A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
2.2. Preparation of ZnONF
2.3. Preparation of the Modified Electrode
2.4. Analytical Procedure
3. Results and Discussion
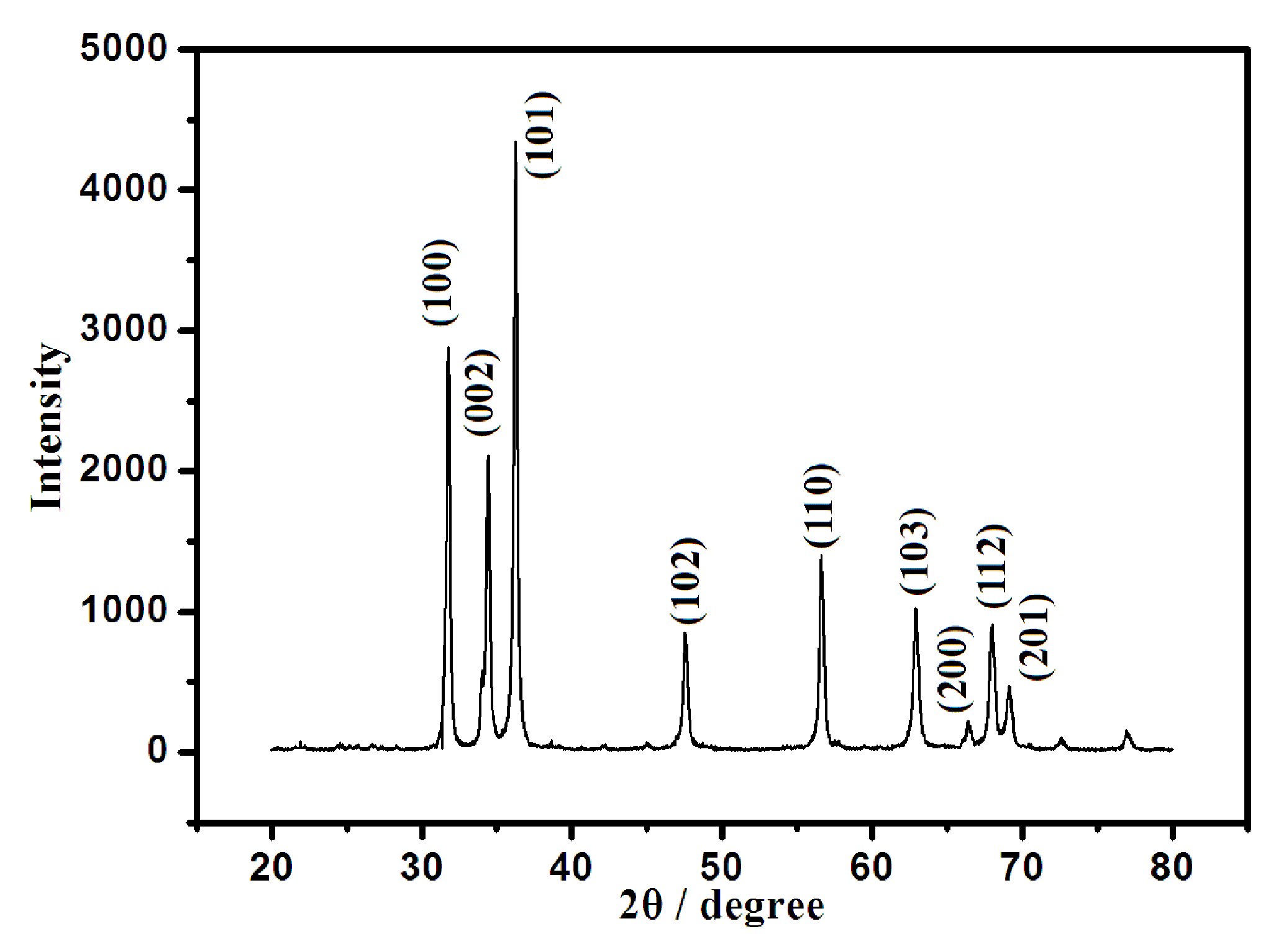
3.1. Characterization of ZnONF and Modified Electrode
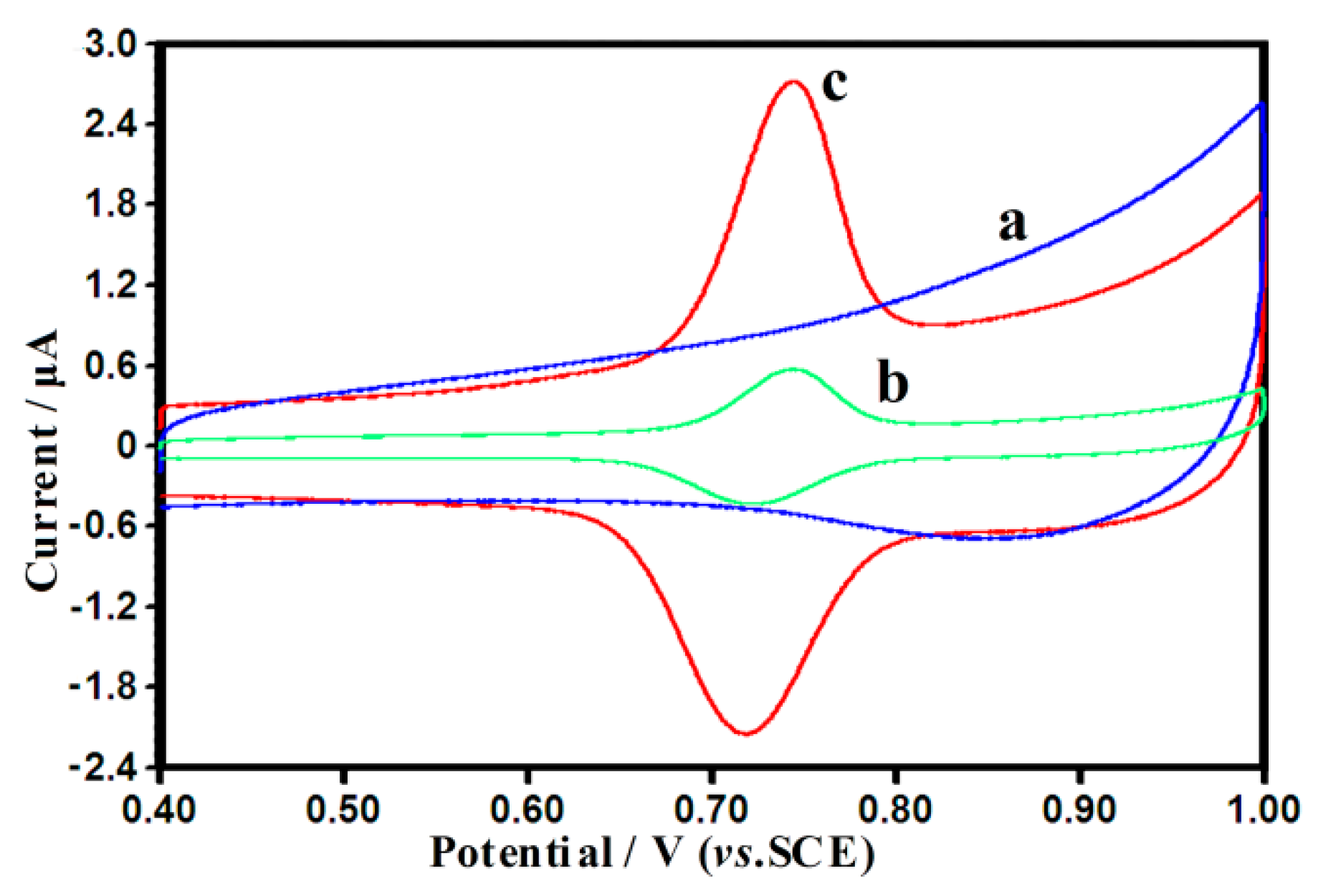
3.2. Cyclic Voltammetric Behavior of SY
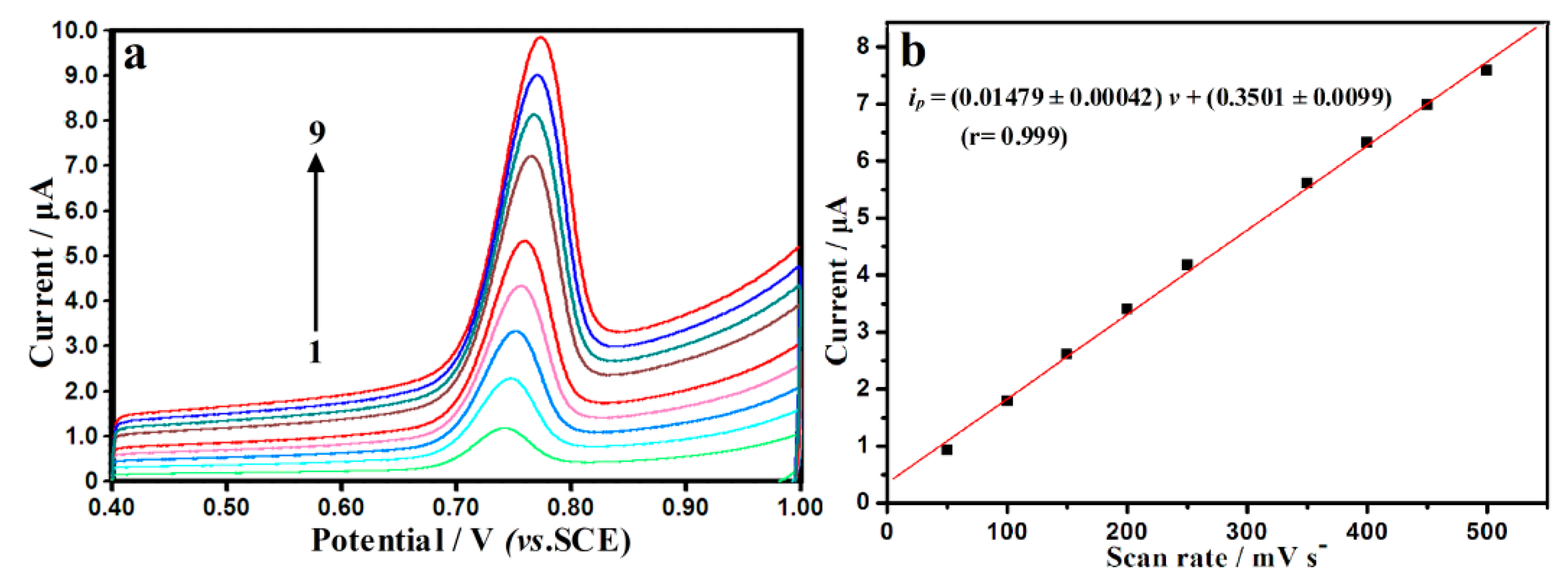
3.3. Influence of Scan Rate
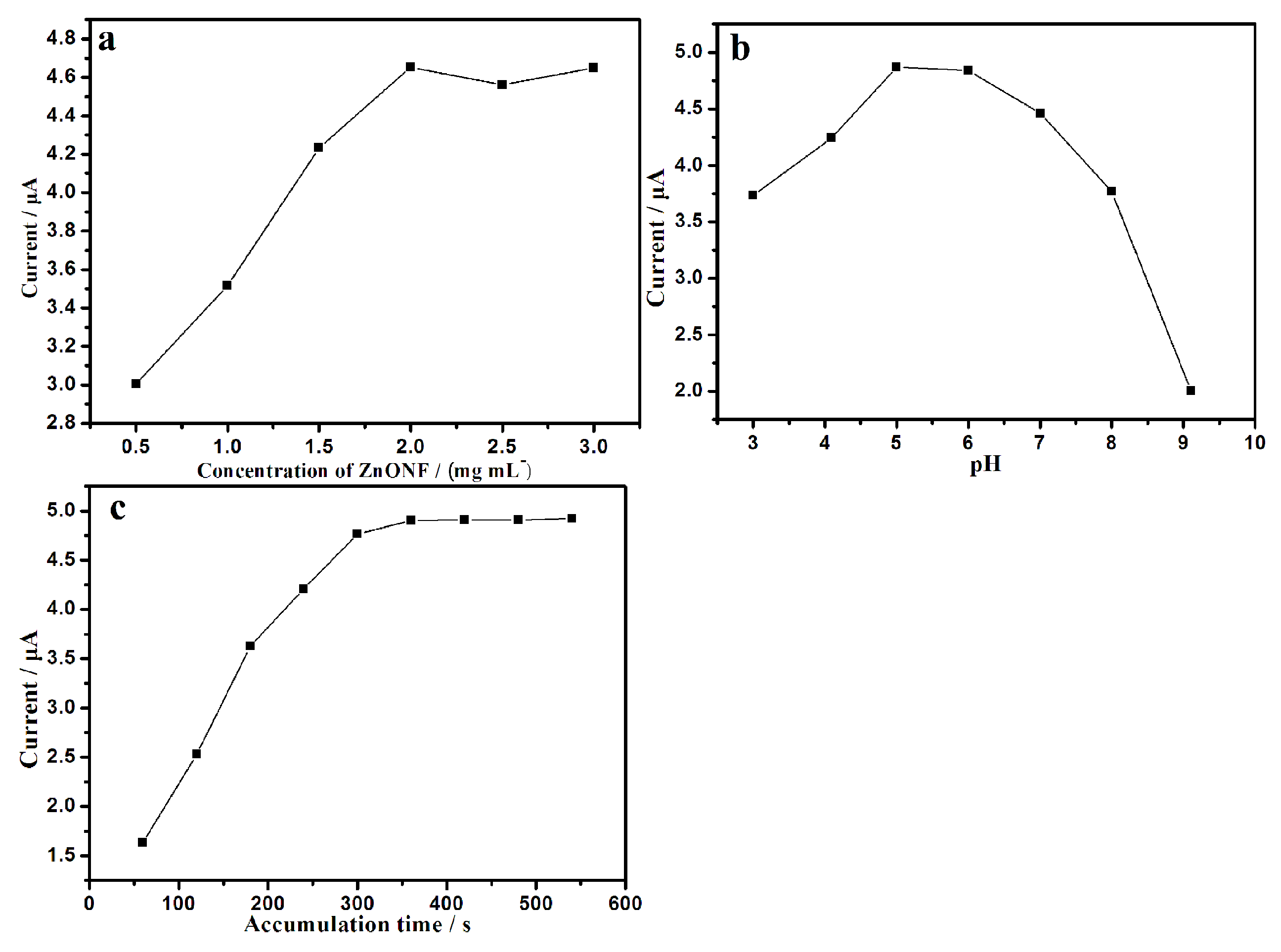
3.4. Optimization of Detection Variables
3.5. Analytical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sardi, M.; Haldemann, Y.; Mordmann, H.; Bottex, B.; Safford, B.; Smith, B.; Tennant, D.; Howlett, J.; Jasti, P.R. Use of retailer fidelity card schemes in the assessment of food additive intake: Sunset yellow a case study. Food Addit. Contam. A 2010, 27, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Tripathi, A.; Das, M. Sunset yellow FCF, a permitted food dye, alters functional responses of splenocytes at non-cytotoxic dose. Toxicol. Lett. 2013, 217, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; He, P.; Yasen, A.; Li, Z. Determination of seven synthetic dyes in animal feeds and meat by high performance liquid chromatography with diode array and tandem mass detectors. Food Chem. 2013, 138, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.P.; Brum, D.M.; De Andrate, É.C.B.; Netto, A.D.P. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem. 2008, 107, 489–496. [Google Scholar] [CrossRef]
