SERS Taper-Fiber Nanoprobe Modified by Gold Nanoparticles Wrapped with Ultrathin Alumina Film by Atomic Layer Deposition
Abstract
:1. Introduction
2. Experiment Procedures and Methods
2.1. Fabrication of the Nanoscale Taper-Fiber
2.2. Preparation of Gold NPs
2.3. Modified Tapered Fiber Nanoprobe with Gold NPs
2.4. Wrap the Modified Nanoprobe with Alumina Layers
2.5. SERS Measurements
3. Results and Discussions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tamor, M.A.; Vassell, W.C. Raman “fingerprinting” of amorphous carbon films. J. Appl. Phys. 1994, 76, 3823–3830. [Google Scholar] [CrossRef]
- Cao, Y.W.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Gessner, R.; Rösch, P.; Petry, R.; Schmitt, M.; Strehle, M.A.; Kiefer, W.; Popp, J. The application of a SERS fiber probe for the investigation of sensitive biological samples. Analyst 2004, 129, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.F.S.; Fan, M.K.; Brolo, A.G. Multilayer silver nanoparticles-modified optical fiber tip for high performance SERS remote sensing. Biosens. Bioelectron. 2010, 25, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Nguyen, J.T.; Rutledge, S.; Zhang, J.; Wang, C.; Walker, G.C. Detection of chronic lymphocytic leukemia cell surface markers using surface enhanced Raman scattering gold nanoparticles. Cancer Lett. 2010, 292, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic etching of silver nanoparticles for plasmonic structures capable of single-particle SERS. J. Am. Chem. Soc. 2009, 132, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Que, R.; Shao, M.; Cheng, L.; Lee, S.T. Copper Nanoparticles Grafted on a Silicon Wafer and Their Excellent Surface-Enhanced Raman Scattering. Adv. Funct. Mater. 