Capacitive Biosensors and Molecularly Imprinted Electrodes
Abstract
:1. Introduction
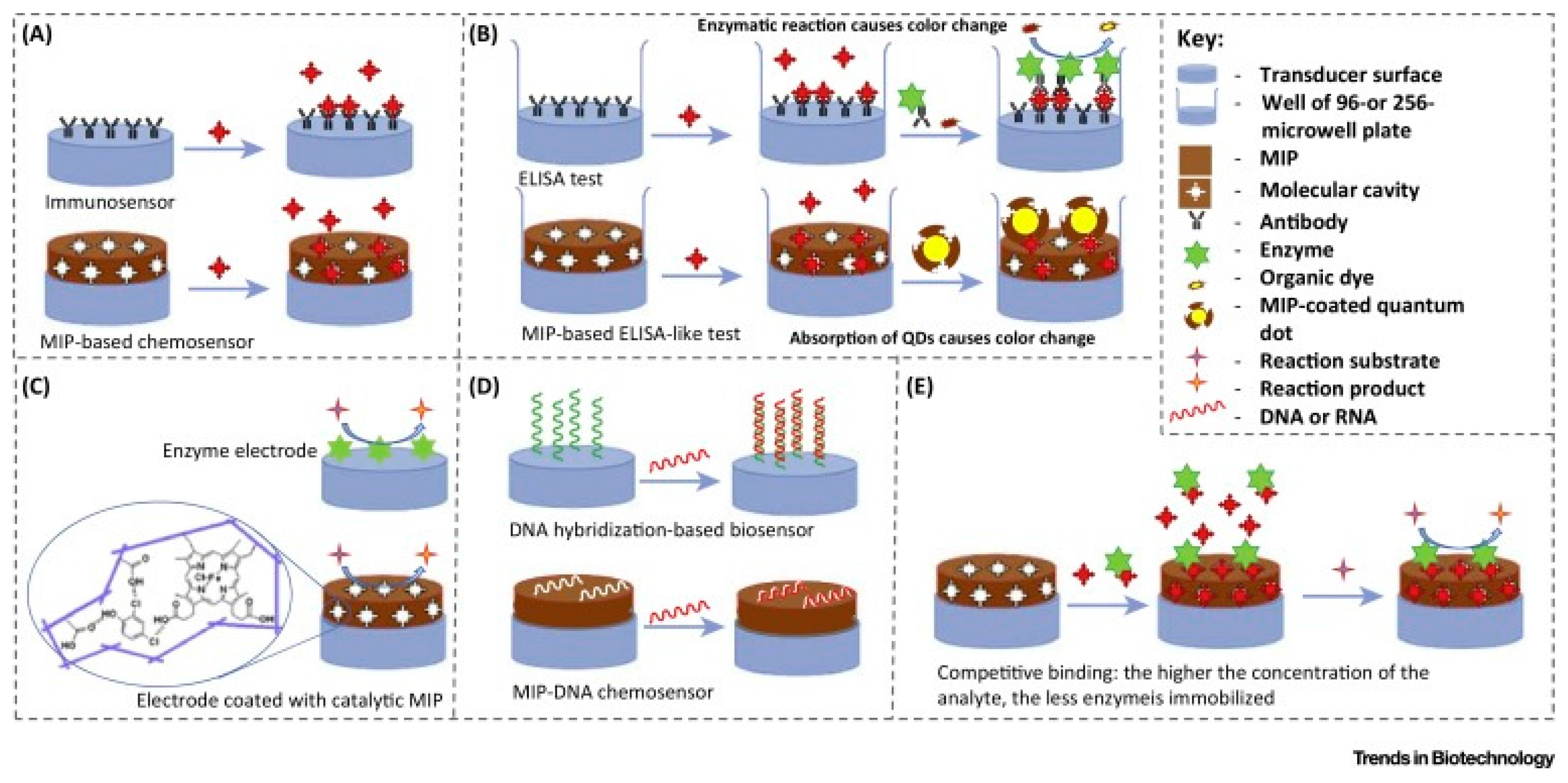
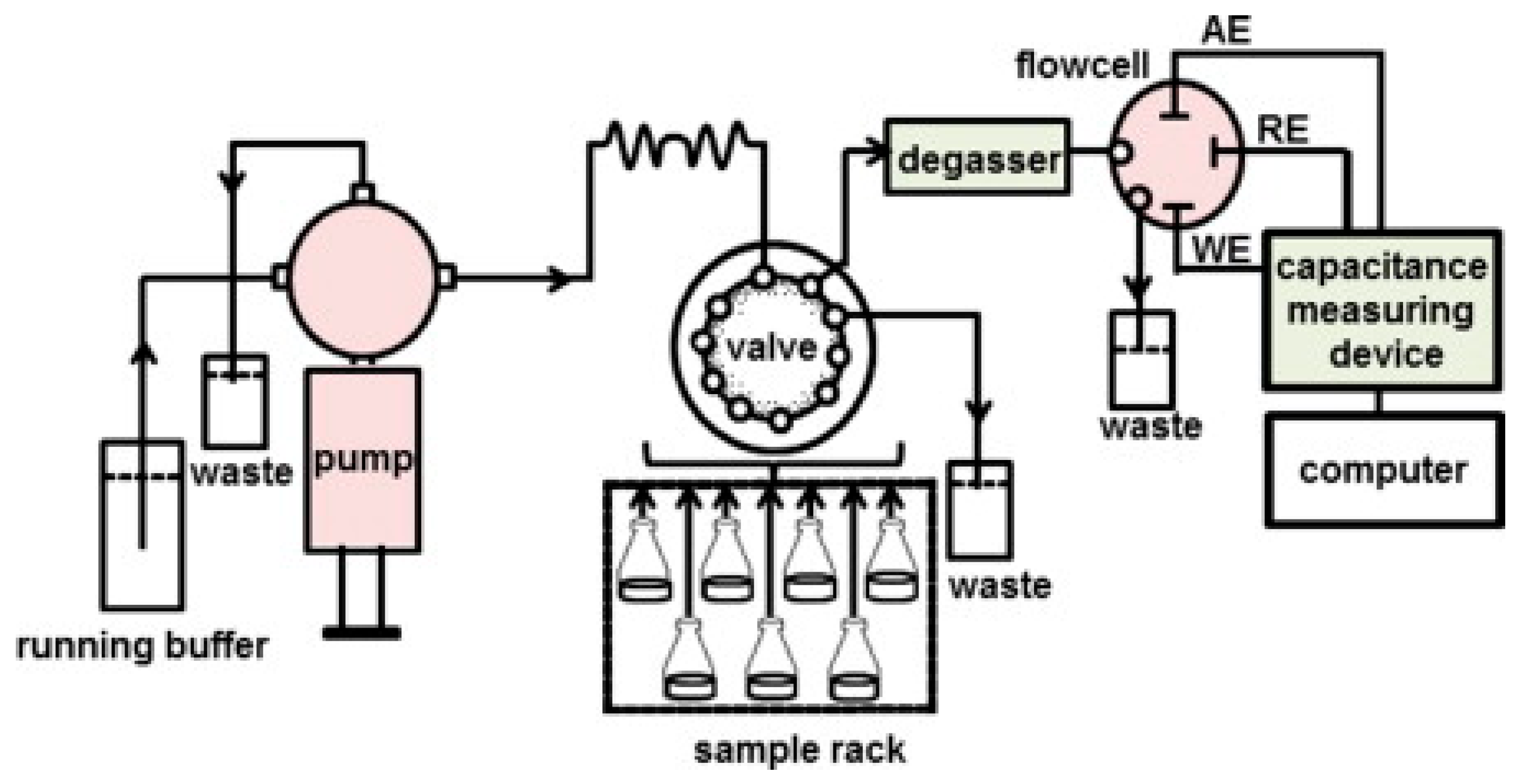
2. Capacitive Biosensors
3. Different Applications of Capacitive Biosensors
3.1. Protein Detection
3.2. Nucleic Acid Detection
3.3. Cell Detection
3.4. Heavy Metal Detection
3.5. Saccharide Detection
3.6. Small Organic Molecules
- (1)
- Unfolding the conformation of the starting protein under acidic conditions;
- (2)
- Addition of template molecule and allow interaction between the template molecule and the denatured protein in order to form new molecular configurations;
- (3)
- Cross-linking of the protein to stabilize the new molecular protein conformation; and
- (4)
- Dialysis to remove the template molecule.
4. Molecular Imprinting
- (1)
- Pre-complexation of functional monomers around the template molecule in solution either by forming covalent bonds or by self-assembling with non-covalent bonds;
- (2)
- Polymerization of the resulting complex in the presence of cross-linking monomers and suitable solvents/ionic liquids as porogens; and
- (3)
- Removal of template molecule from the synthesized polymer.
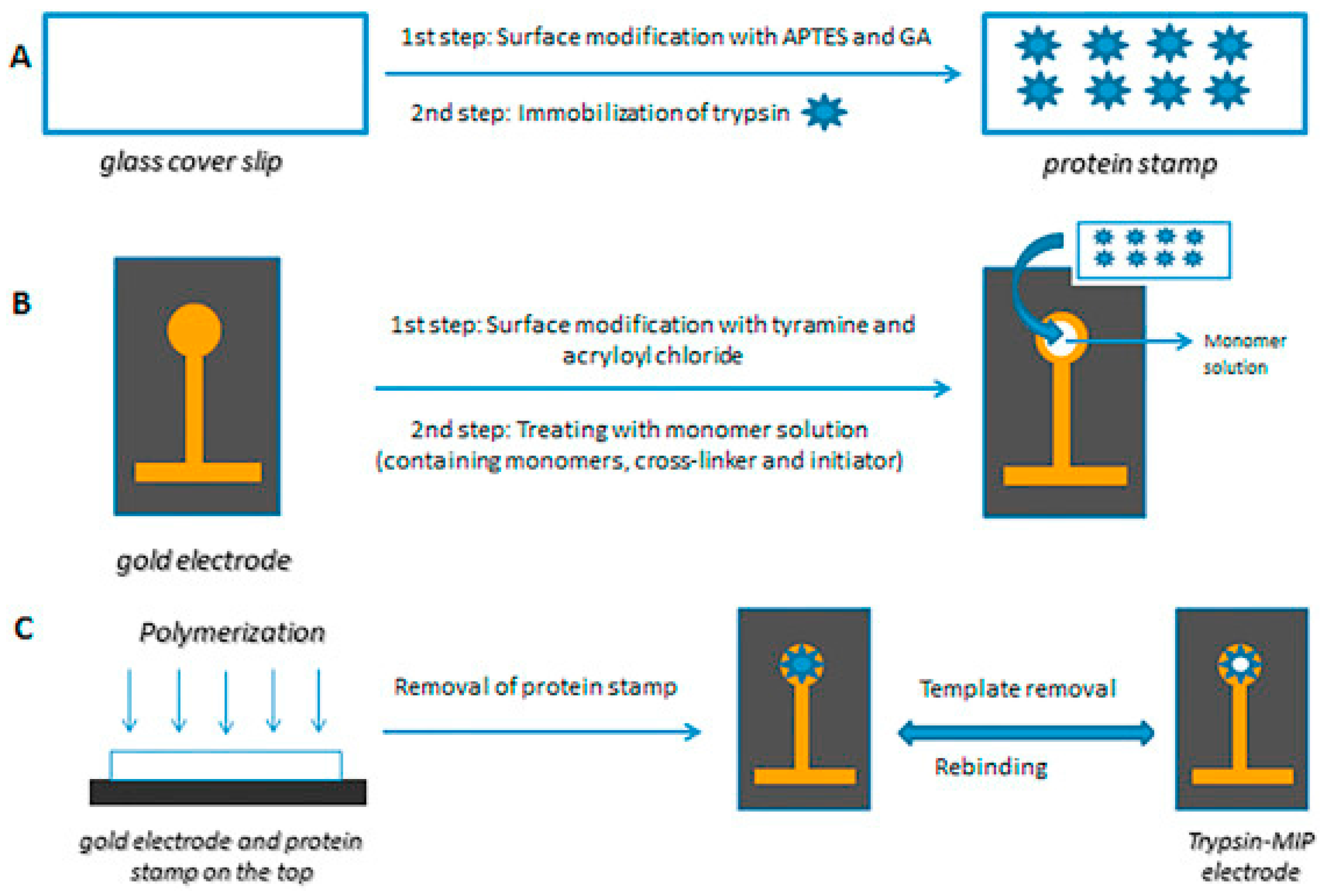
5. Microcontact Imprinting
6. Applications of Microcontact Imprinting Method with Capacitive Biosensors
- (1)
- A current source;
- (2)
- An electro-chemical flow-cell which includes three electrodes: the working electrode which is a thin gold film coated with an insulating layer which functions as a bio-recognition layer to immobilize the ligand, the auxiliary and reference electrodes which are made from a platinum wire;
- (3)
- A potential differential amplifier; and
- (4)
- A processor which converts the analogue potential to digital signal.
7. Concluding Remarks
Conflicts of Interest
References
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Waggoner, P.S.; Craighead, H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Tsouti, V.; Boutopoulos, C.; Zergioti, I.; Chatzandroulis, S. Capacitive microsystems for biological sensing. Biosens. Bioelectron. 2011, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mattiasson, B.; Teeparuksapun, K.; Hedström, M. Immunochemical binding assays for detection and quantification of trace impurities in biotechnological production. Trends Biotechnol. 2010, 28, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Korotkaya, E.V. Biosensors: Design, classification and applications in the food industry. Food Raw Mater. 2014, 2, 161–171. [Google Scholar] [CrossRef]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Mattiasson, B.; Hedström, M. Capacitive biosensors for ultra-sensitive assays. TrAC Trends Anal. Chem. 2016, 79, 233–238. [Google Scholar] [CrossRef]
