Comparison between a Direct-Flow SPR Immunosensor for Ampicillin and a Competitive Conventional Amperometric Device: Analytical Features and Possible Applications to Real Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Flow SPR or Amperometric Apparatus
2.3. Flow-Immunosensor SPR
2.3.1. Functionalization of the Gold Plate
2.3.2. Immobilization of Anti-Ampicillin
2.3.3. Association of Ampicillin
2.4. The Conventional Amperometric Immunosensor
2.4.1. Ampicillin Biotinylation
2.4.2. Anti-Ampicillin Immobilization on the Immobilon Membrane
2.4.3. Ampicillin Determination with the “Competitive Format”
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Microbiologia Medica, 6th ed.; Edra Masson: Milan, Italy, 2015; pp. 167–169, 187–190. [Google Scholar]
- Benito-Peña, E.; Partal-Rodera, A.I.; Léon-González, M.E.; Moreno-Bondi, M.C. Evaluation of mixed mode solid phase extraction cartridges for the preconcentration of beta-lactam antibiotics in wastewater using liquid chromatography with UV-DAD detection. Anal. Chim. Acta 2006, 556, 415–422. [Google Scholar] [CrossRef]
- Marazuela, M.D.; Bogialli, S.A. A review of novel strategies of sample preparation for the determination of antibacterial residues in foodstuffs using liquid chromatography-based analytical methods. Anal. Chim. Acta 2009, 645, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, S.E.; McWhinney, B.C.; Lipman, J.; Roberts, J.A.; Ungerer, J.P.J. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 15, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Evaggelopoulou, E.N.; Papadoyannis, I.N. Development of a validated HPLC method for the determination of four penicillin antibiotics in pharmaceuticals and human biological fluids. J. Sep. Sci. 2006, 2, 1550–1560. [Google Scholar] [CrossRef]