2012, 22, 2067–2070. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.F.; Wei, W.Y.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS substrates: Powerful, portable, and full of potential. Phys. Chem. Chem. Phys. 2014, 16, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-Enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.P.; Gregas, M.K.; Seewaldt, V.; Vo-Dinh, T. SERS-based plasmonic nanobiosensing in single living cells. Anal. Bioanal. Chem. 2009, 393, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.R.; White, D.J. Optical fibre SERS sensors. Anal. Bioanal. Chem. 2009, 394, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, C.; Gu, C.; Seballos, L.; Zhang, J.Z. Liquid core photonic crystal fiber sensor based on surface enhanced Raman scattering. Appl. Phys. Lett. 2007, 90, 193504. [Google Scholar] [CrossRef]
- Yang, X.; Ileri, N.; Larson, C.C.; Carlson, T.C.; Britten, J.A.; Chang, A.S.; Bond, T.C. Nanopillar array on a fiber facet for highly sensitive surface-enhanced Raman scattering. Opt. Express 2012, 20, 24819–24826. [Google Scholar] [CrossRef] [PubMed]
- Viets, C.; Hill, W. Single-fibre surface-enhanced Raman sensors with angled tips. J. Raman Spectrosc. 2000, 31, 625–631. [Google Scholar] [CrossRef]