- Labib, M.; Hedström, M.; Amin, M.; Mattiasson, B. A capacitive immunosensor for detection of cholera toxin. Anal. Chim. Acta 2009, 634, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Loyprasert, S.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Sub-attomolar detection of cholera toxin using a label-free capacitive immunosensor. Biosens. Bioelectron. 2010, 25, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Teeparuksapun, K.; Hedström, M.; Wong, E.Y.; Tang, S.; Hewlett, I.K.; Mattiasson, B. Ultrasensitive detection of HIV-1 p24 antigen using nanofunctionalized surfaces in a capacitive immunosensor. Anal. Chem. 2010, 82, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sens. Actuators B Chem. 2015, 209, 645–651. [Google Scholar] [CrossRef]
- Mahadhy, A. Development of an Ultrasensitive Capacitive DNA-Sensor: A Promising Tool towards Microbial Diagnostics. Ph.D. Thesis, Lund University, Lund, Sweden, 2015. [Google Scholar]
- Tsekenis, G.; Chatzipetrou, M.; Tanner, J.; Chatzandroulis, S.; Thanos, D.; Tsoukalas, D.; Zergioti, I. Surface functionalization studies and direct laser printing of oligonucleotides toward the fabrication of a micromembrane DNA capacitive biosensor. Sens. Actuators B Chem. 2012, 175, 123–131. [Google Scholar] [CrossRef]
- Park, J.W.; Kallempudi, S.S.; Niazi, J.H.; Gurbuz, Y.; Youn, B.-S.; Gu, M.B. Rapid and sensitive detection of Nampt (PBEF/visfatin) in human serum using an ssDNA aptamer-based capacitive biosensor. Biosens. Bioelectron. 2012, 38, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Thipmanee, O.; Samanman, S.; Sankoh, S.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Vilaivan, T.; Thavarungkul, P. Label-free capacitive DNA sensor using immobilized pyrrolidinyl PNA probe: effect of the length and terminating head group of the blocking thiols. Biosens. Bioelectron. 2012, 38, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jantra, J.; Kanatharana, P.; Asawatreratanakul, P.; Hedström, M.; Mattiasson, B.; Thavarungkul, P. Real-time label-free affinity biosensors for enumeration of total bacteria based on immobilized concanavalin A. J. Environ. Sci. Health Part A 2011, 46, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Pandey, A.; Chouhan, R.S.; Gurbuz, Y.; Niazi, J.H. Whole-cell based label-free capacitive biosensor for rapid nanosize-dependent toxicity detection. Biosens. Bioelectron. 2015, 67, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bontidean, I.; Lloyd, J.R.; Hobman, J.L.; Wilson, J.R.; Csöregi, E.; Mattiasson, B.; Brown, N.L. Bacterial metal-resistance proteins and their use in biosensors for the detection of bioavailable heavy metals. J. Inorg. Biochem. 2000, 79, 225–229. [Google Scholar] [CrossRef]
