Compliment Graphene Oxide Coating on Silk Fiber Surface via Electrostatic Force for Capacitive Humidity Sensor Applications
Abstract
:1. Introduction
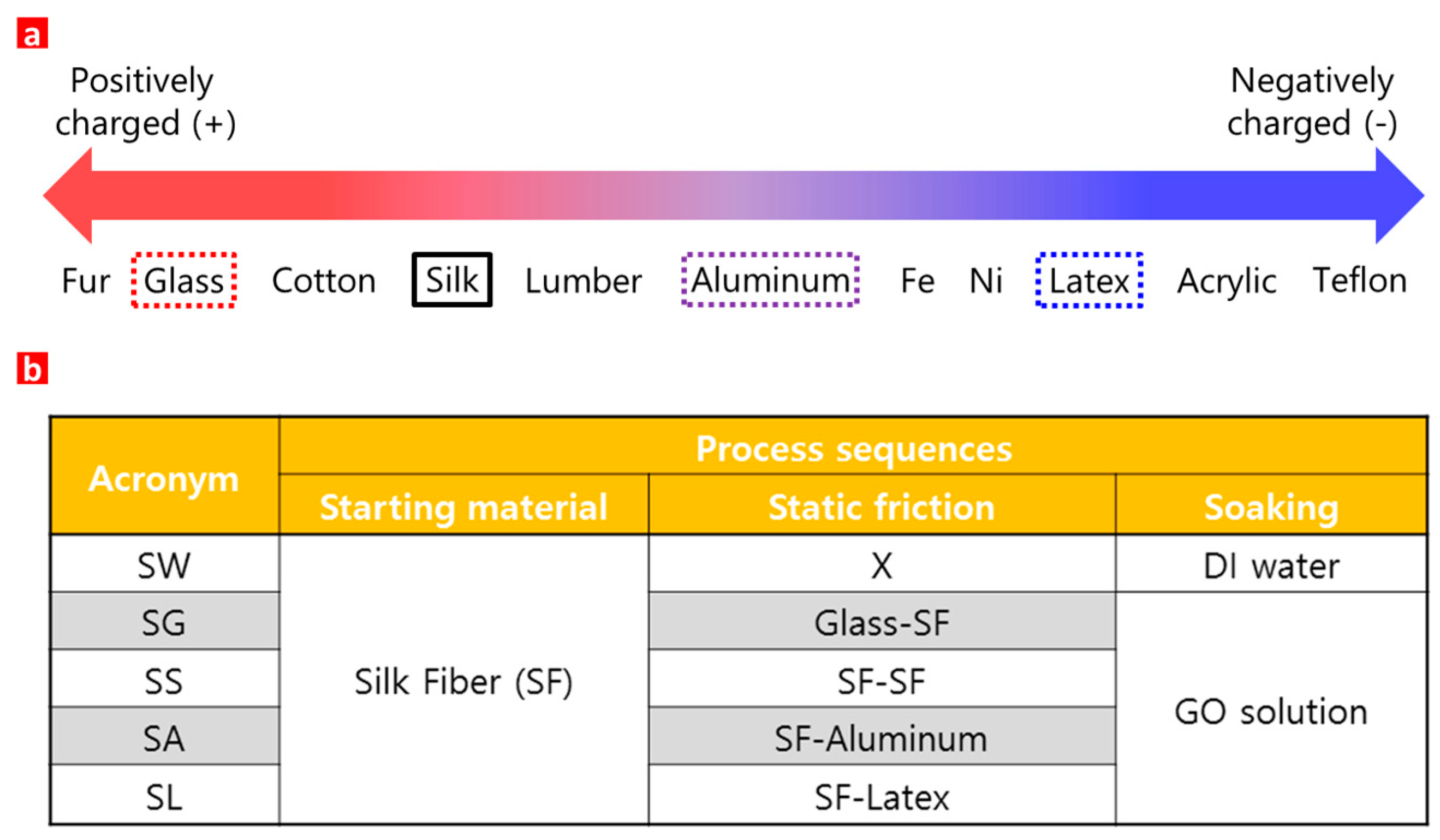
2. Materials and Methods
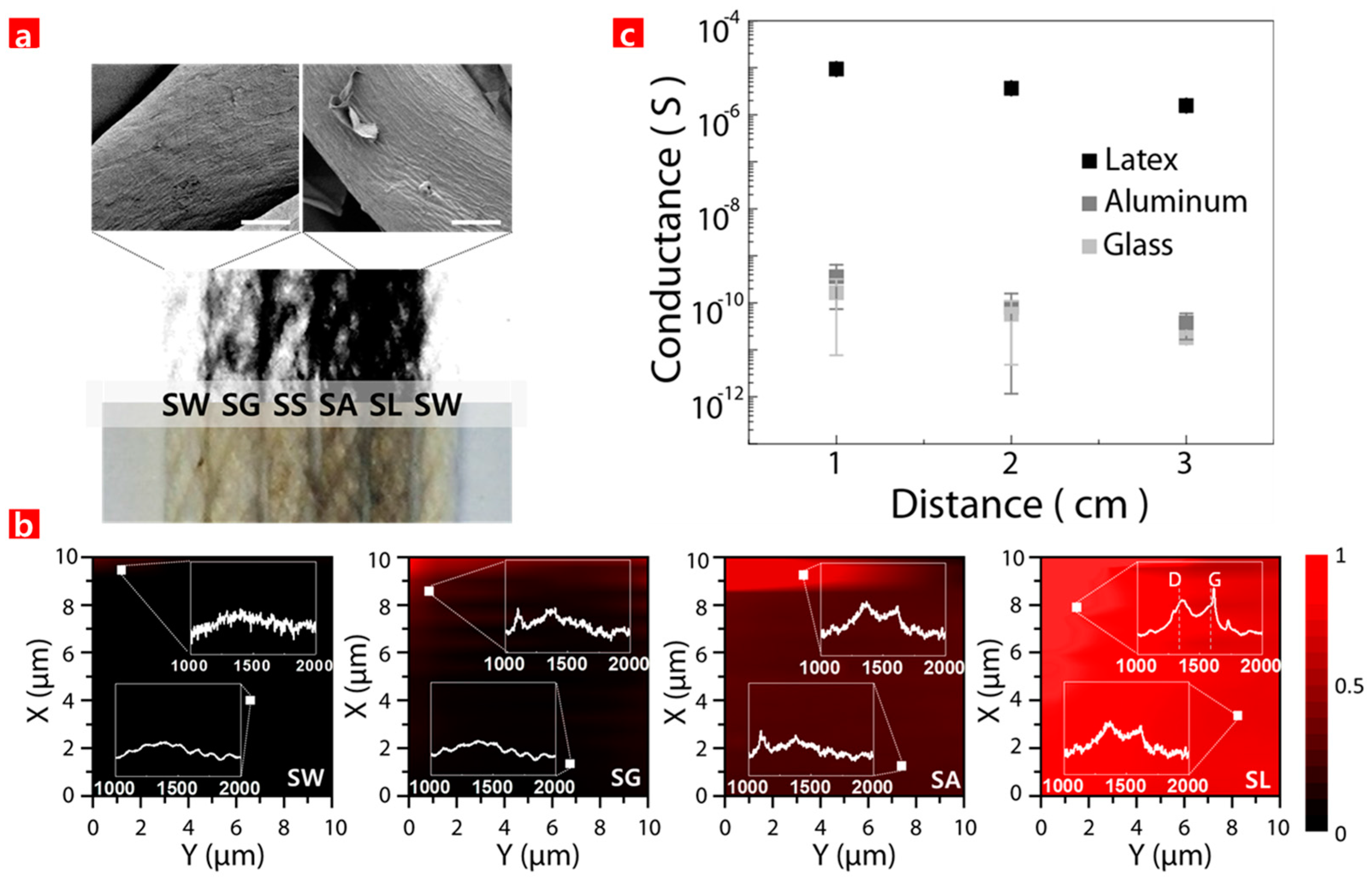
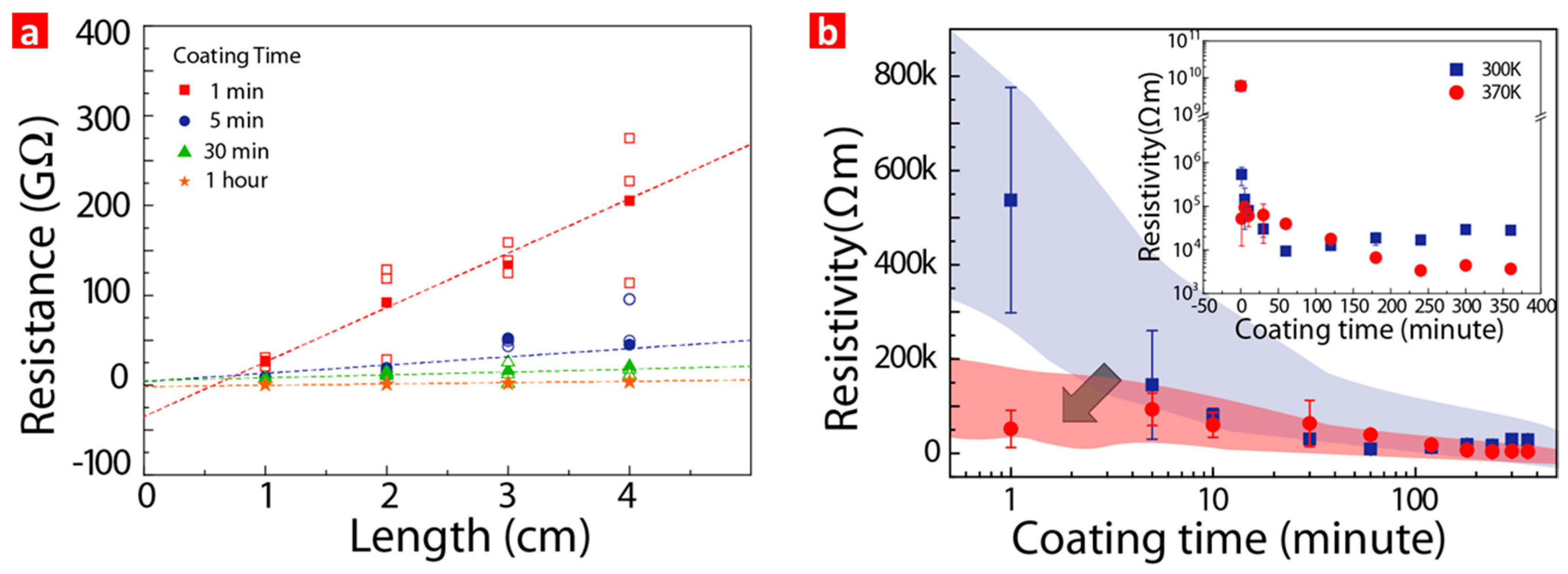
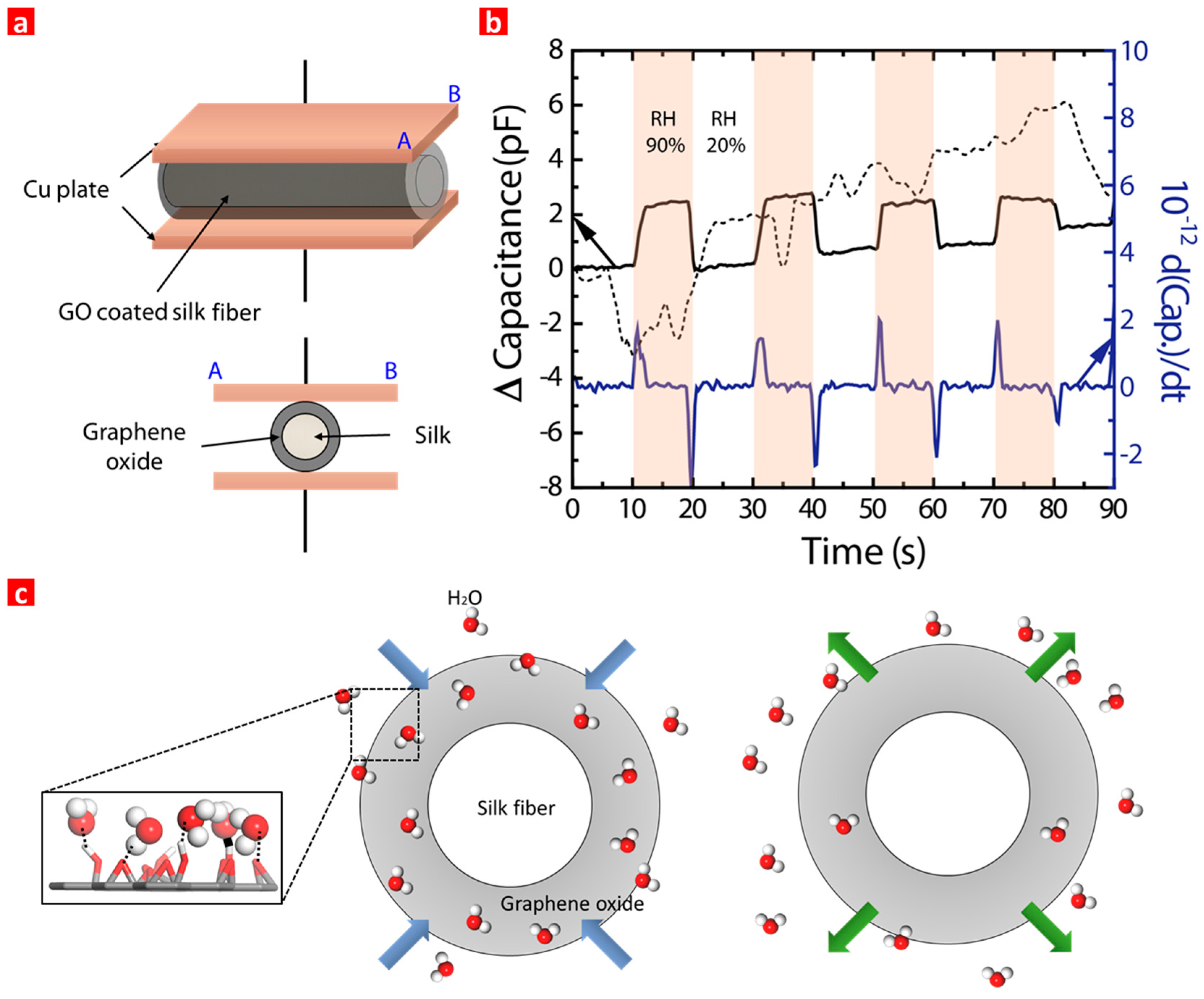
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swan, M. Sensor mania! The internet of things, wearable computing, objective metrics, and the quantified self 2.0. J. Sens. Actuator Netw. 2012, 1, 217–253. [Google Scholar] [CrossRef]
- Suzuki, D.; Oda, S.; Kawano, Y. A flexible and wearable terahertz scanner. Nat. Photonics 2016, 10, 809–813. [Google Scholar] [CrossRef]
- Su, P.G.; Wang, C.P. Flexible humidity sensor based on TiO2 nanoparticles-polypyrrole-poly-[3-(methacrylamino)prolyl] trimethyl ammonium chloride composite materials. Sens. Actuators B Chem. 2008, 129, 538–543. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Yun, S.; Kim, J. Flexible humidity and temperature sensor based on cellulose-polypyrrole nanocomposite. Sens. Actuators A Phys. 2011, 165, 194–199. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed]
- Rubinger, C.P.L.; Martins, C.R.; de Paoli, M.-A.; Rubinger, R.M. Sulfonated polystyrene polymer humidity sensor: Synthesis and characterization. Sens. Actuators B Chem. 2007, 123, 42–49. [Google Scholar] [CrossRef]