- Lan, X.; Han, Y.; Wei, T.; Zhang, Y.; Jiang, L.; Tsai, H.L.; Xiao, H. Surface-enhanced Raman-scattering fiber probe fabricated by femtosecond laser. Opt. Lett. 2009, 34, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, D.; Lei, X.; Liu, Y.; Mao, Q. One-pot hydrothermal synthesis of silver nanoplates on optical fiber tip for surface-enhanced Raman scattering. Appl. Phys. Lett. 2014, 104, 201906. [Google Scholar] [CrossRef]
- Lucotti, A.; Zerbi, G. Fiber-optic SERS sensor with optimized geometry. Sens. Actuators B Chem. 2007, 121, 356–364. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Jayawardhana, S.; Mazzolini, A.P.; Stoddart, P.R. Collection efficiency of scattered light in single-ended optical fiber sensors. Opt. Lett. 2012, 37, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhong, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Whitney, A.V.; Elam, J.W.; Stair, P.C.; Van Duyne, R.P. Toward a thermally robust operando surface-enhanced Raman spectroscopy substrate. J. Phys. Chem. C 2007, 111, 16827–16832. [Google Scholar] [CrossRef]
- Formo, E.V.; Mahurin, S.M.; Dai, S. Robust SERS substrates generated by coupling a bottom-up approach and atomic layer deposition. ACS Appl. Mater. Interfaces 2010, 2, 1987–1991. [Google Scholar] [CrossRef]
- John, J.F.; Mahurin, S.; Dai, S.; Sepaniak, M.J. Use of atomic layer deposition to improve the stability of silver substrates for in situ, high-temperature SERS measurements. J. Raman Spectrosc. 2010, 41, 4–11. [Google Scholar] [CrossRef]
- Bachenheimer, L.; Elliott, P.; Stagon, S.; Huang, H. Enhanced thermal stability of Ag nanorods through capping. Appl. Phys. Lett. 2014, 105, 213104. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, J.C.; Li, C.Y.; Chen, S.; Zhan, C.; Panneerselvam, R.; Yang, Z.L.; Li, J.F.; Zhou, Y.L. Large scale synthesis of pinhole-free shell-isolated nanoparticles (SHINs) using improved atomic layer deposition (ALD) method for practical applications. J. Raman Spectrosc. 2015, 46, 1200–1204. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Xie, Z.; Zhang, Z.J. Silver nanorods wrapped with ultrathin Al2O3 layers exhibiting excellent SERS sensitivity and outstanding SERS stability. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahurin, S.M.; John, J.; Sepaniak, M.J.; Dai, S. A reusable surface-enhanced Raman scattering (SERS) substrate prepared by atomic layer deposition of alumina on a multi-layer gold and silver film. Appl. Spectrosc. 2011, 65, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Dai, Z.M.; Chen, N.; Liu, S.P.; Pang, F.F.; Lu, B.; Wang, T.Y. Gold nanoparticles-modified tapered fiber nanoprobe for remote SERS detection. IEEE Photonics Technol. Lett. 2014, 26, 777–780. [Google Scholar] [CrossRef]
- Lazarev, A.; Fang, N.; Luo, Q. Formation of fine near-field scanning optical microscopy tips. Part II. By laser heated pulling and bending. Rev. Sci. Instrum. 2003, 74, 3684–3688. [Google Scholar] [CrossRef]
- Lazarev, A.; Fang, N.; Luo, Q.; Zhang, X. Formation of fine near-field scanning optical microscopy tips. Part I. By static and dynamic chemical etching. Rev. Sci. Instrum. 2003, 74, 3679–3683. [Google Scholar] [CrossRef]