- Corbisier, P.; van der Lelie, D.; Borremans, B.; Provoost, A.; de Lorenzo, V.; Brown, N.L.; Lloyd, J.R.; Hobman, J.L.; Csöregi, E.; Johansson, G. Whole cell-and protein-based biosensors for the detection of bioavailable heavy metals in environmental samples. Anal. Chim. Acta 1999, 387, 235–244. [Google Scholar] [CrossRef]
- Labib, M.; Hedström, M.; Amin, M.; Mattiasson, B. A novel competitive capacitive glucose biosensor based on concanavalin A-labeled nanogold colloids assembled on a polytyramine-modified gold electrode. Anal. Chim. Acta 2010, 659, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Hedström, M.; Amin, M.; Mattiasson, B. Competitive capacitive biosensing technique (CCBT): A novel technique for monitoring low molecular mass analytes using glucose assay as a model study. Anal. Bioanal. Chem. 2010, 397, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Lenain, P.; de Saeger, S.; Mattiasson, B.; Hedström, M. Affinity sensor based on immobilized molecular imprinted synthetic recognition elements. Biosens. Bioelectron. 2015, 69, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Hedström, M.; Mattiasson, B. Bioimprinting as a tool for the detection of aflatoxin B1 using a capacitive biosensor. Biotechnol. Rep. 2016, 11, 12–17. [Google Scholar] [CrossRef]
- Bougrini, M.; Baraket, A.; Jamshaid, T.; El Aissari, A.; Bausells, J.; Zabala, M.; El Bari, N.; Bouchikhi, B.; Jaffrezic-Renault, N.; Abdelhamid, E. Development of a novel capacitance electrochemical biosensor based on silicon nitride for ochratoxin A detection. Sens. Actuators B Chem. 2016, 234, 446–452. [Google Scholar] [CrossRef]
- Mahadhy, A.; Ståhl-Wernersson, E.; Mattiasson, B.; Hedström, M. Use of a capacitive affinity biosensor for sensitive and selective detection and quantification of DNA—A model study. Biotechnol. Rep. 2014, 3, 42–48. [Google Scholar] [CrossRef]
- Rydosz, A.; Brzozowska, E.; Gorska, S.; Wincza, K.; Gamian, A.; Gruszczynski, S. A broadband capacitive sensing method for label-free bacterial LPS detection. Biosens. Bioelectron. 2016, 75, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, E.; Smietana, M.; Koba, M.; Gorska, S.; Pawlik, K.; Gamian, A.; Bock, W.J. Recognition of bacterial lipopolysaccharide using bacteriophage-adhesin-coated long-period gratings. Biosens. Bioelectron. 