- Llamas, N.E.; Garrido, M.; Nezio, M.S.D.; Band, B.S.F. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV-vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2009, 655, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, X.; Qiao, M.; Zhu, J.; Liu, S.; Yang, J.; Hu, X. Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dots. Spectrochim. Acta A 2016, 167, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Zeynali, K.A.; Manafi-Khoshmanesh, S. Simultaneous spectrophotometric determination of sunset yellow and quinoline yellow in a single step. J. Chin. Chem. Soc. 2015, 62, 772–779. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Yang, L. Designing of the functional paper-based surface-enhanced raman spectroscopy substrates for colorants detection. Mater. Res. Bull. 2015, 63, 199–204. [Google Scholar] [CrossRef]
- Cheng, Q.; Xia, S.; Tong, J.; Wu, K. Highly-sensitive electrochemical sensing platforms for food colourants based on the property-tuning of porous carbon. Anal. Chim. Acta 2015, 887, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of sunset yellow based on gold nanoparticles/graphene electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Zhang, K.; Bin, D.; Shiraish, Y.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of sunset yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2016, 81, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Sun, Q.; Zhao, J. Sensitively simultaneous determination of sunset yellow and tartrazine in foods based on polypyrrole modified oxidized single-walled carbon nanotubes. J. Electrochem. Soc. 2014, 161, B297–B304. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, P.; Wu, J.; Wang, W.; Ye, B. Highly sensitive determination of sunset yellow in drink using a poly(l-cysteine) modified glassy carbon electrode. Anal. Methods 2013, 5, 5044–5050. [Google Scholar] [CrossRef]
- Królicka, A.; Bobrowski, A.; Zarębski, J.; Tesarowicz, I. Bismuth film electrodes for adsorptive stripping voltammetric determination of sunset yellow FCF in soft drinks. Electroanalysis 2014, 26, 756–765. [Google Scholar] [CrossRef]
- Songyang, Y.; Yang, X.; Xie, S.; Hao, H.; Song, J. Highly-sensitive and rapid determination of sunset yellow using functionalized montmorillonite-modified electrode. Food Chem. 2015, 173, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Márquez, F.; Morant, C. Nanomaterials for sensor applications. Soft Nanosci. Lett. 2015, 5, 1–2. [Google Scholar] [CrossRef]
- Lim, W.Q.; Gao, Z. Metal oxide nanoparticles in electroanalysis. Electroanal. 2015, 27, 2074–2090. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A.; et al. Metal oxide nanoscience and nanotechnology for chemical sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- Taherkhani, A.; Jamali, T.; Hadadzadeh, H.; Karimi-Maleh, H.; Beitollahi, H.; Taghavi, M.; Karimi, F. ZnO