. Experimental Setup and Aerosol Generation
4. Results and Discussion
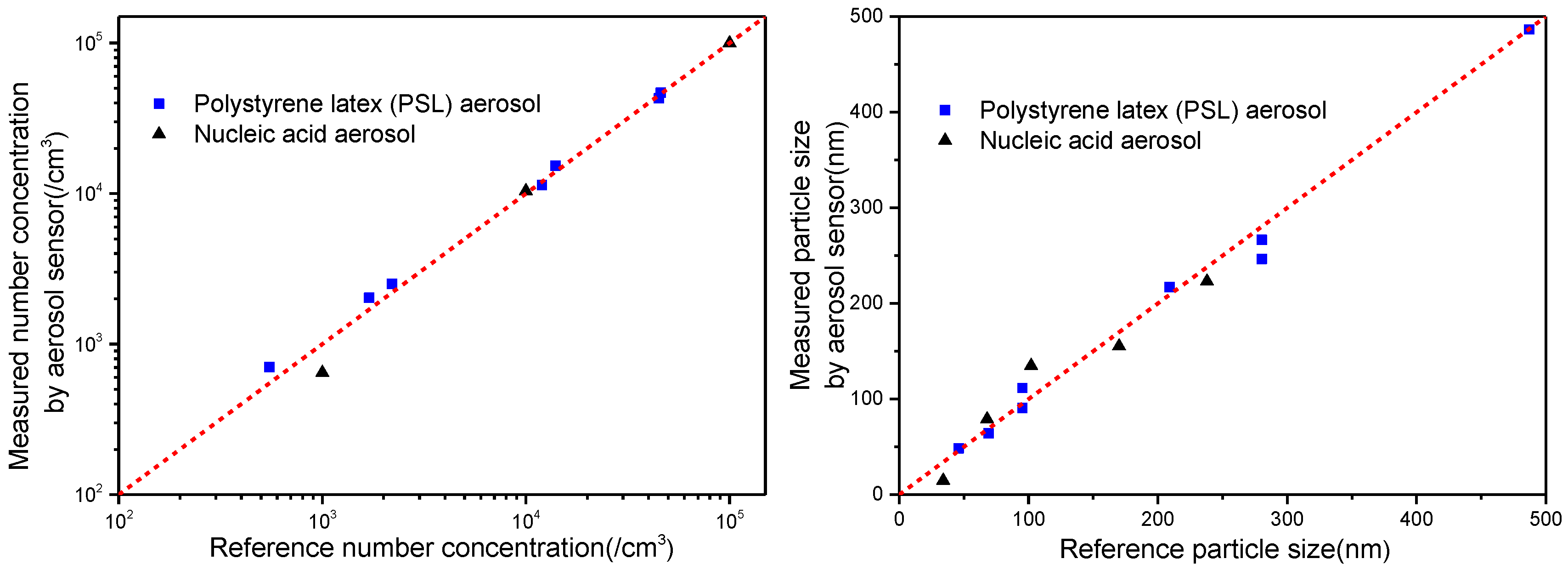
4.1. Preliminary Experiments
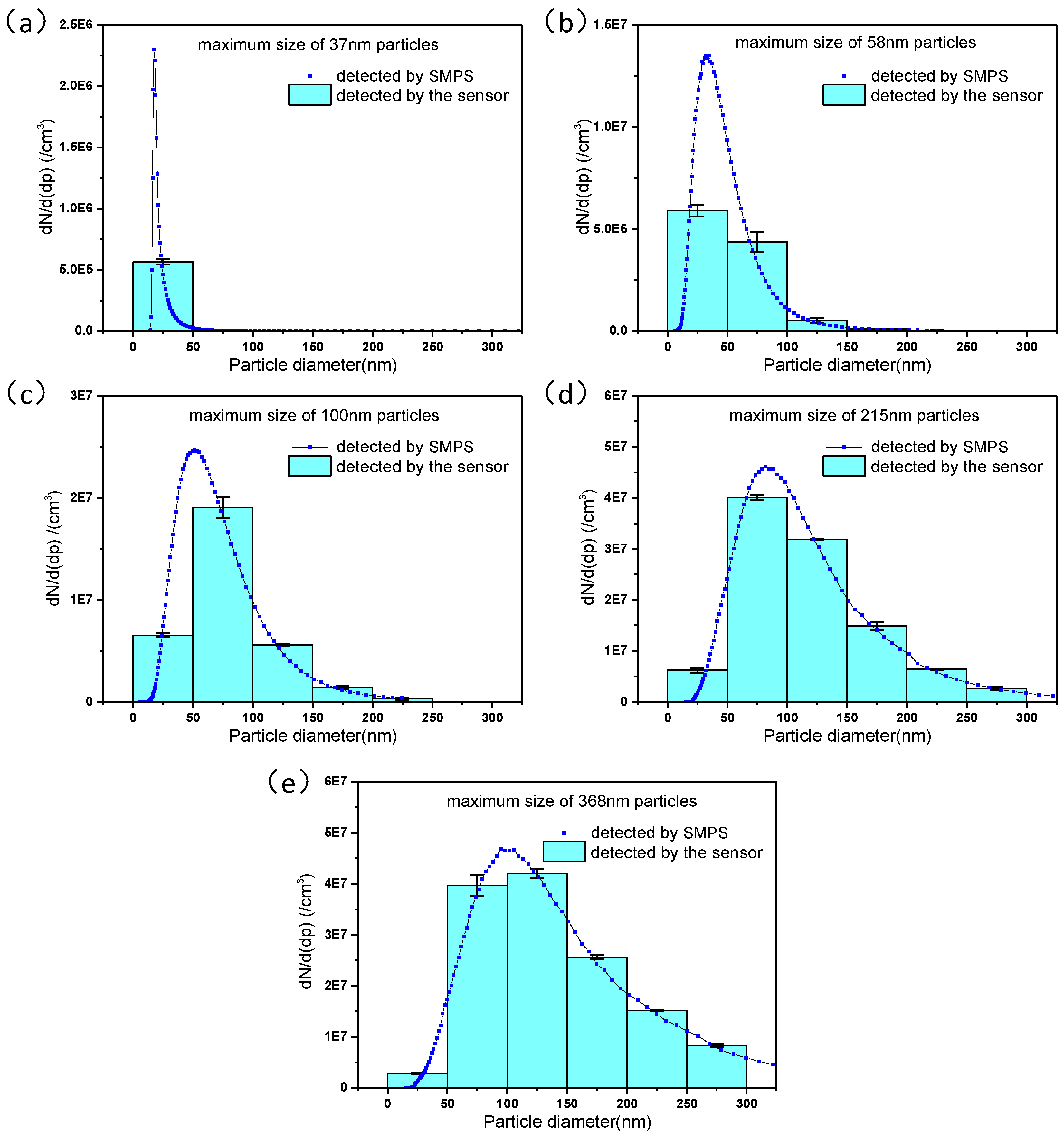
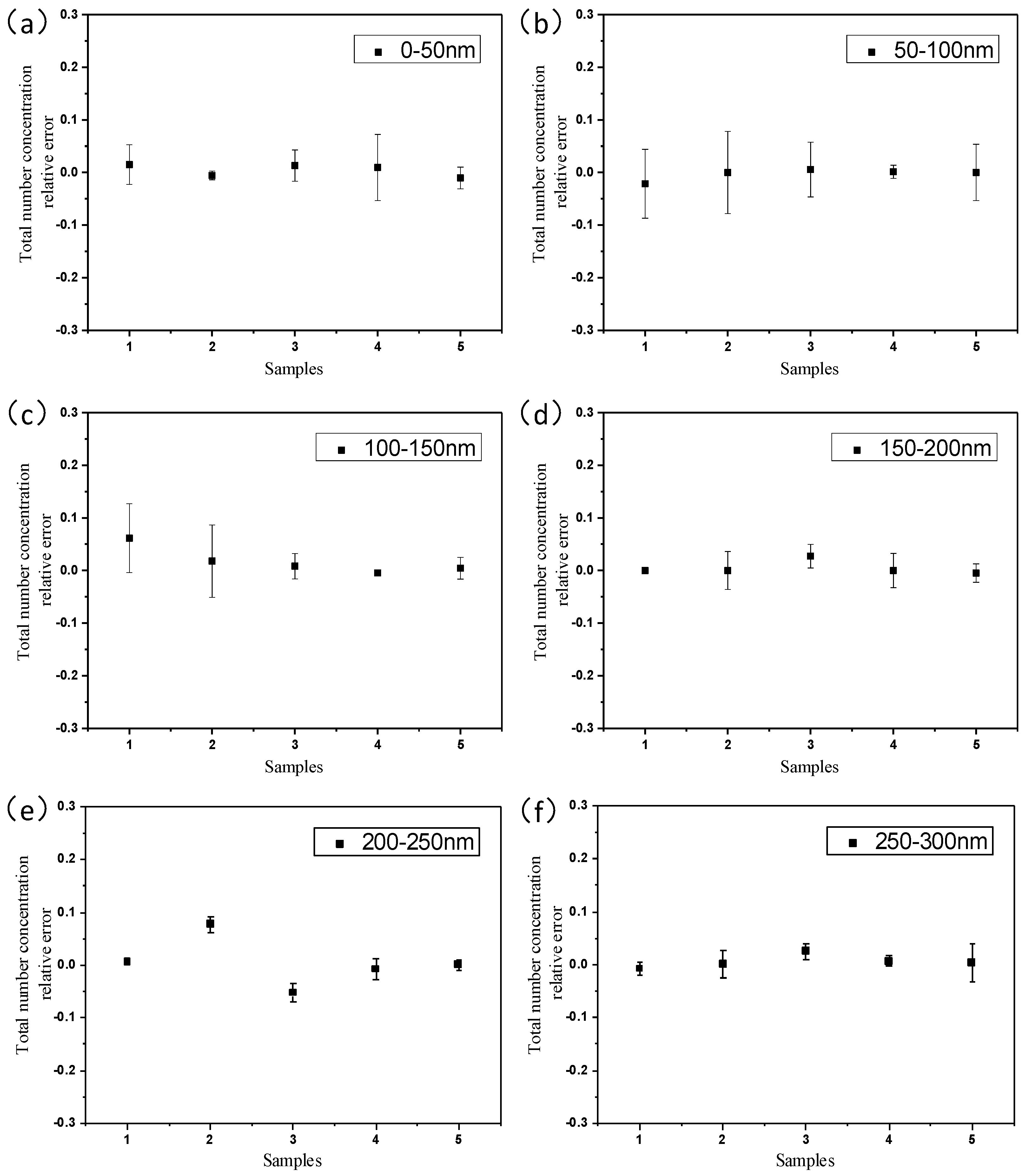
4.2. Measurement Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- William, C.H. Aerosol Technology, Properties, Behavior and Measurement of Airborne Particles, 2nd ed.; John, W.S., Ed.; John Wiley & Sons, Inc: New York, NY, USA, 1998; p. 483. [Google Scholar]
- John, W. Aerosol Measurement, Principles, Techniques and Applications, 4th ed.; Pramod, K., Paul, A.B., Klaus, W., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 41–54. [Google Scholar]
- World Health Organization. Health Risks of Particulate Matter from Long-Range Transboundary Air Pollution; European Centre for Environment and Health: Bonn, Germany, 2006. [Google Scholar]
- Maynard, A.D.; Kuempl, E.M. Airborne nanostructured particles and occupational health. J. Nanoparticle Res. 2005, 7, 587–614. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe Handling of Nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdörster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health Part A. 2002, 65, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Celein, R.M.; Ferin, J.; Weiss, B. Association of particulate air pollution and acute mortality: involvement of ultrafine particles. Inhal. Toxic. 1995, 7, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Li, X.Y.; MacNee, W. Ultrafine (nanometer) particle mediated lung injury. J. Aerosol Sci. 1998, 29, 553–560. [Google Scholar] [CrossRef]
- Jakobsson, J.K.F.; Hedlund, J.; Kumlin, J.; Wollmer, P.; Löndahl, J. A new method for measuring lung deposition efficiency of airborne nanoparticles in a single breath. Sci. Rep. 2016, 6, 36147. [Google Scholar] [CrossRef] [PubMed]
