Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Solutions Preparation
2.3. Synthesis of Magnetic Particles
2.3.1. Preparation of Maghemite
2.3.2. Preparation of MAN-34
2.3.3. Preparation of MAN-37-NH2
2.3.4. Preparation of MAN-38-1-NH2
2.3.5. Preparation of MAN-161-NH2
2.3.6. Preparation of MAN-164
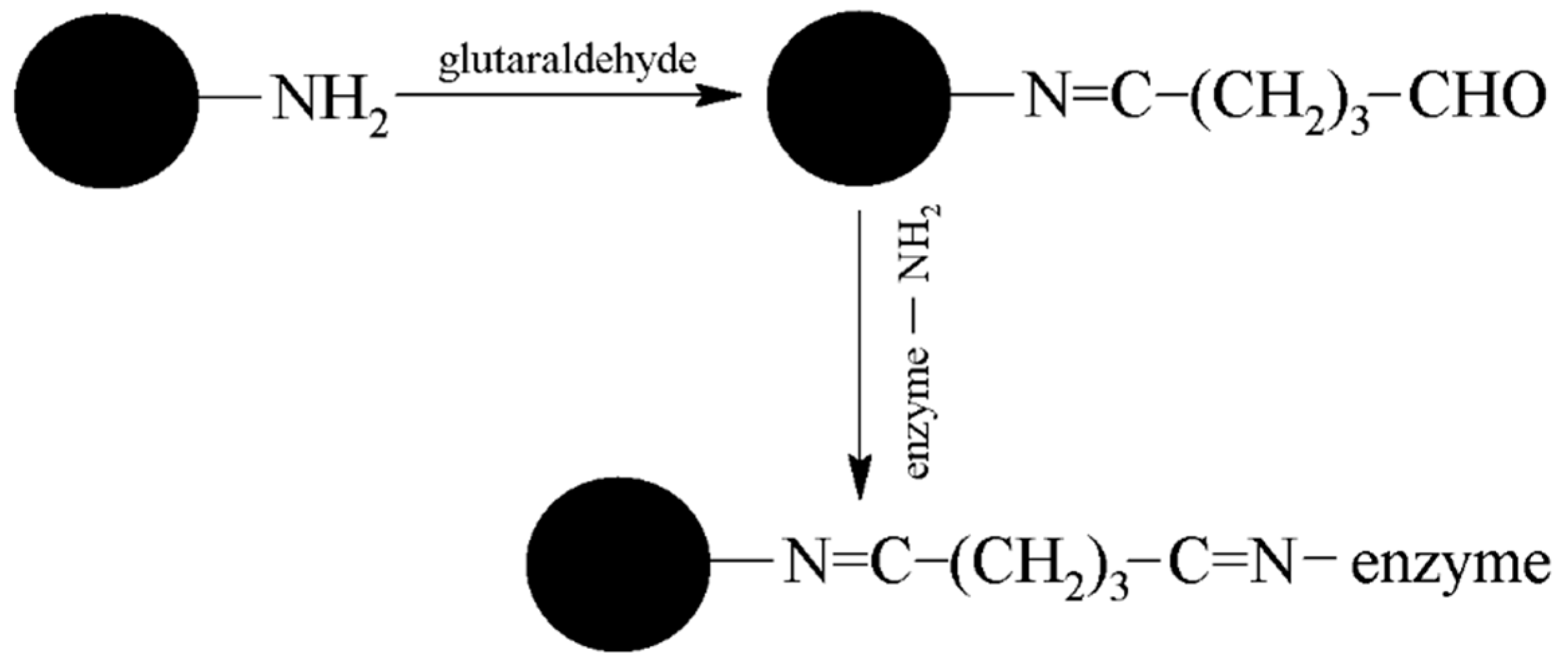
2.4. Preparation and Comparison of Particles with Bound AChE (MPs-AChE)
2.5. Electrochemical Assay
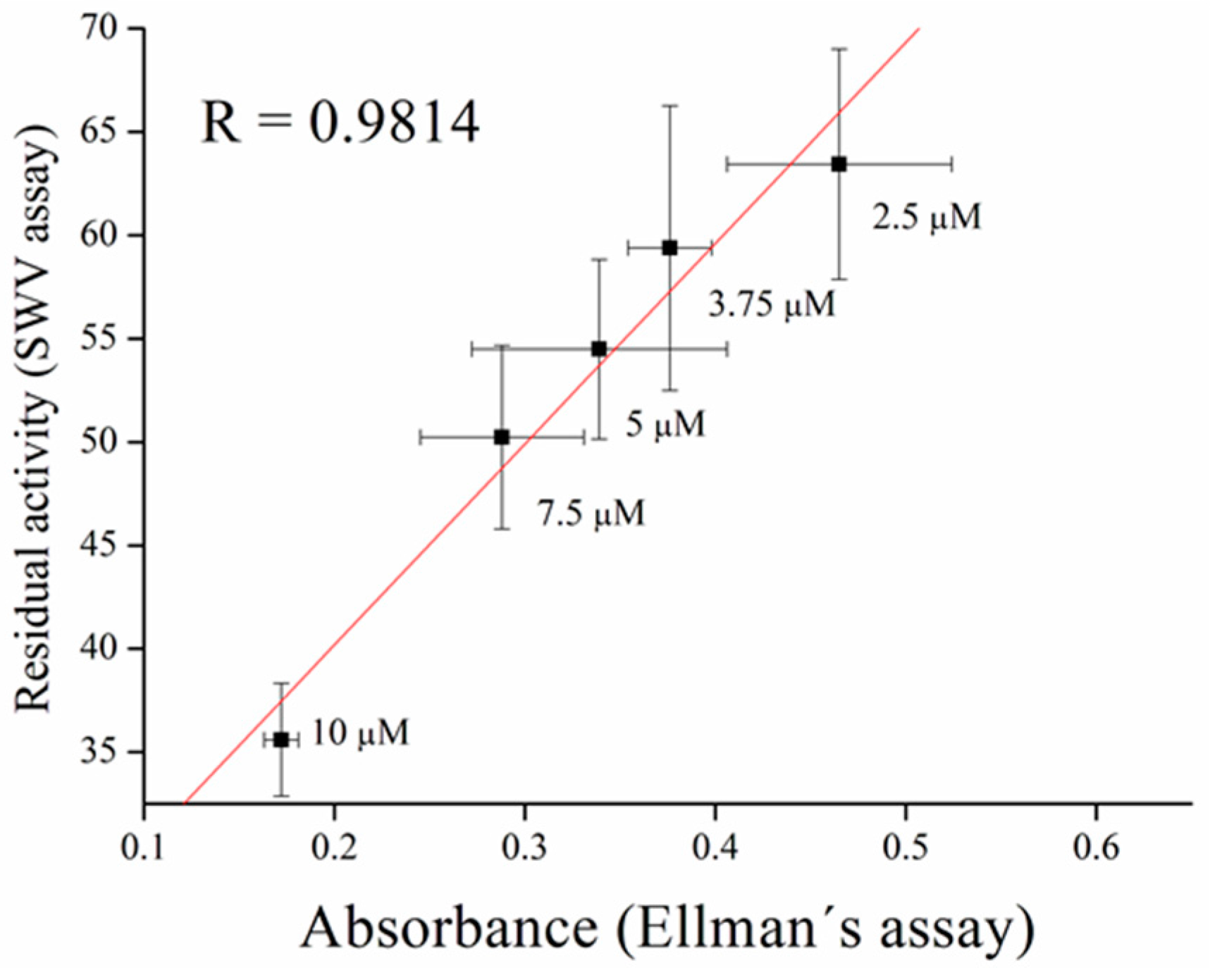
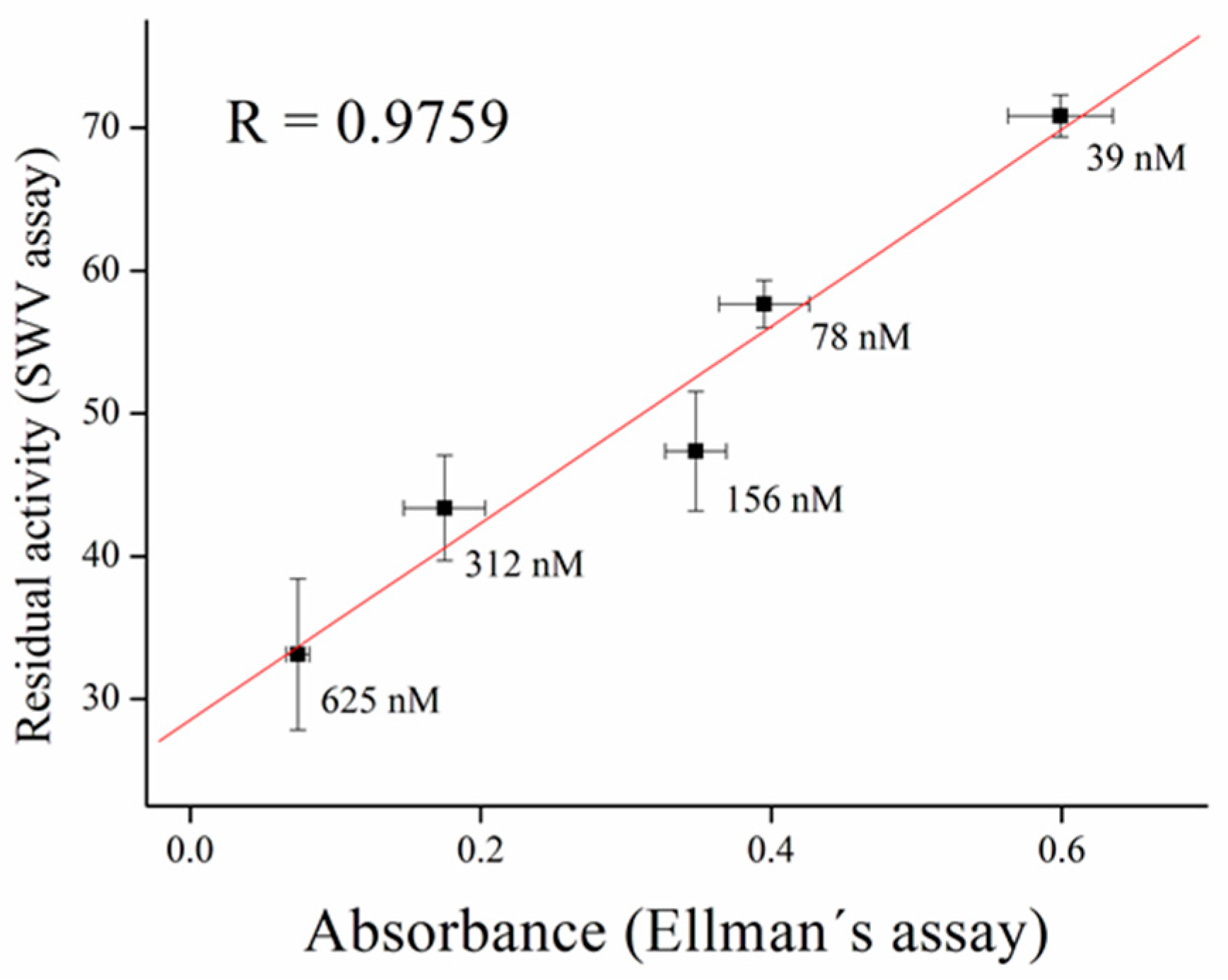
2.6. Validation Measurement
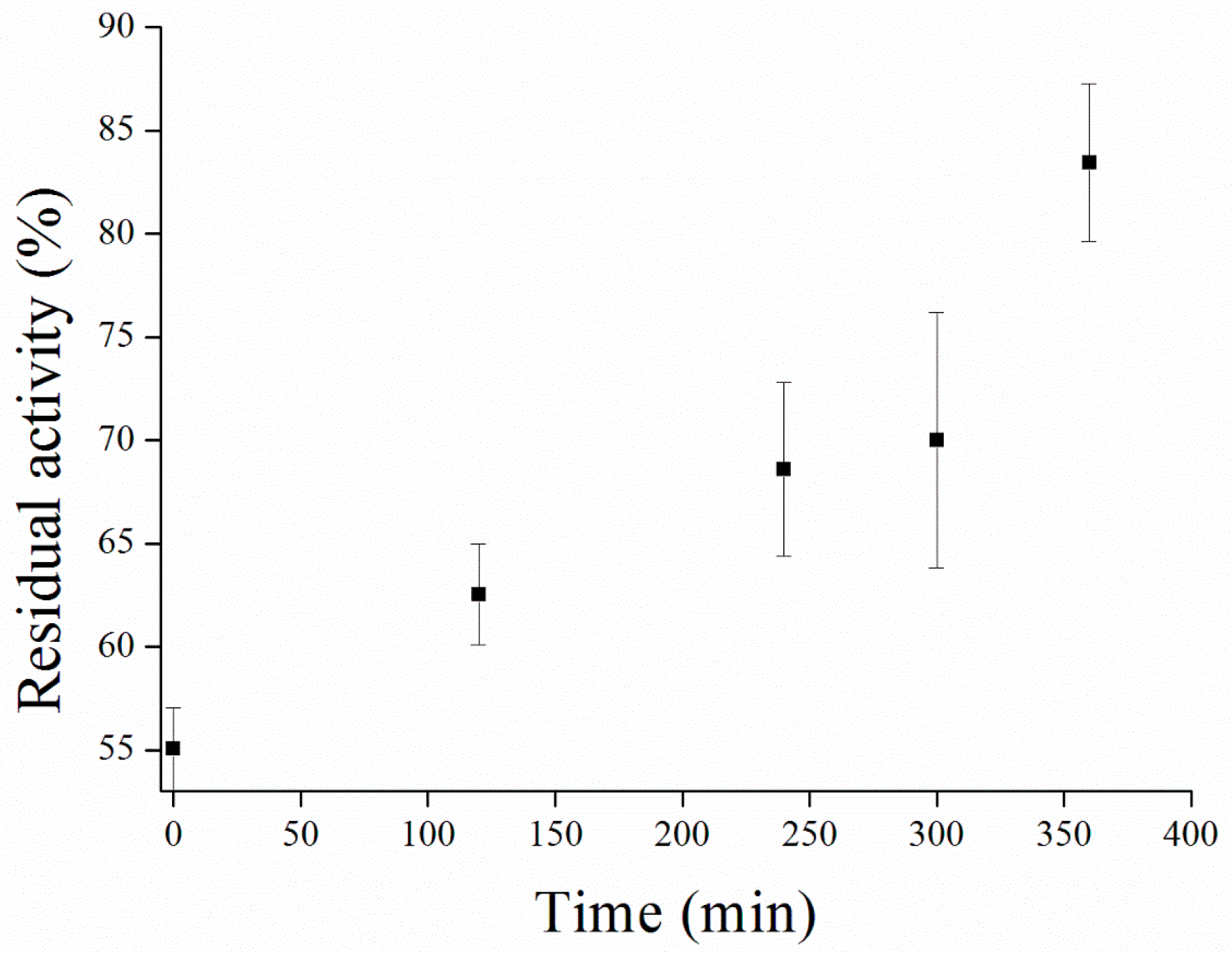
2.7. Galantamine Inhibited MPs Activity Restore
3. Results and Discussion
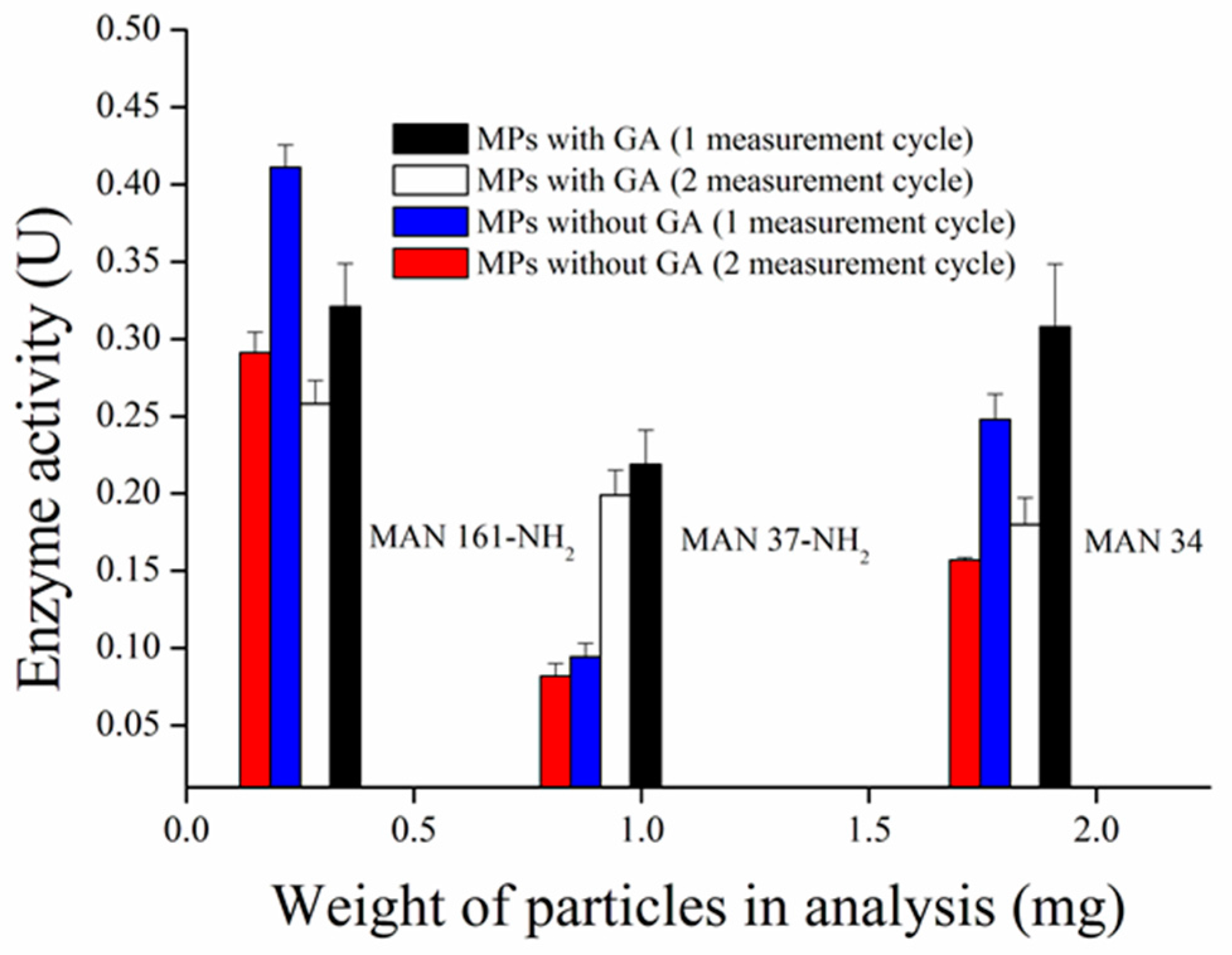
3.1. Synthesis and Comparison of Magnetic Particles
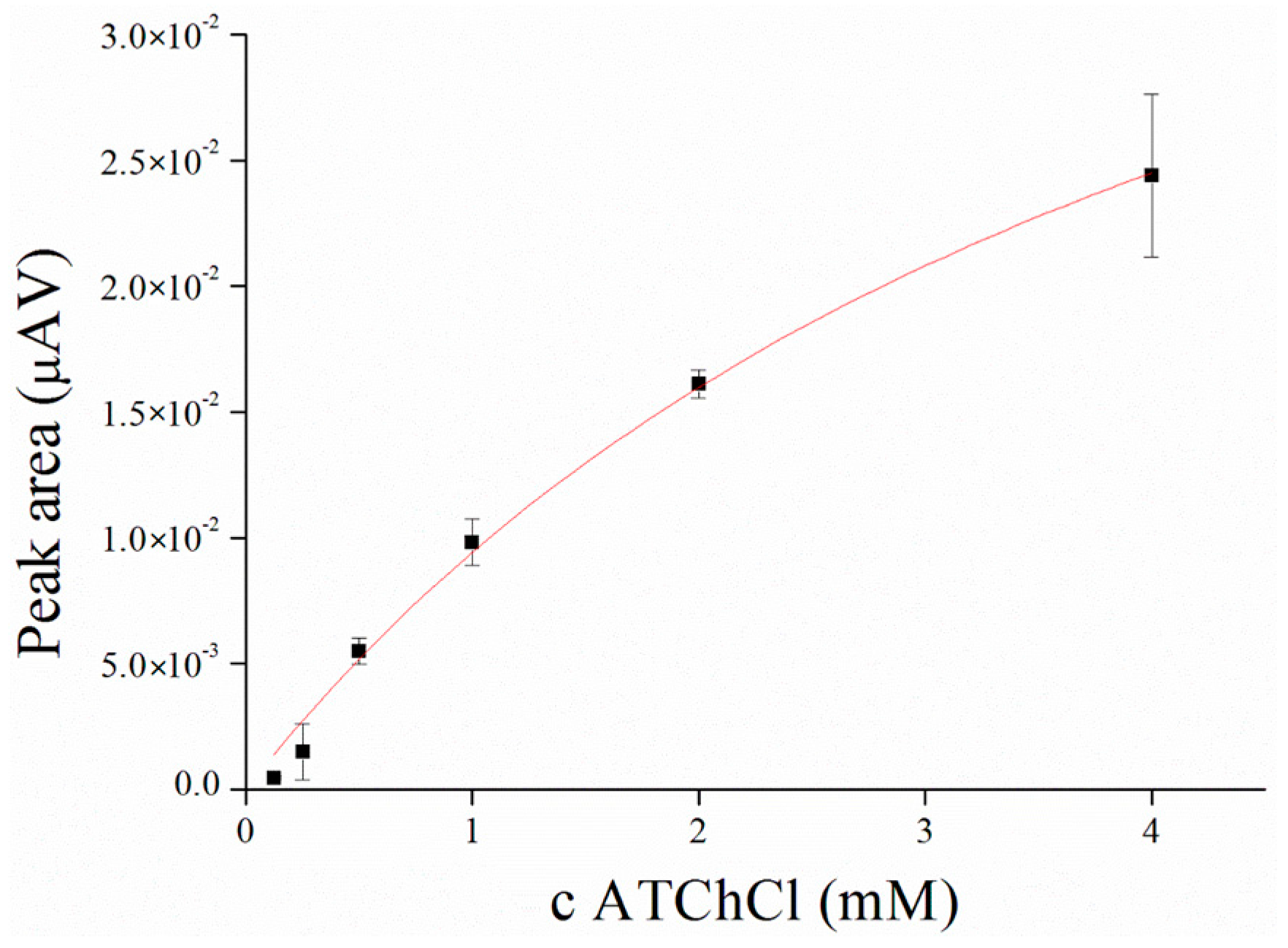
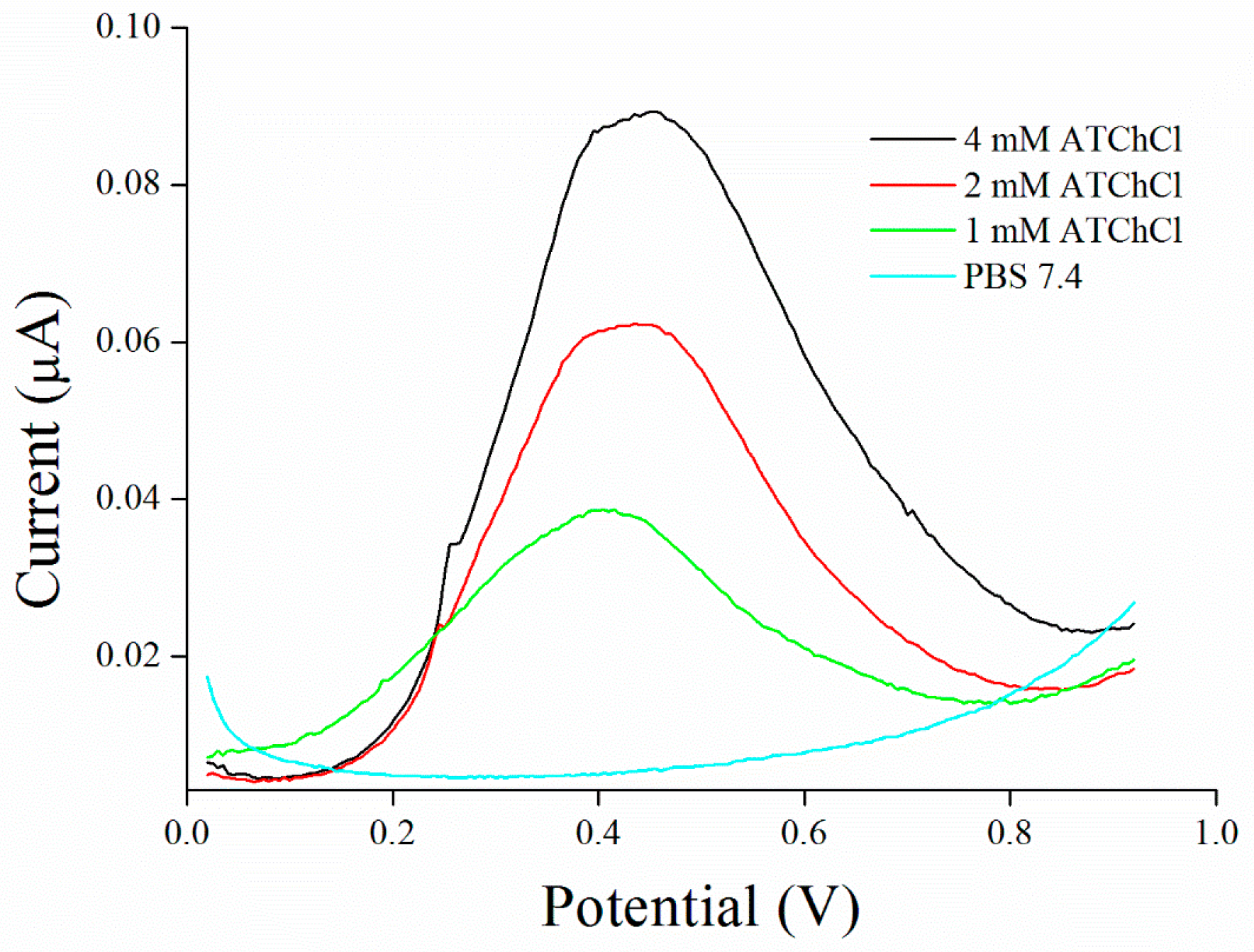
3.2. Substrate Measurement
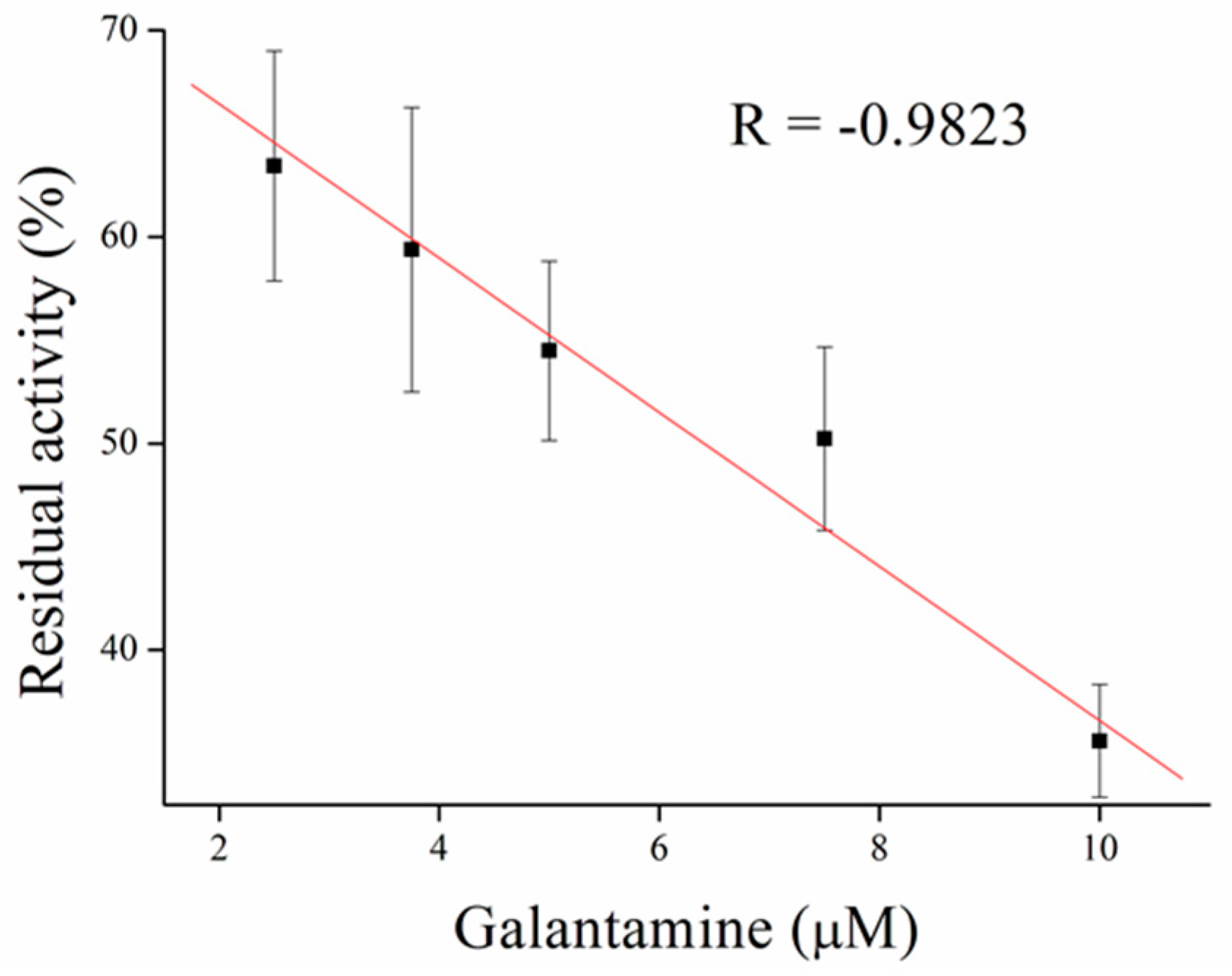
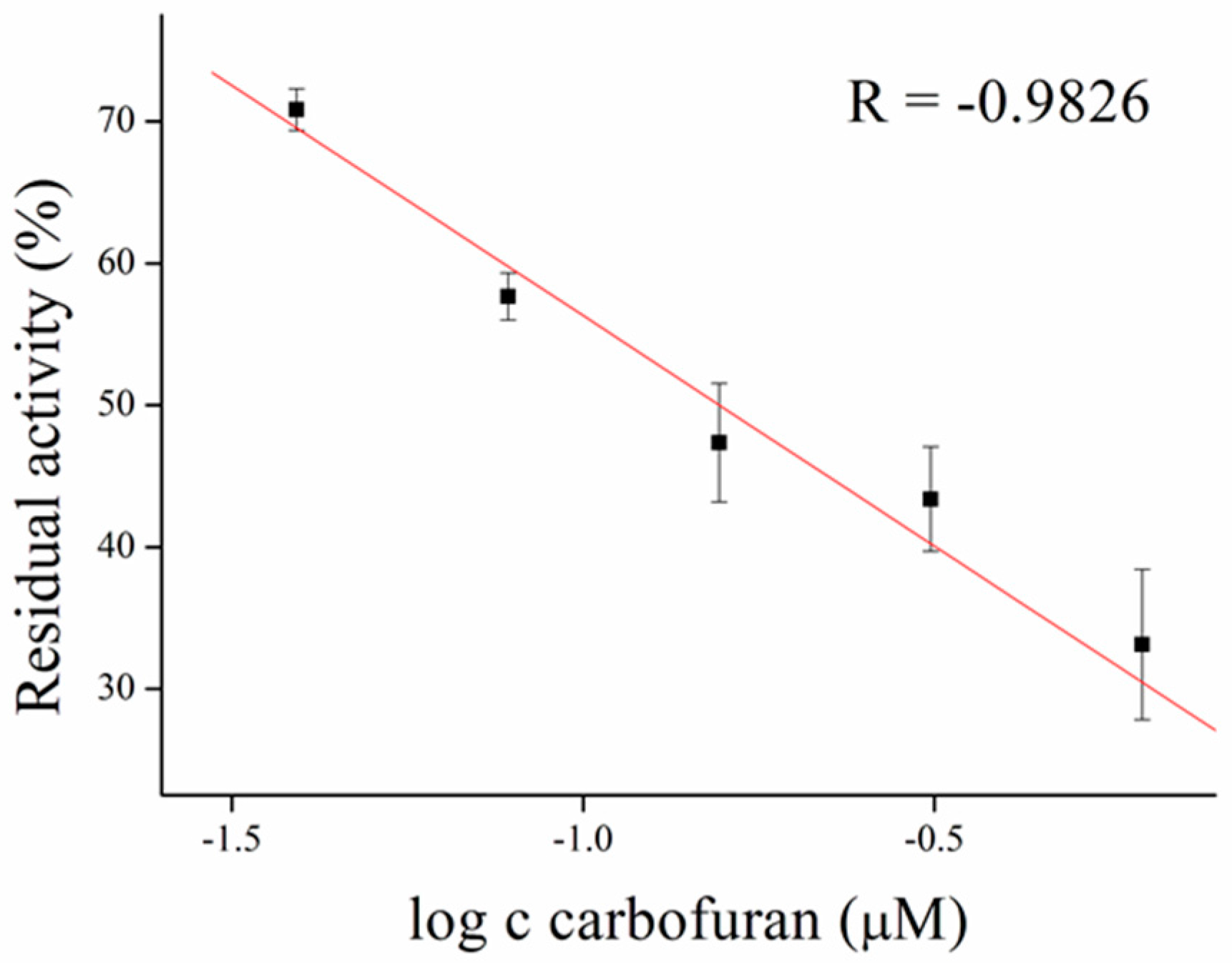
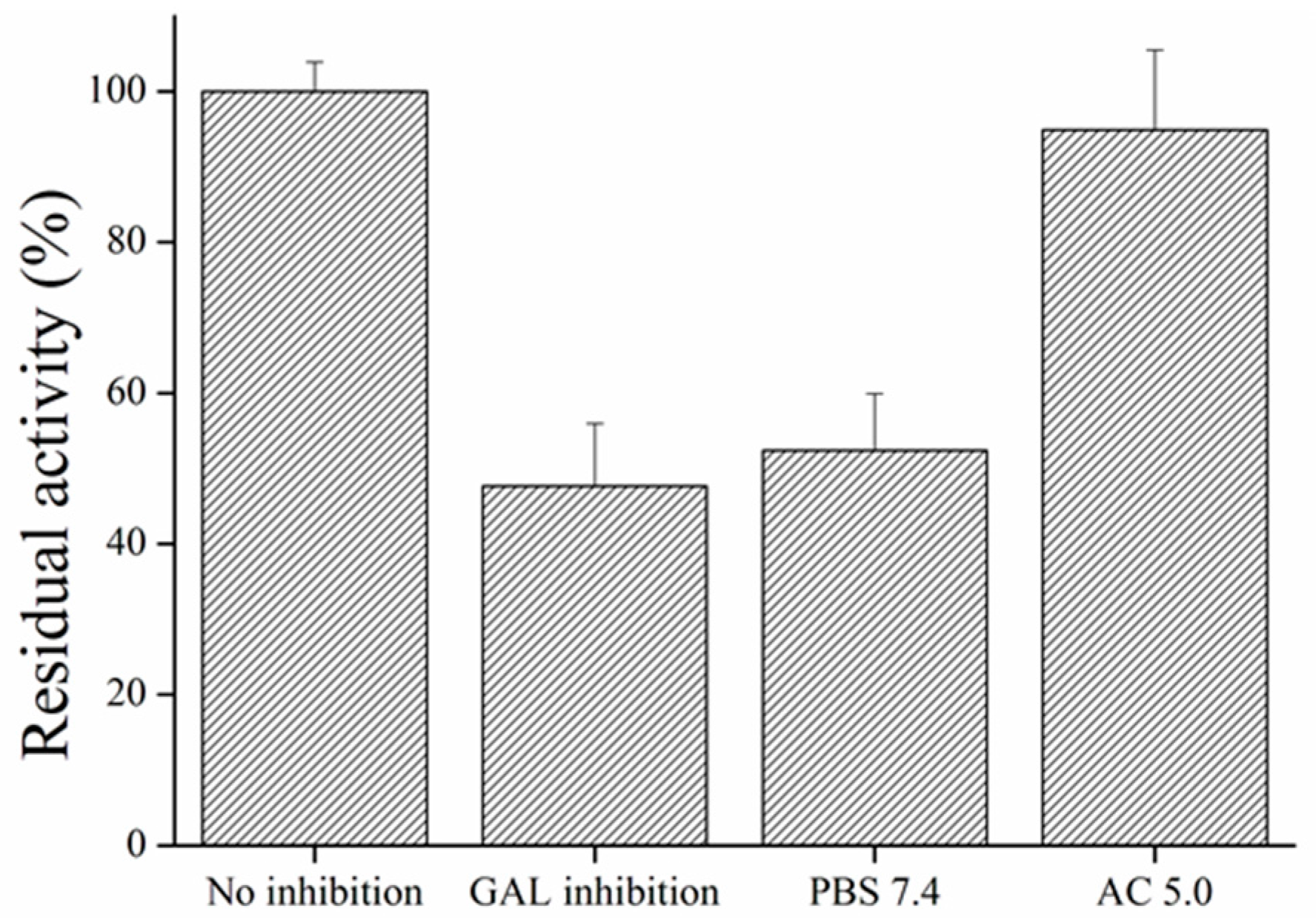
3.3. Inhibitors Measurement
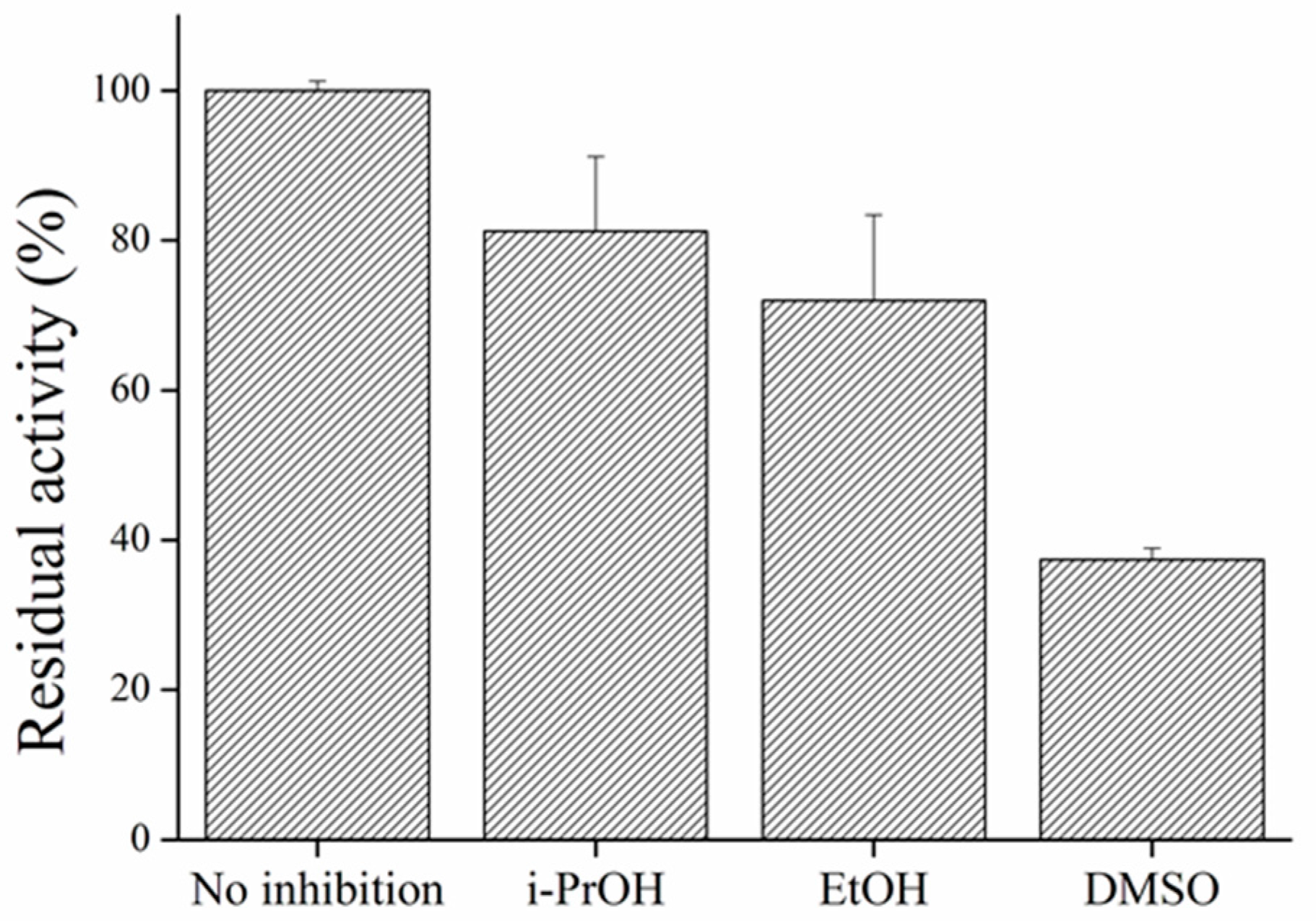
3.4. Influence of Organic Solvents
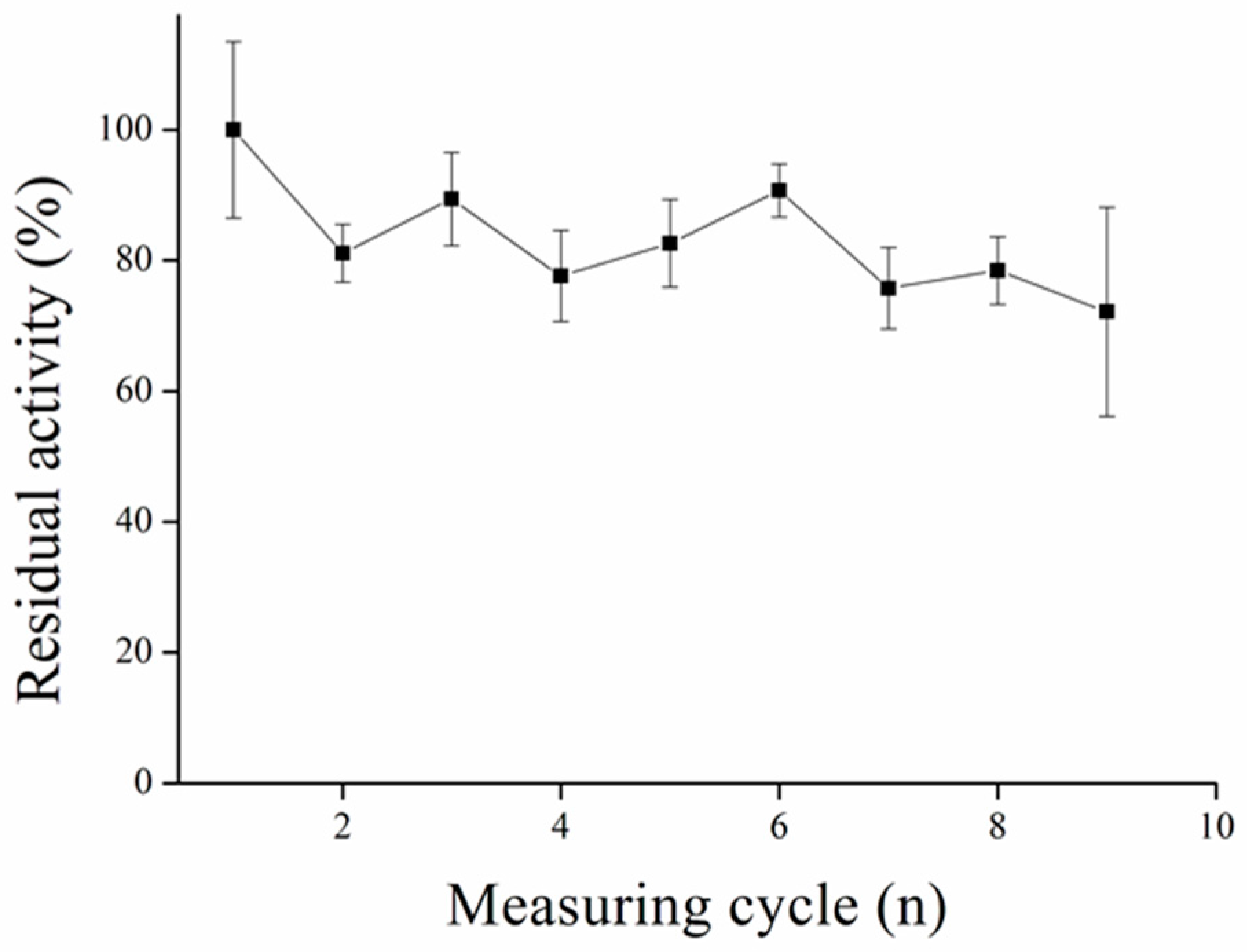
3.5. Repeated Measurement with MPs-AChE