- Hwang, L.S.; Ko, J.M.; Rhee, H.W.; Kim, C.Y. A polymer humidity sensor. Synth. Met. 1993, 55, 3671–3676. [Google Scholar] [CrossRef]
- Varghese, O.K.; Grimes, C.A. Metal oxide nanoarchitectures for environmental sensing. J. Nanosci. Nanotechnol. 2003, 3, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Steele, J.J.; Taschuk, M.T.; Brett, M.J. Nanostructured metal oxide thin films for humidity sensors. IEEE Sens. J. 2007, 7, 955–956. [Google Scholar] [CrossRef]
- Qu, W.; Meyer, J.U. Thick-film humidity sensor based on porous MnWO4 material. Meas. Sci. Technol. 1997, 8, 593–600. [Google Scholar] [CrossRef]
- Connolly, E.J.; O’Halloran, G.M.; Pham, H.T.M.; Sarro, P.M.; French, P.J. Comparison of porous silicon, porous polysilicon and porous silicon carbide as materials for humidity sensing applications. Sens. Actuators B Chem. 2002, 99, 25–30. [Google Scholar] [CrossRef]
- Chou, K.S.; Lee, T.K.; Liu, F.J. Sensing mechanism of a porous ceramic as humidity sensor. Sens. Actuators B Chem. 1999, 56, 106–111. [Google Scholar] [CrossRef]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuators B Chem. 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Q.; Zhou, R.; Xu, B. Humidity sensors based on composite materials of nano-BaTiO3 and polymer RMX. Sens. Actuators B Chem. 2002, 81, 248–254. [Google Scholar] [CrossRef]