nanoparticle-modified ionic liquid-carbon paste electrodefor voltammetric determination of folic acid in food and pharmaceutical samples. Ionics 2014, 20, 421–429. [Google Scholar] [CrossRef]
- Haarindraprasad, R.; Hashim, U.; Gopinath, S.C.B.; Perumal, V.; Liu, W.W.; Balakrishnana, S.R. Fabrication of interdigitated high-performance zinc oxide nanowire modified electrodes for glucose sensing. Anal. Chim. Acta 2016, 925, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, B.; Zhu, G.; Wu, Y.; Liu, S.; Xu, C. Electron transfer properties and electrocatalytic behavior of tyrosinase on ZnO nanorod. J. Electroanal. Chem. 2008, 617, 7–13. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Zhang, J.; Guo, J.; Cao, B. Direct hydrothermal growth of ZnO nanosheets on electrode for ethanol sensing. Sens. Actuators B Chem. 2014, 201, 444–451. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, C.; Zhang, W.; Wang, G. A novel hydrazine electrochemical sensor based on a carbon nanotube-wired ZnO nanoflower-modified electrode. Electrochim. Acta 2009, 55, 178–182. [Google Scholar] [CrossRef]
- He, J.Q.; Yin, J.; Liu, D.; Zhang, L.X.; Cai, F.S.; Bie, L.J. Enhanced acetone gas-sensing performance of La2O3-doped flowerlike ZnO structure composed of nanorods. Sens. Actuators B Chem. 2013, 182, 170–175. [Google Scholar] [CrossRef]



| Electrode | Linearity Range (μg/L) | Detection Limit (μg/L) | Reference |