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pohanka, M. Cholinesterase, a target of pharmacology and toxicology. Biomed. Pap. 2011, 155, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Biosensors containing acetylcholinesterase and butyrylcholinesterase as recognition tools for detection of various compounds. Chem. Pap. 2015, 69, 4–16. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kostelnik, A.; Cegan, A.; Pohanka, M. Electrochemical determination of activity of acetylcholinesterase immobilized on magnetic particles. Int. J. Electrochem. Sci. 2016, 11, 4840–4849. [Google Scholar] [CrossRef]
- Kostelnik, A.; Cegan, A.; Pohanka, M. Color change of phenol red by integrated smart phone camera as a tool for the determination of neurotoxic compounds. Sensors 2016, 16, 1212. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Voltammetric assay of butyrylcholinesterase in plasma samples and its comparison to the standard spectrophotometric test. Talanta 2014, 119, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Biosensors based on cholinesterases. Chem. Listy 2013, 107, 121–125. [Google Scholar]
- Pohanka, M. Cholinesterases in biorecognition and biosensors construction: A review. Anal. Lett. 2013, 46, 1849–1868. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Handa, H.; Abe, M. Synthesis and applications of magnetic nanoparticles for biorecognition and point of care medical diagnostics. Nanotechnology 2010, 21, 442001. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Ma, C.; Wang, F.; Xi, Z.J.; Wang, Z.F.; Deng, Y.; He, N.Y. Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Borlido, L.; Azevedo, A.M.; Roque, A.C.A.; Aires-Barros, M.R. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2015, 78, 016601. [Google Scholar] [CrossRef] [PubMed]
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2009, 110, 1518–1563. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Laschi, S.; Mascini, M. Improvement of analytical performances of a disposable electrochemical immunosensor by using magnetic beads. Talanta 2007, 73, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, Y.; Ma, Z.; Liu, H. Surface modification and characterization of magnetic polymer nanospheres prepared by miniemulsion polymerization. Langmuir 2004, 20, 10278–10282. [Google Scholar] [CrossRef] [PubMed]
- Istamboulie, G.; Andreescu, S.; Marty, J.-L.; Noguer, T. Highly sensitive detection of organophosphorus insecticides using magnetic microbeads and genetically engineered acetylcholinesterase. Biosens. Bioelectron. 2007, 23, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Bilitewski, U. Characterisation of inhibitors of acetylcholinesterase by an automated amperometric flow-injection system. Anal. Chim. Acta 1995, 300, 117–125. [Google Scholar] [CrossRef]
- Lui, J.; Günther, A.; Bilitewski, U. Detection of methamidophos in vegetables using a photometric flow injection system. Environ. Monit. Assess. 1997, 44, 375–382. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Horák, D.; Babič, M.; Macková, H.; Beneš, M.J. Preparation and properties of magnetic nano- and microsized particles for biological and environmental separations. J. Sep. Sci. 2007, 30, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Cernei, N.; Heger, Z.; Matousek, M.; Kopel, P.; Kynicky, J.; Masarik, M.; Kizek, R.; Adam, V. Microfluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urine. Electrophoresis 2013, 34, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Sinigaglia, G.; Nodari, L.; Tucek, J.; Polakova, K.; Marusak, Z.; Cardillo, S.; Salviulo, G.; Russo, U.; Stevanato, R.; et al. Charge binding of rhodamine derivative to oh- stabilized nanomaghemite: Universal nanocarrier for construction of magnetofluorescent biosensors. Acta Biomater. 