- Gubbi, J.; Buyya, R.; Marusic, S.; Palaniswami, M. Internet of Things (IoT): A vision, architectural elements, and future directions. FGCS 2013, 29, 1645–1660. [Google Scholar] [CrossRef]
- Chi, Q.; Yan, H.; Zhang, C.; Pang, Z.; Xu, L.D. A reconfigurable smart sensor interface for industrial WSN in IoT environment. IEEE Trans. Ind. Inform. 2014, 10, 1417–1425. [Google Scholar]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhänen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Yan, L. Free-standing dried foam films of graphene oxide for humidity sensing. Sens. Actuators B Chem. 2015, 215, 316–322. [Google Scholar] [CrossRef]
- Xuan, W.; He, X.; Chen, J.; Wang, W.; Wang, X.; Xu, Y.; Xu, Z.; Fu, Y.Q.; Luo, K.J. High sensitivity flexible Lamb-wave humidity sensors with a graphene oxide sensing layer. Nanoscale 2015, 7, 7430–7436. [Google Scholar] [CrossRef] [PubMed]
- Han, K.I.; Kim, S.D.; Yang, W.S.; Kim, H.S.; Shin, M.; Kim, J.P.; Lee, I.G.; Cho, B.J.; Hwang, W.S. Material characteristics and equivalent circuit models of stacked graphene oxide for capacitive humidity sensors. AIP Adv. 2016, 6, 035203. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B Chem. 