- Jin, H.; Kumar, A.P.; Paik, D.H.; Ha, K.C.; Yoo, K.C.; Lee, Y.I. Trace analysis of tetracycline antibiotics in human urine using UPLC-QTOF mass spectrometry. Microchem. J. 2010, 94, 139–147. [Google Scholar] [CrossRef]
- Mottier, P.; Parisod, V.; Gremaud, E.; Guy, P.A.; Stadler, R.H. Determination of the antibiotic chloramphenicol in meat and seafood products by liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2003, 994, 75–84. [Google Scholar] [CrossRef]
- Becker, M.; Zittlau, E.; Petz, M. Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2004, 520, 19–32. [Google Scholar] [CrossRef]
- Ohmori, T.; Suzuki, A.; Niwa, T.; Ushikoshi, H.; Shirai, K.; Yoshida, S.; Ogura, S.; Itoh, Y. Simultaneous determination of eight b-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Rasic Misic, I.; Miletic, G.; Mitic, S.; Mitic, M.; Pecev-Marinkovic, E. A Simple Method for the Ampicillin Determination in Pharmaceuticals and Human Urine. Chem. Pharm. Bull. 2013, 61, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Pikkemaat, M.G.; Rapallini, M.L.; Dijk, S.O.; Elferink, J.W. Comparison of three microbial screening methods for antibiotics using routine monitoring samples. Anal. Chim. Acta 2009, 637, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Musser, M.B.; Anderson, K.L.; Rushing, J.E.; Moats, W.A. Potential for milk containing penicillin G or amoxicillin to cause residues in calves. J. Dairy Sci. 2001, 84, 126–133. [Google Scholar] [CrossRef]
- Brand, U.; Reinhardt, B.; Rüther, F.; Scheper, T.; Schügerl, K. Bio-field-effect transistors as detectors in flow-injectin analysis. Anal. Chim. Acta 1990, 238, 201–210. [Google Scholar] [CrossRef]