|---|---|---|---|
| Porous carbon-modified GCE | 2.5–500 | 1.4 | [10] |
| Gold nanoparticles-modified GCE | 23–723 | 0.90 | [11] |
| Nanoparticles/graphene-modified GCE | 0.9–49,372 | 0.90 | [12] |
| Au-Pd and reduced graphene oxide Nanocomposites-modified GCE | 310–150,048 | 0.68 | [13] |
| Polypyrrole-modified oxidized single-walled Carbon Nanotubes-modified GCE | 2.3–452 | 0.32 | [14] |
| Poly (l-cysteine)-modified GCE | 3.6–317 | 1.8 | [15] |
| Bismuth film-modified GCE | 4.4–87 | 1.0 | [16] |
| Functionalized montmorillonite-modified CPE | 1.1–90 | 0.32 | [17] |
| ZnONF/CPE | 0.50–10 and 10–70 | 0.10 | This work |
| Original (mg/L) | Added (mg/L) | Found (mg/L) | Recovery (%) | By HPLC (mg/L) | Relative Error (%) |
|---|---|---|---|---|---|
| 20.00 | 33.07 | 97.5 | 31.42 | −4.99 | |
| 13.57 | 30.00 | 42.25 | 95.6 | 45.05 | 6.63 |
| 40.00 | 54.71 | 103 | 53.12 | −2.91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow. Sensors 2017, 17, 545. https://doi.org/10.3390/s17030545
Ya Y, Jiang C, Li T, Liao J, Fan Y, Wei Y, Yan F, Xie L. A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow. Sensors. 2017; 17(3):545. https://doi.org/10.3390/s17030545
Chicago/Turabian StyleYa, Yu, Cuiwen Jiang, Tao Li, Jie Liao, Yegeng Fan, Yuning Wei, Feiyan Yan, and Liping Xie. 2017. "A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow" Sensors 17, no. 3: 545. https://doi.org/10.3390/s17030545