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xia, B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Li, P.; Zhang, Y. Facile fabrication of MoS2-modified SnO2 hybrid nanocomposite for ultrasensitive humidity sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, Y.; Park, C.; Fukushima, T.; Aida, T. Ultrahigh-throughput exfoliation of graphite into pristine ‘single-layer’ graphene using microwaves and molecularly engineered ionic liquids. Nat. Chem. 2015, 7, 10730–10736. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Feng, C.; Zhao, G.; Wang, C.; Wang, Z. Graphene-coated fiber for solid-phase microextraction of triazine herbicides in water samples. J. Sep. Sci. 2012, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Ye, X. Fabrication and application of flexible graphene silk composite film electrodes decorated with spiky Pt nanospheres. Nanoscale 2014, 6, 4264–4274. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.J.; Hong, W.G.; Choi, N.J.; Park, H.J.; Moon, S.E.; Kim, B.H.; Song, K.B.; Jun, Y.; Lee, H.K. A 3D scaffold for ultra-sensitive reduced graphene oxide gas sensors. Nanoscale 2014, 6, 6511–6514. [Google Scholar] [CrossRef] [PubMed]
- Oprea, A.; Courbat, J.; Bârsan, N.; Briand, D.; de Rooij, N.F.; Weimar, U. Temperature, humidity and gas sensors integrated on plastic foil for low power applications. Sens. Actuators B Chem. 2009, 140, 227–232. [Google Scholar] [CrossRef]