- Goldfinch, M.J.; Lowe, C.R. Solid-phase optoelectronic sensors for biochemical analysis. Anal. Biochem. 1984, 138, 430–436. [Google Scholar] [CrossRef]
- Adrian, J.; Pasche, S.; Voirin, G.; Adrian, J.; Pinacho, D.G.; Font, H.; Sanchez-Baeza, F.; Marco, J.M.P.; Diserens, J.M.; Granier, B. Wavelength-interrogated optical biosensor for multi-analyte screening of sulphonamide, fluoroquinolone, beta-lactam and tetracycline antibiotics in milk. Trends Anal. Chem. 2009, 28, 769–777. [Google Scholar] [CrossRef]
- Thavarungkul, P.; Dawan, S.; Kanatharana, P.; Asawatreratanakul, P. Detecting penicillin G in milk with Impedimetric label-free immunosensor. Biosens. Bioelectron. 2007, 23, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Moreno-Bondi, M.C.; Orellana, G.; Maquieira, A.; Van Amerongen, A. Development of a novel and automated fluorescent immunoassay for the analysis of beta-lactam antibiotics. J. Agric. Food Chem. 2005, 53, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, W.C.; Lee, H.J. Application of a solid-phase fluorescence immunoassay to determine amoxicillin residues in fish tissue. Acta Vet. Hung. 2010, 58, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Conzuelo, F.; Esteban-Torres, M.; De Las Rivas, A.B.; Reviejo, A.J.; Muñoz, R.; Pingarrón, J.M. An amperometric affinity penicillin-binding protein magnetosensor for the detection of beta-lactam antibiotics in milk. Analyst 2013, 138, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.P.; Warholic, P.S.; Devou, J.M.; Chaney, L.K.; Clark, G.H. Parallux™ beta-lactam: A capillary-based fluorescent immunoassay for the determination of penicillin-G, ampicillin, amoxicillin, cloxacillin, cephapirin, and ceftiofur in bovine milk. J. AOAC Int. 2002, 85, 355–364. [Google Scholar] [PubMed]