2015, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.M.; Yuan, Q.; Close, D.M.; O’Dell, K.B.; Fortney, J.L.; Wu, J.; Hazen, T.C. Rapid Detection of Microbial Cell Abundance in Aquatic Systems. Biosens. Bioelectron. 2016, 85, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Bontidean, I.; Berggren, C.; Johansson, G.; Csöregi, E.; Mattiasson, B.; Lloyd, J.R.; Jakeman, K.J.; Brown, N.L. Detection of heavy metal ions at femtomolar levels using protein-based biosensors. Anal. Chem. 1998, 70, 4162–4169. [Google Scholar] [CrossRef] [PubMed]
- Tekin, K.; Uzun, L.; Şahin, Ç.A.; Bektaş, S.; Denizli, A. Preparation and characterization of composite cryogels containing imidazole group and use in heavy metal removal. React. Funct. Polym. 2011, 71, 985–993. [Google Scholar] [CrossRef]
- Say, R.; Birlik, E.; Ersöz, A.; Yılmaz, F.; Gedikbey, T.; Denizli, A. Preconcentration of copper on ion-selective imprinted polymer microbeads. Anal. Chim. Acta 2003, 480, 251–258. [Google Scholar] [CrossRef]
- Ersöz, A.; Say, R.; Denizli, A. Ni(II) ion-imprinted solid-phase extraction and preconcentration in aqueous solutions by packed-bed columns. Anal. Chim. Acta 2004, 502, 91–97. [Google Scholar] [CrossRef]
- Birlik, E.; Ersöz, A.; Açıkkalp, E.; Denizli, A.; Say, R. Cr(III)-imprinted polymeric beads: Sorption and preconcentration studies. J. Hazard. Mater. 2007, 140, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Esen, C.; Andac, M.; Bereli, N.; Say, R.; Henden, E.; Denizli, A. Highly selective ion-imprinted particles for solid-phase extraction of Pb 2+ ions. Mater. Sci. Eng. C 2009, 29, 2464–2470. [Google Scholar] [CrossRef]
- Say, R.; Ersöz, A.; Denizli, A. Selective separation of uranium containing glutamic acid molecular-imprinted polymeric microbeads. Sep. Sci. Technol. 2003, 38, 3431–3447. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Jung, H.I. A symmetric metamaterial element-based RF biosensor for rapid and label-free detection. Appl. Phys. Lett. 2011, 99, 163703. [Google Scholar] [CrossRef]
- Kim, N.Y.; Dhakal, R.; Adhikari, K.K.; Kim, E.S.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Altunbaş, C.; Uygun, M.; Uygun, D.A.; Akgöl, S.; Denizli, A. Immobilization of inulinase on concanavalin A-attached super macroporous cryogel for production of high-fructose syrup. Appl. Biochem. Biotechnol. 