- Puers, R. Capacitive sensors: When and how to use them. Sens. Actuators A Phys. 1993, 37, 93–105. [Google Scholar] [CrossRef]
- Wilson, J.S. Sensor Technology Handbook; Newnes: Waltham, MA, USA, 2005; pp. 217–273. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, J.C. Disputatio Physica Experimentalis, de Electricitatibus Contrariis; Rostochii: Mecklenburg, Germany, 1757. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.I.; Kim, S.; Lee, I.G.; Kim, J.P.; Kim, J.-H.; Hong, S.W.; Cho, B.J.; Hwang, W.S. Compliment Graphene Oxide Coating on Silk Fiber Surface via Electrostatic Force for Capacitive Humidity Sensor Applications. Sensors 2017, 17, 407. https://doi.org/10.3390/s17020407
Han KI, Kim S, Lee IG, Kim JP, Kim J-H, Hong SW, Cho BJ, Hwang WS. Compliment Graphene Oxide Coating on Silk Fiber Surface via Electrostatic Force for Capacitive Humidity Sensor Applications. Sensors. 2017; 17(2):407. https://doi.org/10.3390/s17020407
Chicago/Turabian StyleHan, Kook In, Seungdu Kim, In Gyu Lee, Jong Pil Kim, Jung-Ha Kim, Suck Won Hong, Byung Jin Cho, and Wan Sik Hwang. 2017. "Compliment Graphene Oxide Coating on Silk Fiber Surface via Electrostatic Force for Capacitive Humidity Sensor Applications" Sensors 17, no. 2: 407. https://doi.org/10.3390/s17020407