2012, 8, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Cernei, N.; Guran, R.; Michalek, P.; Milosavljevic, V.; Kopel, P.; Zitka, O.; Kynicky, J.; Lany, P.; Adam, V.; et al. Gamma-Fe2O3 magnetic core functionalized with tetraethyl orthosilicate and 3-aminopropyl triethoxysilane for an isolation of H7N7 influenza serotype virions. Int. J. Electrochem. Sci. 2014, 9, 3374–3385. [Google Scholar]
- Heger, Z.; Cernei, N.; Krizkova, S.; Masarik, M.; Kopel, P.; Hodek, P.; Zitka, O.; Adam, V.; Kizek, R. Paramagnetic nanoparticles as a platform for fret-based sarcosine picomolar detection. Sci. Rep. 2015, 5, 8868. [Google Scholar] [CrossRef] [PubMed]
- Nejdl, L.; Kudr, J.; Cihalova, K.; Chudobova, D.; Zurek, M.; Zalud, L.; Kopecny, L.; Burian, F.; Ruttkay-Nedecky, B.; Krizkova, S.; et al. Remote-controlled robotic platform orpheus as a new tool for detection of bacteria in the environment. Electrophoresis 2014, 35, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
- Gabrovska, K.; Marinov, I.; Godjevargova, T.; Portaccio, M.; Lepore, M.; Grano, V.; Diano, N.; Mita, D.G. The influence of the support nature on the kinetics parameters, inhibition constants and reactivation of immobilized acetylcholinesterase. Int. J. Biol. Macromol. 2008, 43, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Barteri, M.; Pala, A.; Rotella, S. Structural and kinetic effects of mobile phone microwaves on acetylcholinesterase activity. Biophys. Chem. 