- ICRP, International Commission on Radiological Protection. ICRP Publication 66: Human Respiratory Tract Model for Radiological Protection. Elsevier Health Sci. 1994, 24, 1–482. [Google Scholar]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Asbach, C.; Fissan, H.; Hülser, T.; Kuhlbusch, T.A.J.; Thompson, D.; Pui, D.Y.H. How can nano-biotechnology oversight advance science and industry: Examples from environmental, health, and safety studies of nanoparticles (nano-EHS). J. Nanoparticle Res. 2011, 13, 1373–1387. [Google Scholar] [CrossRef]
- Asbach, C.; Fissan, H.; Stahlmecke, B.; Kuhlbusch, T.A.J.; Pui, D.Y.H. Conceptual limitation and extensions of lung-deposited Nanoparticle Surface Area Monitor (NSAM). J. Nanoparticle Res. 2009, 11, 101–109. [Google Scholar] [CrossRef]
- Manigrasso, M.; Stabile, L.; Avino, P.; Buonanno, G. Influence of measurement frequency on the evaluation of short-term dose of sub-micrometric particles during indoor and outdoor generation events. Atmos. Environ. 2013, 67, 130–142. [Google Scholar] [CrossRef]
- Buonanno, G.; Morawska, L.; Stabile, L.; Wang, L.; Giovinco, G. A comparison of submicrometer particle dose between Australian and Italian people. Environ. Pollut. 2012, 169, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, N.K.; Lee, S.G.; Hwang, J. Real time Measurement of the Size Distribution of Diesel Exhaust Particles using a Portable 4-stage Electrical Low Pressure Impactor. Part. Part. Syst. Charact. 2009, 26, 179–186. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Pui, D.Y.H. On the performance of the electrical aerosol analyzer. J. Aerosol Sci. 1975, 6, 249–264. [Google Scholar] [CrossRef]
- Sarangi, B.; Aggarwal, S.G.; Gupta, P.K. A Simplified Approach to Calculate Particle Growth Rate Due to Self-Coagulation, Scavenging and Condensation Using SMPS Measurements during a Particle Growth Event in New Delhi. Aerosol Air Qual. Res. 2015, 15, 166–179. [Google Scholar] [CrossRef]