2013, 170, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Perçin, I.; Yavuz, H.; Aksöz, E.; Denizli, A. Mannose-specific lectin isolation from Canavalia ensiformis seeds by PHEMA-based cryogel. Biotechnol. Prog. 2012, 28, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Sarı, M.M.; Armutcu, C.; Bereli, N.; Uzun, L.; Denizli, A. Monosize microbeads for pseudo-affinity adsorption of human insulin. Colloids Surf. B Biointerfaces 2011, 84, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Çorman, M.E.; Armutcu, C.; Uzun, L.; Denizli, A. Rapid, efficient and selective preconcentration of benzo [a] pyrene (BaP) by molecularly imprinted composite cartridge and HPLC. Mater. Sci. Eng. C 2017, 70, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.M.; Üzek, R.; Bereli, N.; Şenel, S.; Denizli, A. Synthesis of novel monolithic cartridges with specific recognition sites for extraction of melamine. React. Funct. Polym. 2016, 109, 33–41. [Google Scholar] [CrossRef]
- Inanan, T.; Tüzmen, N.; Akgöl, S.; Denizli, A. Selective cholesterol adsorption by molecular imprinted polymeric nanospheres and application to GIMS. Int. J. Biol. Macromol. 2016, 92, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Göktürk, I.; Perçin, I.; Denizli, A. Catalase purification from rat liver with iron chelated poly (hydroxyethyl methacrylate-N-methacryloyl-(l)-glutamic acid) cryogel discs. Prep. Biochem. Biotechnol. 2015, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Preparation of imprinted cryogel cartridge for chiral separation of l-phenylalanine. Artif. Cells Nanomed. Biotechnol. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aşır, S.; Derazshamshir, A.; Yilmaz, F.; Denizli, A. Triazine herbicide imprinted monolithic column for capillary electrochromatography. Electrophoresis 2015, 36, 2888–2895. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E.; Kutsal, T.; Denizli, A. Surface imprinted bacterial cellulose nanofibers for cytochrome c purification. Process Biochem. 2015, 50, 2289–2297. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Use of Polymers with Enzyme-Analogous Structures for Resolution of Racemates, Angewandte Chemie-International ed.; Wiley-VCH Verlag Gmbh: Muhlenstrasse, Berlin, Germany, 1972; pp. 33–34. [Google Scholar]
- Takagishi, T.; Klotz, I.M. Macromolecule-small molecule interactions; introduction of additional binding sites in polyethyleneimine by disulfide cross–linkages. Biopolymers 1972, 11, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.C.; Rick, J.; Chou, T.C. C-reactive protein thin-film molecularl imprinted polymers formed using a micro-contact approach. Anal. Chim. Acta 2005, 542, 20–25. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. From imprinting to microcontact imprinting—A new tool to increase selectivity in analytical devices. J. Chromatogr. B 2016, 1021, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Hsu, C.Y.; Thomas, J.L.; Wang, S.E.; Chen, H.C.; Chou, T.C. The microcontact imprinting of proteins: The effect of cross-linking monomers for lysozyme, ribonuclease A and myoglobin. Biosens. Bioelectron. 2006, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Berillo, D.; Hedström, M.; Mattiasson, B. Microcontact-BSA imprinted capacitive biosensor for real-time, sensitive and selective detection of BSA. Biotechnol. Rep. 2014, 3, 65–72. [Google Scholar] [CrossRef]
- Erlandsson, D.; Teeparuksapun, K.; Mattiasson, B.; Hedström, M. Automated flow-injection immunosensor based on current pulse capacitive measurements. Sens. Actuators B Chem. 2014, 190, 295–304. [Google Scholar] [CrossRef]
- Ertürk, G.; Hedström, M.; Tümer, M.A.; Denizli, A.; Mattiasson, B. Real-time prostate-specific antigen detection with prostate-specific antigen imprinted capacitive biosensors. Anal. Chim. Acta 2015, 891, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole cell based microcontact imprinted capacitive biosensor for the detection of Escherichia coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Hedström, M.; Mattiasson, B. A sensitive and real-time assay of trypsin by using molecular imprinting-based capacitive biosensor. Biosens. Bioelectron. 2016, 86, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. An immunological Interleukine-6 capacitive biosensor using perturbation with a potentiostatic step. Biosens. Bioelectron. 1998, 13, 1061–1068. [Google Scholar] [CrossRef]
- Hedström, M.; Galaev, I.Y.; Mattiasson, B. Continuous measurements of a binding reaction using a capacitive biosensor. Biosens. Bioelectron. 2005, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lebogang, L.; Hedström, M.; Mattiasson, B. Development of a real-time capacitive biosensor for cyclic cyanotoxic peptides based on Adda-specific antibodies. Anal. Chim. Acta 2014, 826, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens. Actuators B Chem. 2016, 224, 823–832. [Google Scholar] [CrossRef]
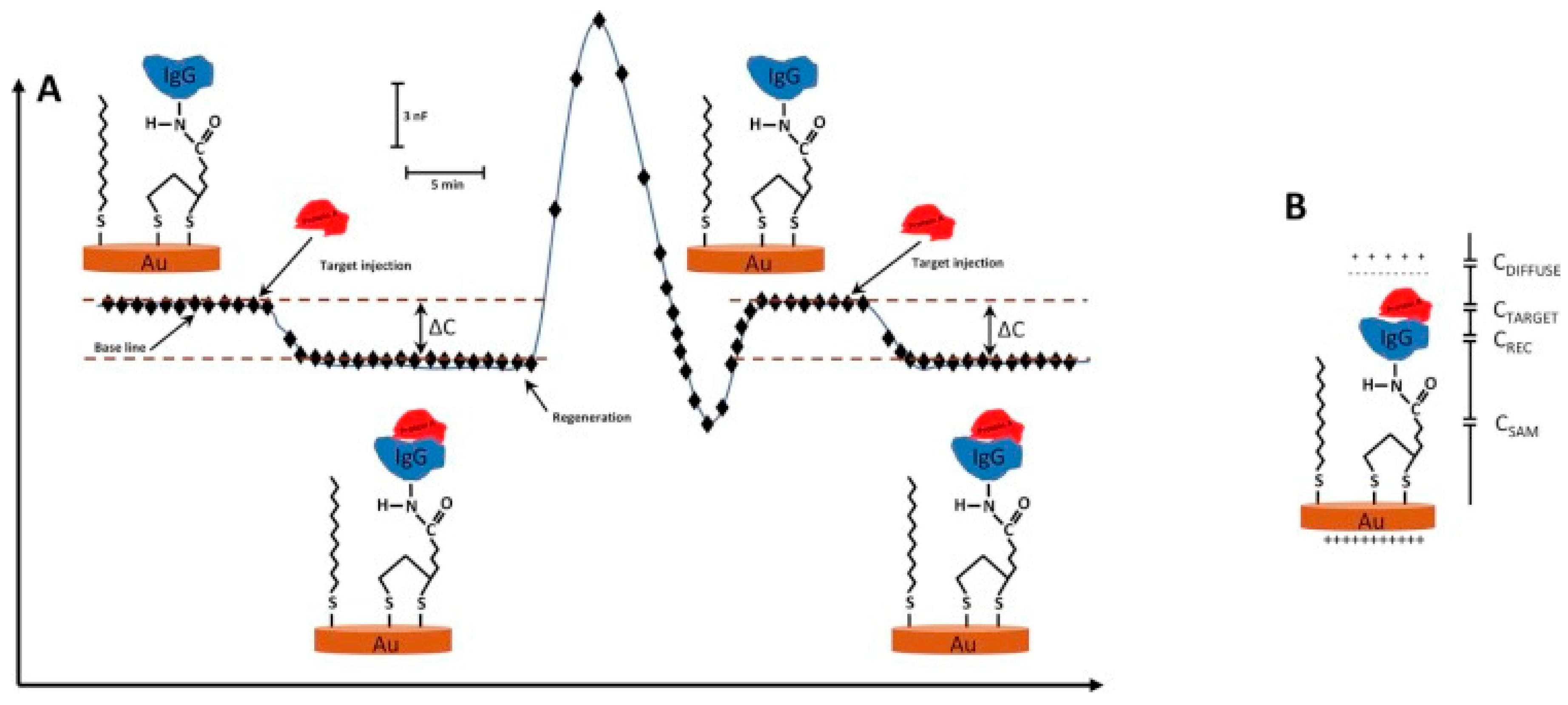
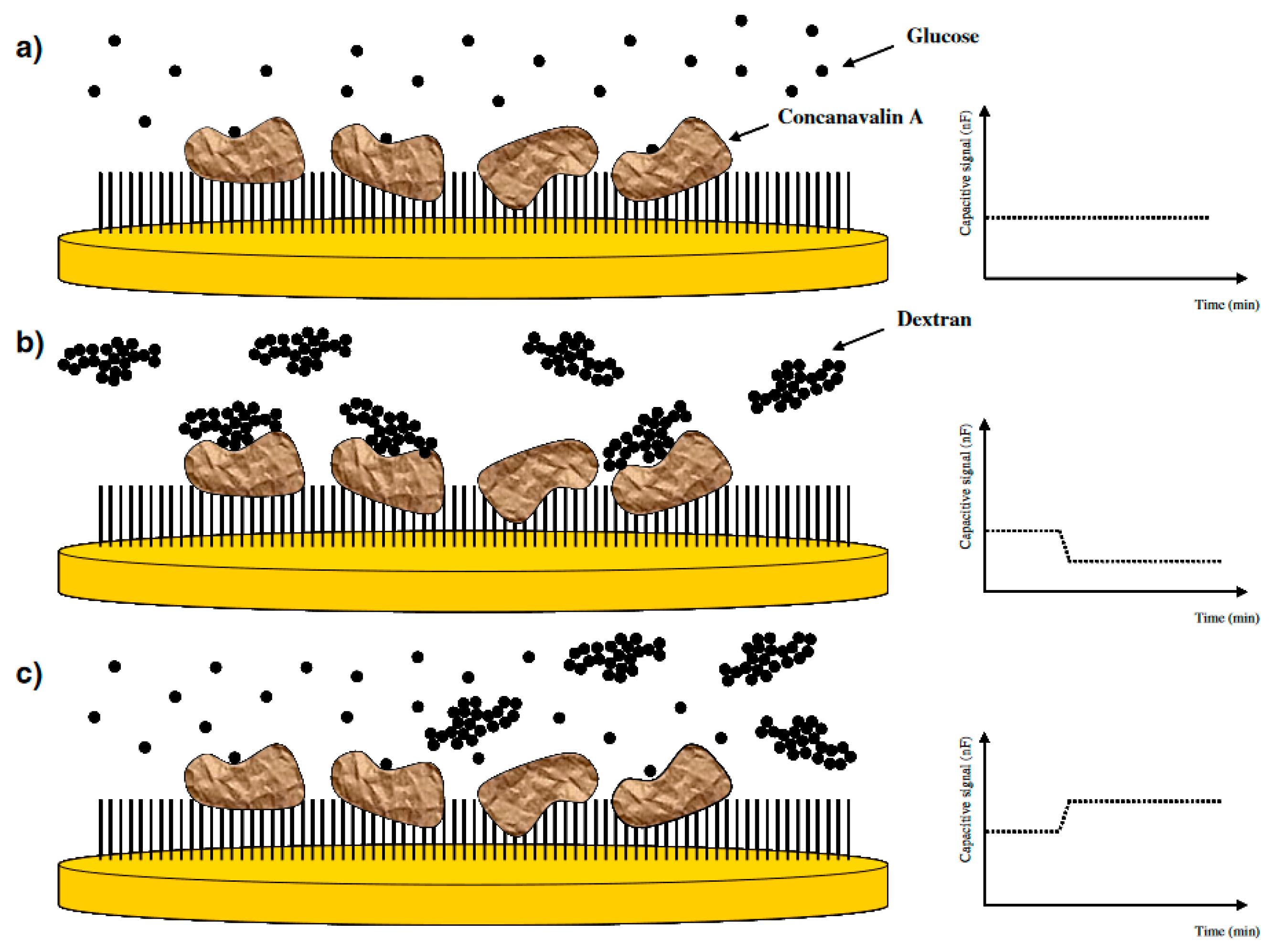
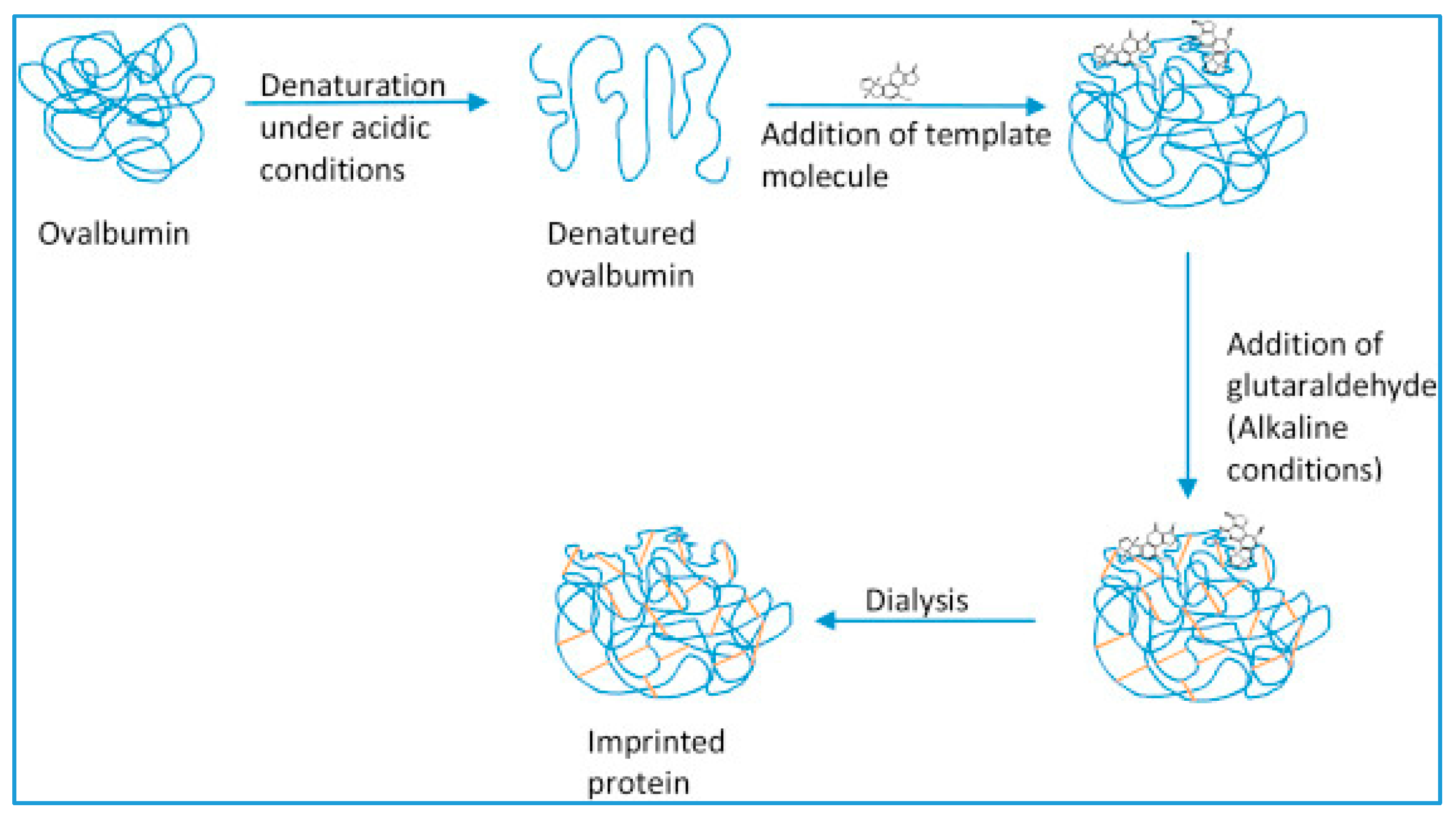

| Target | Sensor Preparation Method | Dynamic range (M) | Limit of Detection (M) | Selectivity | Stability | Ref. | |
|---|---|---|---|---|---|---|---|
| Proteins | Cholera toxin (CT) | Immobilization of anti-CT antibodies on self-assembled monolayer (SAM) of lipoic acid and 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) | 1.0 × 10−13 –1.0 × 10−10 | 1.0 × 10−14uiu | N/D | N/D | [9] |