- Campanella, L.; Tomassetti, M.; Sbrilli, R. Benzylpenicillinate liquid membrane ion-selective electrode: Preparation and application to a real matrix (Drug). Ann. Chim. 1986, 26, 483–497. [Google Scholar]
- Merola, G.; Martini, E.; Tomassetti, M.; Campanella, L. New immunosensor for β-lactam antibiotics determination in river waste waters. Sens. Actuators B Chem. 2014, 199, 301–313. [Google Scholar] [CrossRef]
- Merola, G.; Martini, E.; Tomassetti, M.; Campanella, L. Simple and suitable immunosensor for β-Lactam antibiotics analysis in real matrixes: Milk, serum, urine. J. Pharm. Biomed. Anal. 2015, 106, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Conta, G.; Campanella, L.; Favero, G.; Sanzò, G.; Mazzei, F.; Antiochia, R. A Flow SPR Immunosensor Based on a Sandwich Direct Method. Biosensors 2016, 6, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.N.; Smith, M.L.; Chiesa, O.A. LC/MS/MS measurement of penicillin G in bovine plasma, urine, and biopsy samples taken from kidneys of standing animals. J. Chromatogr. B 2006, 830, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, M.; Hu, J.; Zhang, Y.; Chang, H.; Jin, F. Determination of ampicillin and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Grubelnik, A.; Padeste, C.; Tiefenauer, L. Highly Sensitive Enzyme Immunoassays for the Detection of β-Lactam Antibiotics. Food Agric. Immunol. 2001, 13, 161–169. [Google Scholar] [CrossRef]
- Bogdanov, S. Current status of analytical methods for the detection of residues in bee products. Apiacta 2003, 38, 190–197. [Google Scholar]