2005, 113, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Zhang, Y.; Eslami, A.C.; Butler, J.R.; McCammon, J.A. Studying the roles of W86, E202, and Y337 in binding of acetylcholine to acetylcholinesterase using a combined molecular dynamics and multiple docking approach. Protein Sci. 2003, 12, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.; Barak, D.; Ordentlich, A.; Kronman, C.; Velan, B.; Shafferman, A. Is aromaticity essential for trapping the catalytic histidine 447 in human acetylcholinesterase? Biochemistry 2004, 43, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Adam, V.; Kizek, R. Comparison of an alzheimer disease drug ability to bind acetylcholinesterase using both electrochemical and spectrophotometric assays. Res. Opin. Anim. Vet. Sci. 2014, 4, 203–207. [Google Scholar]
- Stoytcheva, M.; Zlatev, R.; Velkova, Z.; Valdez, B.; Ovalle, M. Analytical characteristics of electrochemical biosensors. Port. Electrochim. Acta 2009, 27, 353–362. [Google Scholar] [CrossRef]
- Cuartero, M.; García, M.S.; García-Cánovas, F.; Ortuño, J.Á. New approach for the potentiometric-enzymatic assay of reversible-competitive enzyme inhibitors. Application to acetylcholinesterase inhibitor galantamine and its determination in pharmaceuticals and