| Cholera toxin (CT) | Immobilization of anti-CT on gold nanoparticles incorporated on a poly-tyramine layer | 0.1 × 10−18–10 × 10−12 | 9.0 × 10−20 | N/D | Up to 36 times with an RSD of 2.5% | [10] | |
| HIV-1 p24 antigen | Immobilization of anti-HIV 1 p24 antigen on gold nanoparticles incorporated on a poly-tyramine layer | 10.1 × 10−20–10.1 × 10−17 | 3.32 × 10−20 | N/D | N/D | [11] | |
| VEGF | Immobilization of anti-VEGF aptamer first capturing the VEGF protein then, sandwiching with antibody-conjugated magnetic beads | 13 × 10−14–2.6 × 10−11 | N/D | N/D | N/D | [12] | |
| Nucleic acids | 25-mer oligo C | Covalent attachment of 25-mer oligo C on poly-tyramine modified electrode | 10−8–10−11 | 10−11 | Oligo-T was used as the competing agent, when the temperature was increased from RT to 50 °C, the ΔC value decreased from 48 nF·cm−2 to 3 nF·cm−2 | N/D | [13] |
| ssDNA | Thiol modified oligonucleotides were immobilized on Au and 3-glycidoxypropyl-tri-methoxy silane (GOPTS) | 0.5 × 10−6–1.0 × 10−3 | N/D | N/D | GOPTS functionalized surfaces were more stable at 4 °C. Ten-fold decrease in fluorescence intensity after 1 week even when the substrates were stored at 4 °C. | [14] | |
| Nampt | Immobilization of ssDNA aptamers on SAM of mercaptopropionic acid (MPA) | 0–45 × 10−10 | 1.8 × 10−11 | N/D | N/D | [15] | |
| Target DNA | Immobilization of pyrrolidinyl peptide nucleic acid probes (acpcPNA) | 1.0 × 10−11–1.0 × 10−10 | 6–10 × 10−12 | Complementary DNA provided a much higher ΔC compared to single and double mismatched DNA | Could be reused for 58–73 times with an average residual activity of ≥98% | [16] | |
| Cells | Total bacteria | Based on the interaction between E. coli and concanavalin A immobilized on a modified gold surface | 12 CFU·mL−1–1.2 × 10−6 CFU·mL−1 | 12 CFU·mL−1 | N/D | For the first 35 cycles, the residual activity was 95% ± 3% (RSD = 3.2%). After 35 cycles, it was 85%. | [17] |
| E. coli | E. coli cells immobilized on SAM of Mercaptopropionic acid (MPA) | 8 × 105 CFU·mL−1–8 × 107 CFU·mL−1 | N/D | N/D | N/D | [18] | |
| Heavy metals | Hg(II), Cu(II), Zn(II), Cd(II) | Immobilization of metal resistance and metal regulatory proteins on gold electrode | 10−15–10−3 | N/D | N/D | N/D | [19] |
| Cu(II), Cd(II), Hg(II) | 1. Immobilization of whole bacterial cell to emit a bioluminescent/fluorescent signal in the presence of heavy metal ions | 0–200 × 10−6 | 1.0 × 10−6 | N/D | 84% of the activity loss within 6 days | [20] | |
| 2. Immobilization of heavy metal binding proteins | 10−15–10−1 | Stable over 16 days | |||||