- Adrian, J.; Pinacho, D.G.; Granier, B.; Diserens, J.M.; Sanchez-Baeza, F.; Marco, M.P. A multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and β-lactam antibiotics in milk samples using class-selective bioreceptors. Anal. Bioanal. Chem. 2008, 391, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, S.P.; O’Loan, N.; McConnell, R.I.; Benchikh, E.O.; Kane, N.E. Stable competitive enzyme-linked immunosorbent assay kit for rapid measurement of 11 active beta-lactams in milk, tissue, urine, and serum. J. AOAC Int. 2007, 90, 334–342. [Google Scholar] [PubMed]
- Okerman, L.; De Wasch, K.; Van Hoof, J.; Smedts, W. Simultaneous determination of different antibiotic residues in bovine and in porcine kidneys by solid-phase fluorescence immunoassay. J. AOAC Int. 2003, 86, 236–240. [Google Scholar] [PubMed]
- European Communities. Commission Regulation (EEC) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union L 2010, 15, 1–72. [Google Scholar]

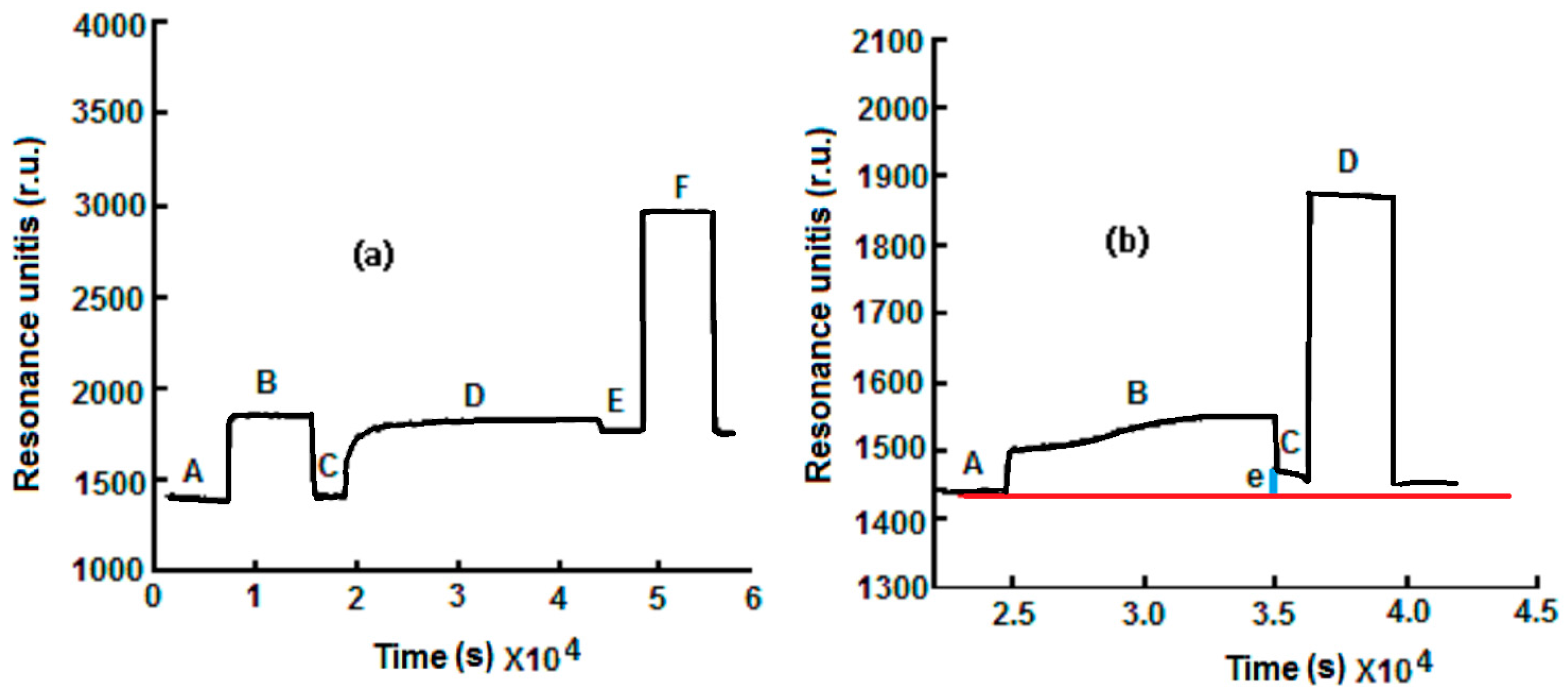
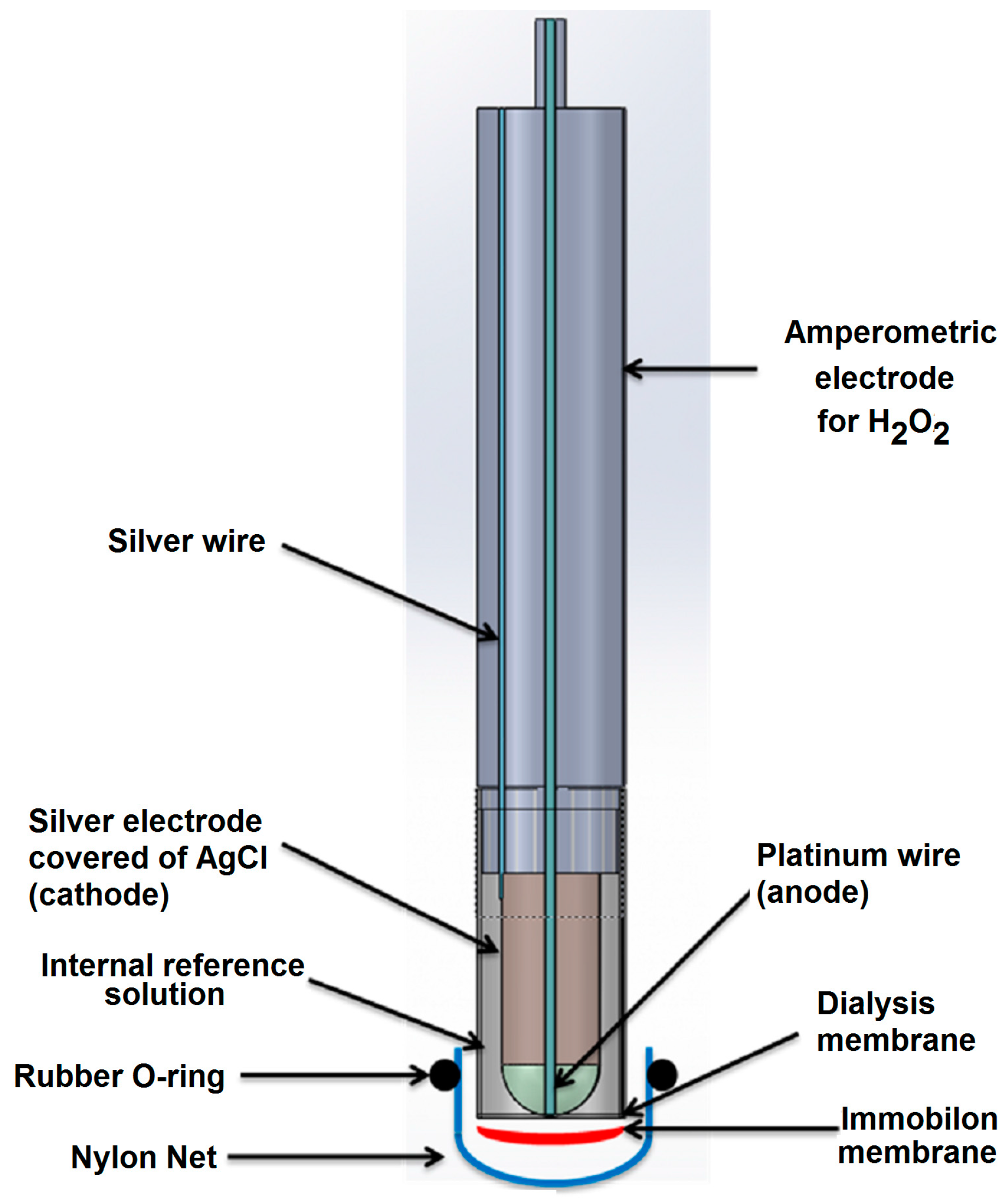
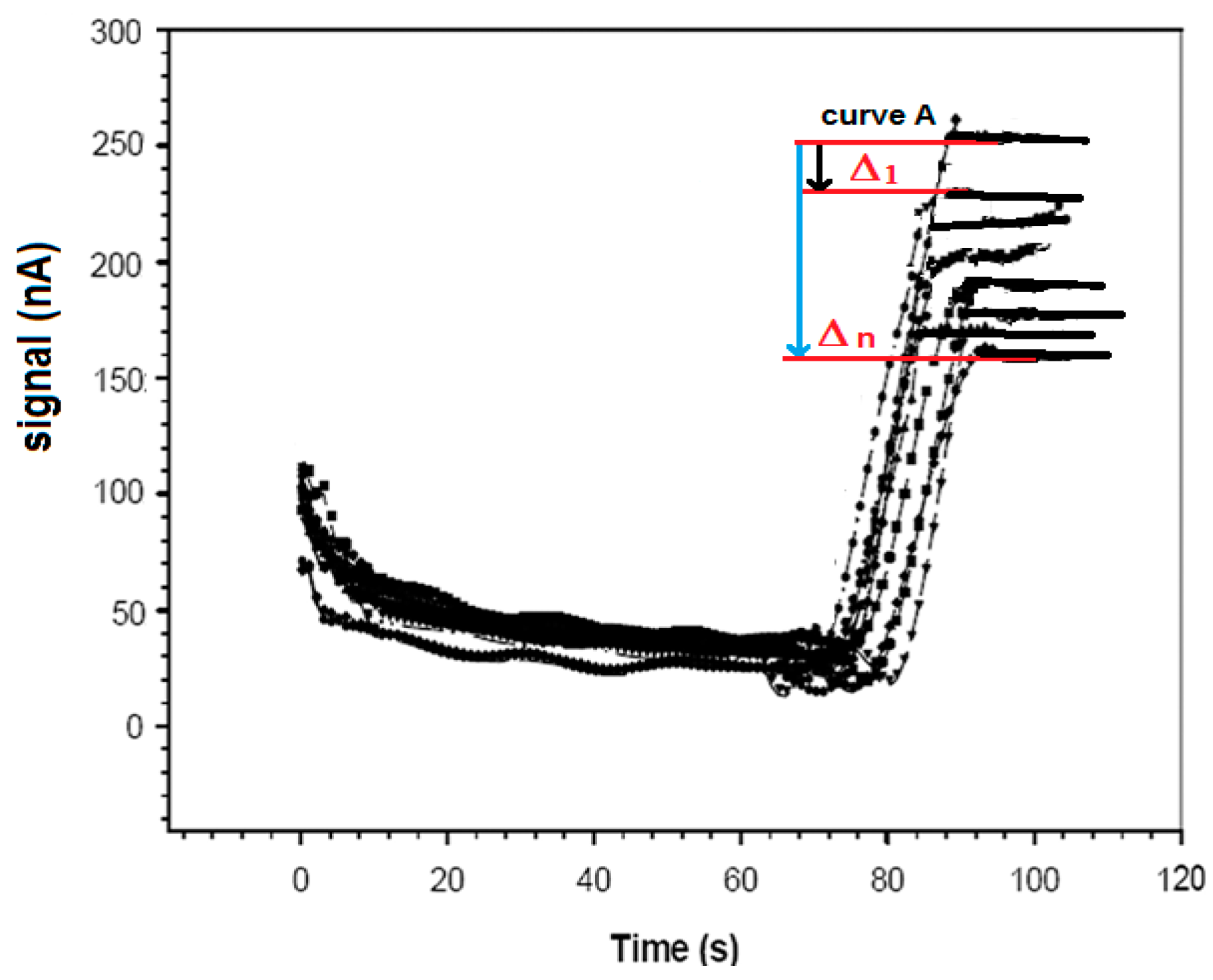
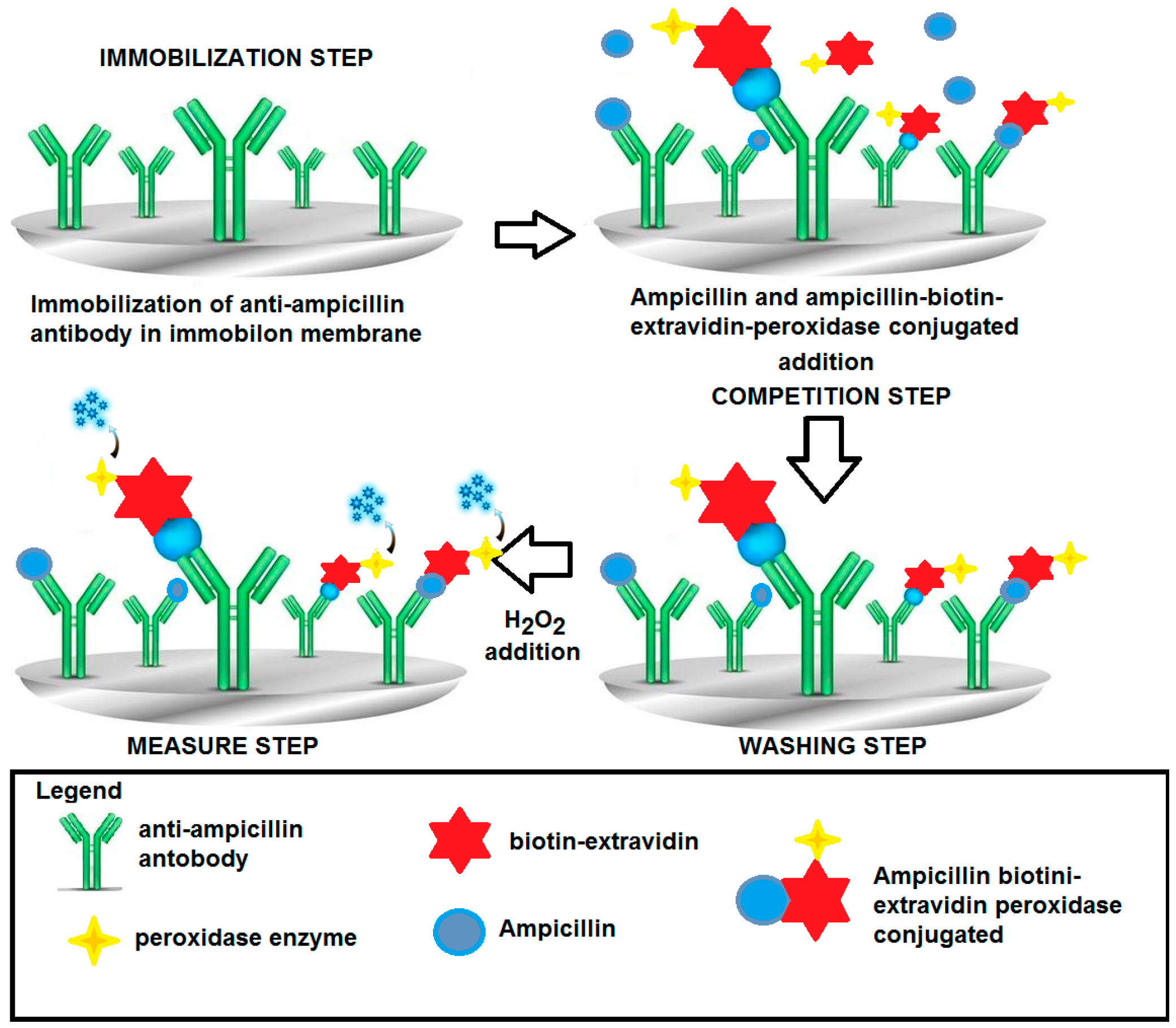
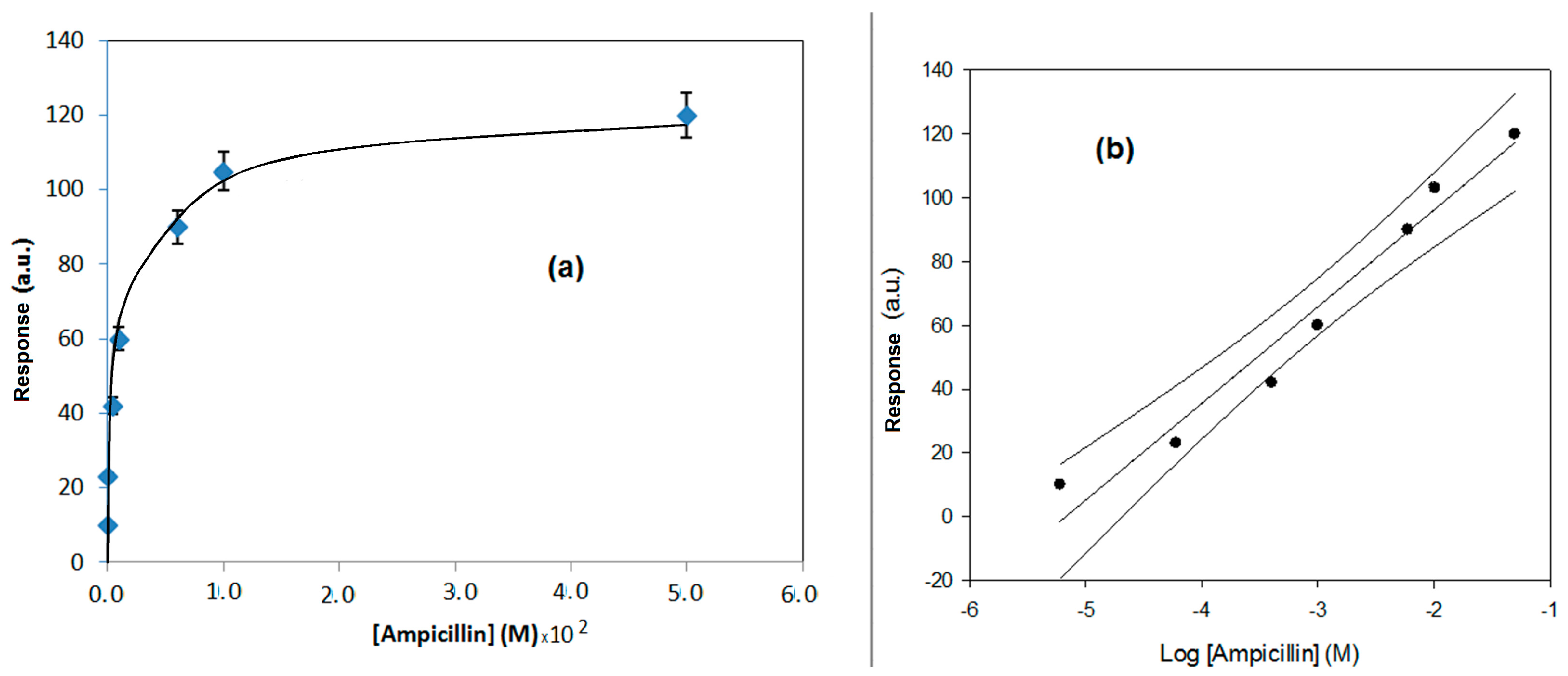
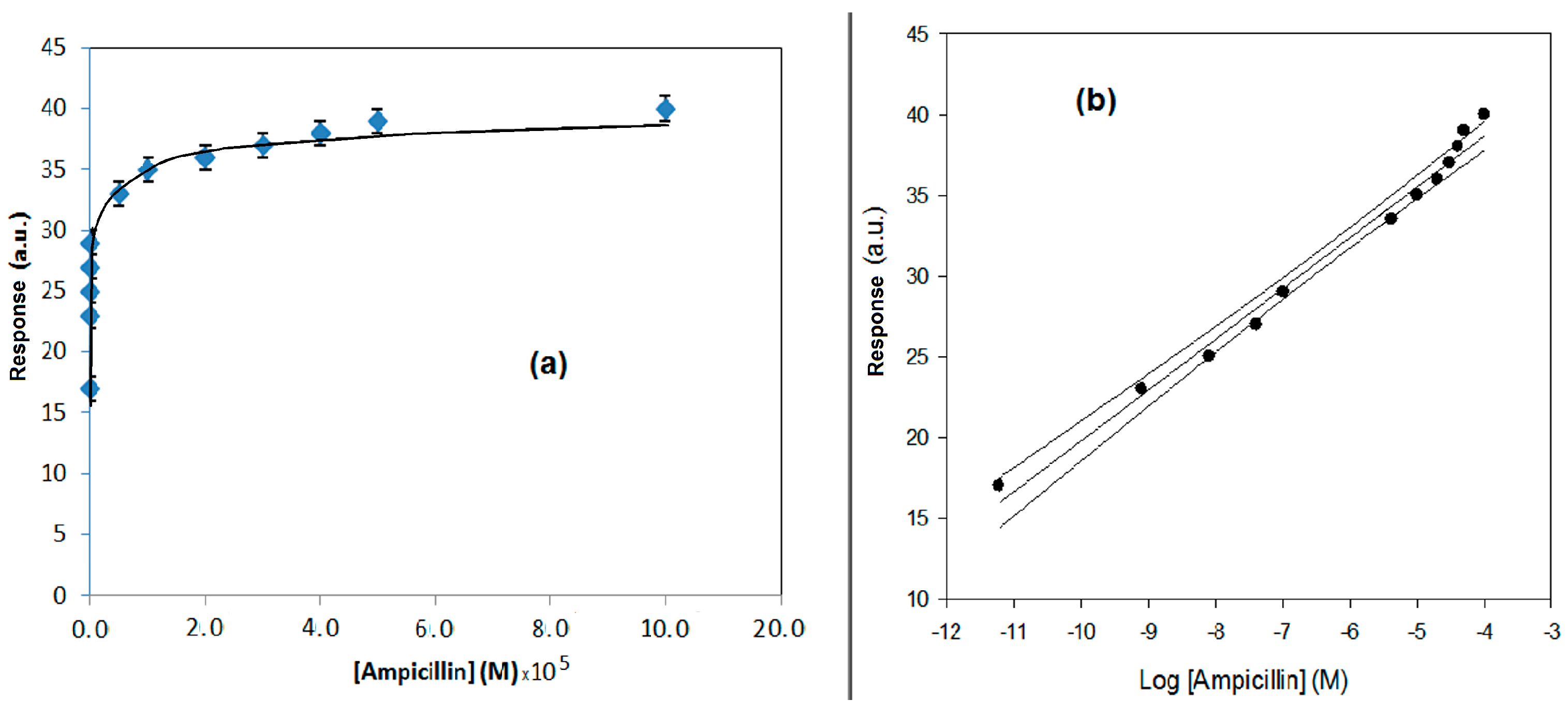
| Method | Determination of Ampicillin with SPR Immunosensor | Determination of Ampicillin with Conventional Amperometric Immunosensor |
|---|---|---|
| Geometry of the test | Direct measurement between ampicillin free in solution and anti-ampicillin immobilized | Competition between ampicillin–biotin–avidin–peroxidase conjugated and ampicillin, both free in solution, for anti-ampicillin immobilized in membrane. |
| Regression equation (Y = a.u., X = M) Confidence level (1 − α) = 0.95 | Y = 13.03 (±0.78) log X + 162.8 (±6.2) (n − ν) = 6; (t = 2.23) | Y = 50.1 (±2.3) log X + 1.3 (±0.1) (n − ν) = 16; (t = 2.23) |
| Linear range (M) | 2.5 × 10−6–3.0 × 10−2 | 5.0 × 10−1–1 × 10−4 |
| Correlation coefficient | 0.9830 | 0.9806 |