- Leskinen, J.; Joutsensaari, J.; Lyyränen, J.; Koivisto, J.; Ruusunen, J.; Järvelä, M.; Tuomi, T.; Hämeri, K.; Auvinen, A.; Jokiniemi, J. Comparison of nanoparticle measurement instruments for occupational health applications. J. Nanoparticle Res. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Stabile, L.; Cauda, E.; Marini, S.; Buonanno, G. Metrological assessment of a portable analyzer for monitoring the particle size distribution of ultrafine particles. Ann. Occup. Hyg. 2014, 58, 860–876. [Google Scholar] [PubMed]
- Price, H.D.; Stahlmecke, B.; Arthur, R.; Kaminski, H.; Lindermann, J.; Däuber, E.; Asbach, C.; Kuhlbusch, T.A.J.; BéruBé, K.A.; Jones, T.P. Comparison of instruments for particle number size distribution measurements in air quality monitoring. J. Aerosol Sci. 2014, 76, 48–55. [Google Scholar] [CrossRef]
- Zimmerman, N.; Pollitt, K.J.G.; Jeong, C.-H.; Wang, J.M.; Jung, T.; Cooper, J.M.; Wallace, J.S.; Evans, G.J. Comparison of three nanoparticle sizing instruments: The influence of particle morphology. Atmos. Environ. 2014, 86, 140–147. [Google Scholar] [CrossRef]
- Marra, J.; Den, B.W.; Goossens, H.; Kessels, S. Nanoparticle monitoring for exposure assessment. Nanotechnol. Mag. IEEE 2009, 3, 6–37. [Google Scholar] [CrossRef]
- Marra, J.; Voetz, M.; Kiesling, H.J. Monitor for detecting and assessing exposure to airborne nanoparticles. J. Nanoparticle Res. 2010, 12, 21–37. [Google Scholar] [CrossRef]
- Fierz, M.; Houle, C.; Steigmeier, P.; Burtscher, H. Design, calibration, and field performance of a miniature diffusion size classifier. Aerosol Sci. Techn. 2011, 45, 1–10. [Google Scholar] [CrossRef]