human urine. Talanta 2013, 110, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Simantiraki, M.G.; Siontorou, C.G.; Toth, K. Flow injection analysis of carbofuran in foods using air stable lipid film based acetylcholinesterase biosensor. Anal. Chim. Acta 2005, 537, 169–177. [Google Scholar] [CrossRef]
- Pohanka, M.; Fusek, J.; Adam, V.; Kizek, R. Carbofuran assay using gelatin based biosensor with acetylcholinesterase as a recogniton element. Int. J. Electrochem. Sci. 2013, 8, 71–79. [Google Scholar]
- Shulga, O.; Kirchhoff, J.R. An acetylcholinesterase enzyme electrode stabilized by an electrodeposited gold nanoparticle layer. Electrochem. Commun. 2007, 9, 935–940. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Jung, C.H.; Choi, S.J.; Seo, W.J.; Cha, S.H.; Sok, D.E. Potentiation effect of choline esters on choline-catalysed decarbamoylation of dimethylcarbamoyl-acetylcholinesterase. Biochem. J. 1992, 284, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.B.; Harrison, M.A.; Ginsburg, S. Carbamyl derivatives of acetylcholinesterase. J. Biol. Chem. 1961, 236, 1498–1500. [Google Scholar] [PubMed]
- Li, H.; Ricordel, I.; Tong, L.; Schopfer, L.M.; Baud, F.; Mégarbane, B.; Maury, E.; Masson, P.; Lockridge, O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J. Appl. Toxicol. 2009, 29, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Solná, R.; Sapelnikova, S.; Skládal, P.; Winther-Nielsen, M.; Carlsson, C.; Emnéus, J.; Ruzgas, T. Multienzyme electrochemical array sensor for determination of phenols and pesticides. Talanta 2005, 65, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Adam, V.; Kizek, R. An acetylcholinesterase-based chronoamperometric biosensor for fast and reliable assay of nerve agents. Sensors 2013, 13, 11498–11506. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Yang, X.; Xie, D.; Wu, Y.; Wen, W. A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen printed carbon electrode. Sensors 2010, 10, 625–638. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostelnik, A.; Kopel, P.; Cegan, A.; Pohanka, M. Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay. Sensors 2017, 17, 676. https://doi.org/10.3390/s17040676
Kostelnik A, Kopel P, Cegan A, Pohanka M. Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay. Sensors. 2017; 17(4):676. https://doi.org/10.3390/s17040676
Chicago/Turabian StyleKostelnik, Adam, Pavel Kopel, Alexander Cegan, and Miroslav Pohanka. 2017. "Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay" Sensors 17, no. 4: 676. https://doi.org/10.3390/s17040676