| Repeatability of each measurement as RSD% | 4.8 (n = 3) | 5.0 (n = 5) |
| Repeatability of the measurement (as pooled SD%) | 6.9 | 7.5 |
| Low detection limit (LOD) (M) | 1.0 × 10−6 | 2.5 × 10−1 |
| Instrumental response time (min) | ≅15 | ≅10 |
| Measurement time (min) | ≤20 | ≅75 |
| Method | IC50 n = 5; RSD% ≤ 5 (M) | Kaff n = 5; RSD% ≤ 5 (M−1) |
|---|---|---|
| Amperometric ampicillin competitive format device | 2.7 × 10−8 | 3.7 × 107 |
| Direct SPR immunosensor for ampicillin | 5.0 × 10−4 | 2.0 × 103 |
| Antibiotics | %Response of Several Antibiotics RSD% ≤ 5.0 | |
|---|---|---|
| SPR Flow-Immunosensor 1 | Conventional Amperometric Immunosensor 2 | |
| Ampicillin | 100.0 | 68.9 |
| Potassium penicillin G | 41.0 | 100 |
| Dicloxacillin | 35.5 | 70.7 |
| Piperacillin | 29.0 | 61.5 |
| Amoxicillin | 23.5 | 52.0 |
| Ceftriaxone | 18.0 | - |
| Cefalexin | 17.6 | 38.7 |
| Cefotaxime | - | 48.5 |
| Fosfomycin | 11.7 | 39.0 |
| Neomycin | 11.7 | 24.2 |
| Erythromycin | - | 30.5 |
| Bacitracin | - | 2.3 |
| Ac. amino 6-penicillamic | - | 18.0 |
| Matrix | Concentration of β-lactam Antibiotic “Pool” Found in Samples Using SPR Immunosensor n = 3; RSD% ≤ 5.5 (M) | Concentration of β-lactam Antibiotic “Pool” Found in Samples Using Conventional Immunosensor n = 5; RSD% ≤ 5.5 (M) | Values of β-Lactam Antibiotics Reported in Literature | References |
|---|---|---|---|---|
| Bovine milk (first sample) | ≈1 × 10−6 | 1.30 × 10−6 | (0.4–17.0) × 10−6 (0.6–17.5) × 10−6 | [25] [11] |
| Bovine milk (second sample) | ≈1 × 10−6 | 2.40 × 10−6 | (0.4–17.0) × 10−6 (0.6–17.5) × 10−6 | [25] [11] |
| “SACCO” river water sample | ≤1 × 10−6 | 1.45 × 10−7 | (0.9–60.0) × 10−8 (0.1–3.0) × 10−8 | [26] [27] |