- Gu, N.; Li, C.; Sun, L.; Liu, Z.H.; Sun, Y.K.; Xu, L.N. Controllable fabrication of fiber nano-tips by dynamic chemical etching based on siphon principle. J. Vac. Sci. Technol. B 2004, 22, 2283–2285. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hu, L.; Chen, Z.Y.; Chen, N.; Zhang, W.; Zhu, H.; Liu, S.P.; Wang, T.Y. Preparation of gold colloid and its surface-enhanced Raman scattering properties. In Proceedings of the Communications and Photonics Conference and Exhibition, Shanghai, China, 13–16 November 2011.
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
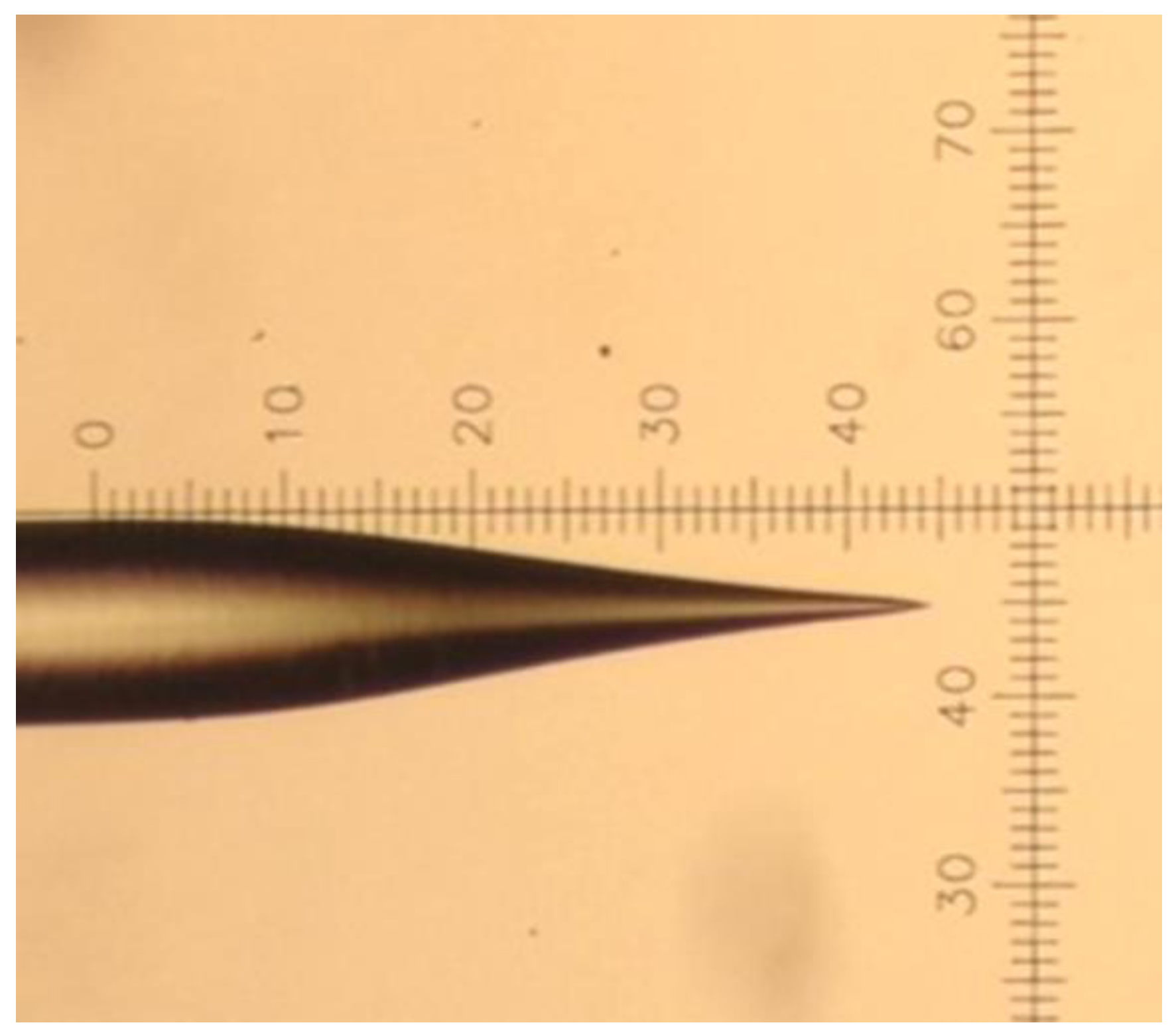
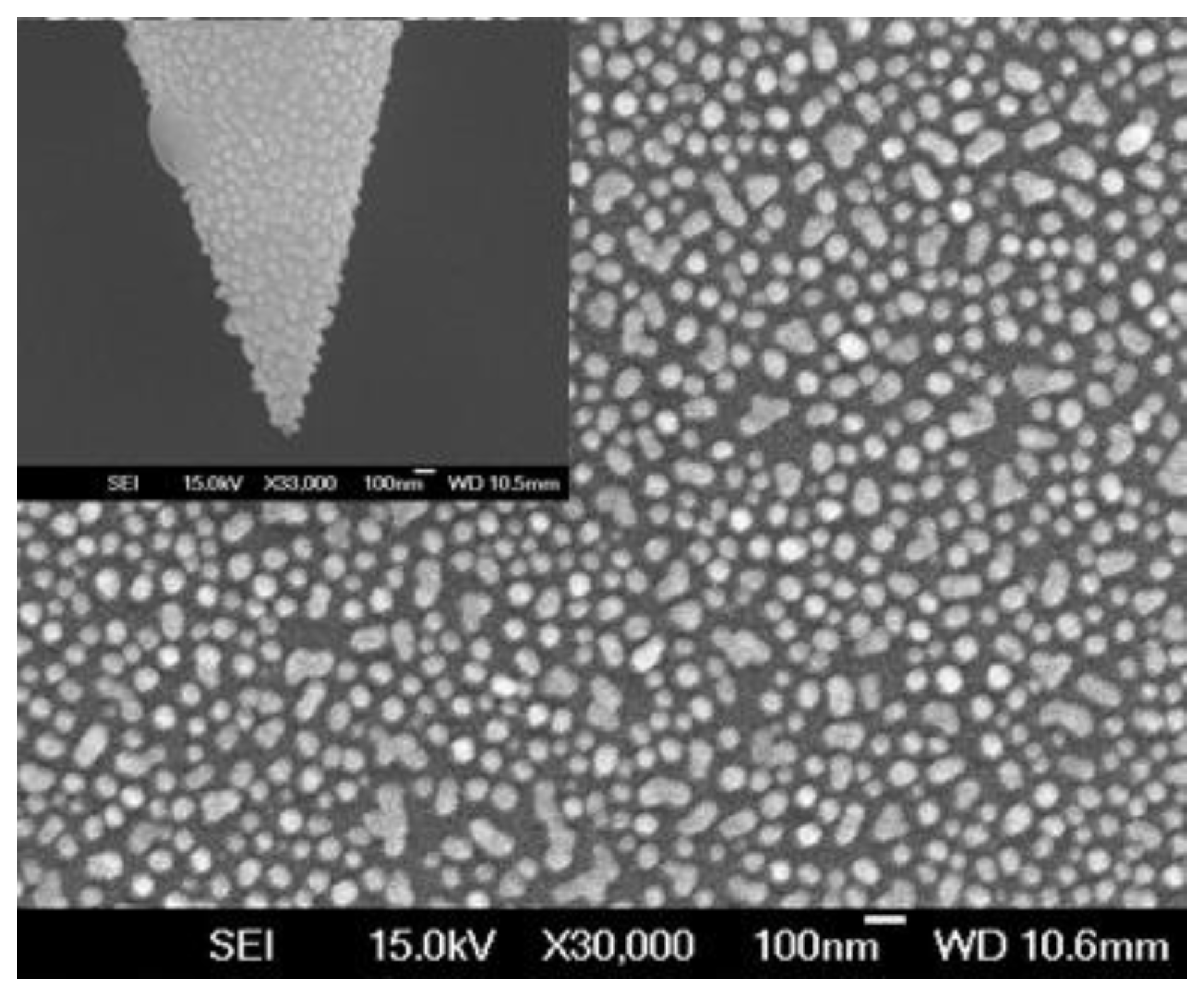

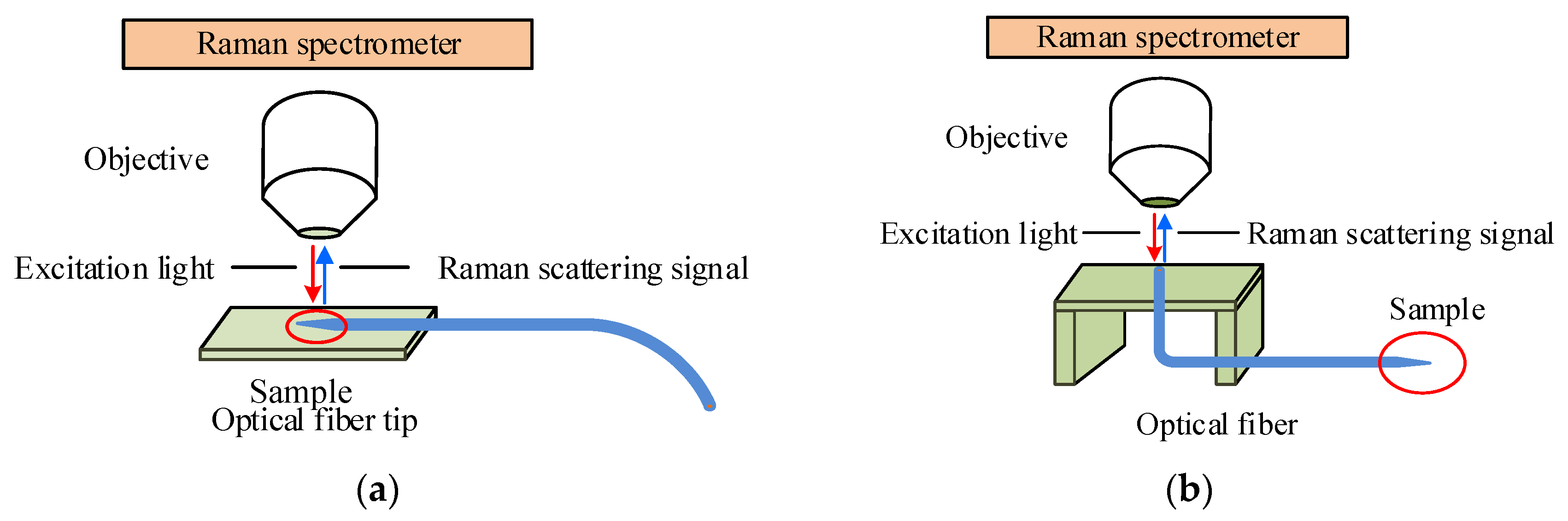

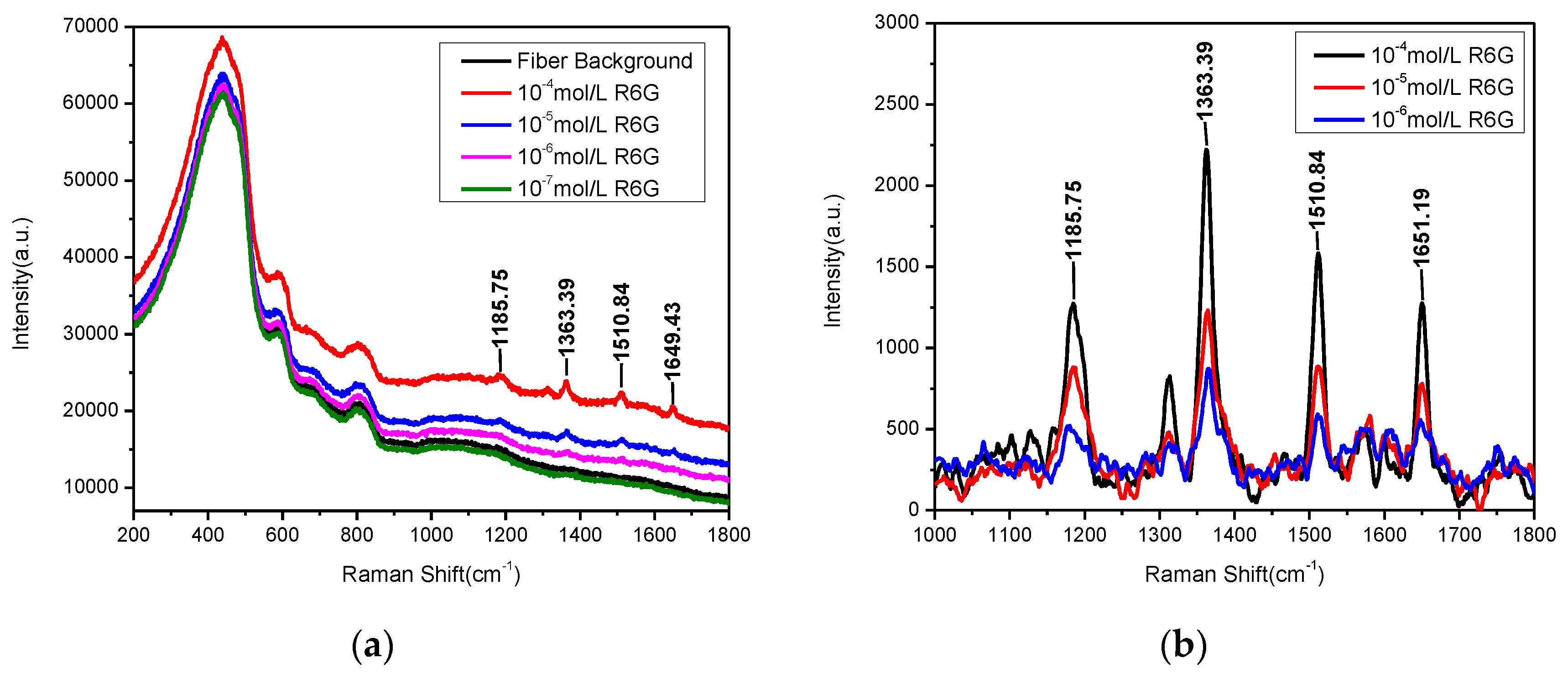
| Position | Thickness (nm) | N (633 nm) |
|---|---|---|
| #1 | 36.618 | 1.658 |
| #2 | 37.083 | 1.658 |
| #3 | 37.822 | 1.658 |
| #4 | 36.672 | 1.658 |
| #5 | 37.021 | 1.658 |
| #6 | 37.706 | 1.658 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Chen, Z.; Chen, N.; Zhang, H.; Liu, S.; Hu, X.; Wen, J.; Wang, T. SERS Taper-Fiber Nanoprobe Modified by Gold Nanoparticles Wrapped with Ultrathin Alumina Film by Atomic Layer Deposition. Sensors 2017, 17, 467. https://doi.org/10.3390/s17030467
Xu W, Chen Z, Chen N, Zhang H, Liu S, Hu X, Wen J, Wang T. SERS Taper-Fiber Nanoprobe Modified by Gold Nanoparticles Wrapped with Ultrathin Alumina Film by Atomic Layer Deposition. Sensors. 2017; 17(3):467. https://doi.org/10.3390/s17030467
Chicago/Turabian StyleXu, Wenjie, Zhenyi Chen, Na Chen, Heng Zhang, Shupeng Liu, Xinmao Hu, Jianxiang Wen, and Tingyun Wang. 2017. "SERS Taper-Fiber Nanoprobe Modified by Gold Nanoparticles Wrapped with Ultrathin Alumina Film by Atomic Layer Deposition" Sensors 17, no. 3: 467. https://doi.org/10.3390/s17030467