- Bau, S.; Zimmermann, B.; Payet, R.; Witschger, O. A laboratory study of the performance of the handheld diffusion size classifier (DiSCmini) for various aerosols in the 15–400 nm range. Environ. Sci. Process. Impacts 2015, 17, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, R.; Yang, W. A Micro Aerosol Sensor for the Measurement of Airborne Ultrafine Particles. Sensors 2016, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Tritscher, T.; Beeston, M.; Zerrath, A.F.; Elzey, S.; Krinke, T.J.; Filimundi, E.; Bischof, O.F. NanoScan SMPS—A Novel, Portable Nanoparticle Sizing and Counting Instrument. J. Phys. Conf. Ser. IOP Publ. 2013, 429, 012061. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Lee, Y.C.; Chen, K.C.; Ye, W.C.; Sopajaree, K.; Tsai, Y.I. Experimental Comparison of Two Portable and Real-Time Size Distribution Analyzers for Nano/Submicron Aerosol Measurements. Aerosol Air Qual. Res. 2016, 16, 919–929. [Google Scholar] [CrossRef]
- Jung, H.; Kittelson, D.B. Characterization of aerosol surface instruments in transition regime. Aerosol Sci. Tech. 2005, 39, 902–911. [Google Scholar] [CrossRef]
- Lau, C. Neural Networks: Theoretical Foundations and Analysis; IEEE Press: Piscataway, NJ, USA, 1991. [Google Scholar]
- Robert, F.W. Molecular Biology, 5th ed.; McGraw-Hill Higher Education: New York, NJ, USA, 2011. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, D.; Zhu, R.; Yang, W.; Jiang, P. A Miniature Aerosol Sensor for Detecting Polydisperse Airborne Ultrafine Particles. Sensors 2017, 17, 929. https://doi.org/10.3390/s17040929
Zhang C, Wang D, Zhu R, Yang W, Jiang P. A Miniature Aerosol Sensor for Detecting Polydisperse Airborne Ultrafine Particles. Sensors. 2017; 17(4):929. https://doi.org/10.3390/s17040929
Chicago/Turabian StyleZhang, Chao, Dingqu Wang, Rong Zhu, Wenming Yang, and Peng Jiang. 2017. "A Miniature Aerosol Sensor for Detecting Polydisperse Airborne Ultrafine Particles" Sensors 17, no. 4: 929. https://doi.org/10.3390/s17040929