| Spring surface water sample | ≤1 × 10−6 | 8.30 × 10−8 | (0.9–60.0) × 10−8 (0.1–3.0) × 10−8 1.0 × 10−9 | [26] [27] [28] |
| Matrix | Concentration of β-Lactam Antibiotic “Pool” Found in Samples Using SPR Immunosensor n = 3; RSD% ≤ 5.5 (M) | Concentration of Ampicillin Added in the Samples (M) | Nominal Concentration of β-Lactam Antibiotic n = 3; RSD% ≤ 5.5 (M) | Experimental Concentration Found in Spiked Samples Using SPR Immunosensor n = 3; RSD% ≤ 5.5 (M) | %Recovery Using SPR Immunosensor RSD% ≤ 5.5 |
|---|---|---|---|---|---|
| Bovine milk (first sample) | ≈1 × 10−6 | 4.00 × 10−6 | 5.00 × 10−6 | 4.81 × 10−6 | 96.2% |
| Bovine milk (second sample) | ≈1 × 10−6 | 4.00 × 10−6 | 5.00 × 10−6 | 4.88 × 10−6 | 97.6% |
| “SACCO” River water | ≈1 × 10−6 | 4.00 × 10−6 | 5.00 × 10−6 | 4.75 × 10−6 | 95.0% |
| Standard Spring water | ≈1 × 10−6 | 4.00 × 10−6 | 5.00 × 10−6 | 4.83 × 10−6 | 96.6% |
| Real Matrix | Concentration of β-Lactam Antibiotic “Pool” Found in Samples Using Conventional Amperometric Immunosensor. n = 5; RSD% ≤ 5.5 (M) | Concentration of Ampicillin Added in the Samples (M) | Nominal Concentration of β-Lactam Antibiotic n = 5; RSD% ≤ 5.5 (M) | Experimental Concentration Found in Spiked Samples Using Amperometric Immunosensor n = 5; RSD% ≤ 5.5 (M) | %Recovery Using Amperometric Immunosensor RSD% ≤ 5.5 |
|---|---|---|---|---|---|
| Bovine milk (first sample) | 1.30 × 10−6 | 1.00 × 10−6 | 2.30 × 10−6 | 2.28 × 10−6 | 99.1% |
| Bovine milk (second sample) | 2.40 × 10−6 | 1.50 × 10−6 | 3.90 × 10−6 | 3.75 × 10−6 | 96.2% |
| “SACCO” river water sample | 1.45 × 10−7 | 1.05 × 10−7 | 2.50 × 10−7 | 2.48 × 10−7 | 99.2% |
| Spring surface water sample | 8.30 × 10−8 | 0.7 × 10−8 | 9.00 × 10−8 | 8.70 × 10−8 | 96.6% |
| Method Assay | Range (μg·L−1) | LOD (μg·L−1) | Reference |
|---|---|---|---|
| EIA | / | 4.6 | [29] |
| ELISA (enzyme-linked immunosorbent assay) | 10–50 | / | [30] |
| ELISA (enzyme-linked immunosorbent assay) | / | 2.97 | [31] |
| Fluorescent immunoassay | 6.0–191 | 2.4 | [17] |
| Solid-Phase Fluorescence Immunoassay | / | 50 | [32] |
| Fluorescent Immunoassay | 2.0–10 | 2.9 | [20] |
| Solid-Phase Fluorescence Immunoassay | / | 1.0 | [33] |
| Our competitive format amperometric method | 0.17–34.9 × 103 | 0.087 | This paper |
| Our direct SPR method | 875–10.5 × 106 | 350 | This paper |
| Our sandwich Flow SPR method | 350 × 103–34.9 × 106 | 350 × 103 | [24] |
| / | / | 4.0 (in milk) | [34] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomassetti, M.; Merola, G.; Martini, E.; Campanella, L.; Sanzò, G.; Favero, G.; Mazzei, F. Comparison between a Direct-Flow SPR Immunosensor for Ampicillin and a Competitive Conventional Amperometric Device: Analytical Features and Possible Applications to Real Samples. Sensors 2017, 17, 819. https://doi.org/10.3390/s17040819
Tomassetti M, Merola G, Martini E, Campanella L, Sanzò G, Favero G, Mazzei F. Comparison between a Direct-Flow SPR Immunosensor for Ampicillin and a Competitive Conventional Amperometric Device: Analytical Features and Possible Applications to Real Samples. Sensors. 2017; 17(4):819. https://doi.org/10.3390/s17040819
Chicago/Turabian StyleTomassetti, Mauro, Giovanni Merola, Elisabetta Martini, Luigi Campanella, Gabriella Sanzò, Gabriele Favero, and Franco Mazzei. 2017. "Comparison between a Direct-Flow SPR Immunosensor for Ampicillin and a Competitive Conventional Amperometric Device: Analytical Features and Possible Applications to Real Samples" Sensors 17, no. 4: 819. https://doi.org/10.3390/s17040819