| Saccharides | Glucose | Immobilization of ConA on gold nanoparticles incorporated on the tyramine modified gold electrode | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−6 | Small sugars including D-fructose, D-mannose, D-maltose, methyl-α-D-glucopyranoside, methyl-α-D-mannopyranoside also bound instead of glucose | A neglectable loss in sensitivity after 10 cycles (7.5%) | [21] |
| Glucose | Immobilization of ConA and replacement of small glucose with the large glucose polymer | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−6 | Small molecules and high molecular weight dextran also bound instead of glucose | N/D | [22] | |
| Small molecules | Metergoline | Immobilization of molecularly imprinted spherical beads on modified gold electrode | 1–50 × 10−6 | 1.0 × 10−6 | Cross reactant contribution was maximum 1.3 nF | N/D | [23] |
| Aflatoxin B1 | Bioimprinting | 3.2 × 10−6–3.2 × 10−9 | 6.0 × 10−12 | Competing agents’ binding was significantly lower than aflatoxin B1 | Little variation over 28 injections with non-reduced Schiff’s bases | [24] | |
| Ochratoxin A (OTA) | Monoclonal anti-OTA immobilization on Si3N4 substrate combined with magnetic nanoparticles (MNPs) | 2.47–49.52 × 10−12 | 4.57 × 10−12 | Differences for ochratoxin B and aflatoxin B1 were not significant | N/D | [25] |
| Template | Method | Matrix | Comments | Ref. |
|---|---|---|---|---|
| Benzo[a]pyrene (BAP) | BAP-imprinted poly (2-hydroxyethylmethacrylate-N-methacryloyl-(L)-phenylalanine composite cryogel cartridge | Aqueous solutions |
| [42] |
| Melamine | Melamine imprinted monolithic cartridges | Water + milk |
| [43] |
| Cholesterol | Cholesterol imprinted polymeric nanospheres | Gastrointestinal mimicking solution |
| [44] |
| Catalase | Iron chelated poly (2-hydroxyethylmethacrylate-N-methacryloyl-(L)-glutamic acid cryogel discs | Rat liver |
| [45] |
| L-phenylalanine (L-Phe) | L-Phe imprinted cryogel cartridges | Aqueous solutions |
| [46] |
| Triazine | Triazine imprinted monolithic columns | Aqueous solutions |
| [47] |
| Cytochrome c | Surface imprinted bacterial cellulose nanofibers | Rat liver |
| [48] |
| Target | Biosensing Method | Monomers | Dynamic Range | LOD | Selectivity | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| Bovine Serum Albumin (BSA) | Capacitive biosensor with current pulse method | Methacrylic acid (MAA); Poly ethyleneglycol-dimethacrylate (PEGDMA) | 1.0 × 10−20 M–1.0 × 10−8 M | 1.0 × 10−19 M | For human serum albumin (HSA): 5%; For IgG: 3% | >70 assays during 2 months | [56] |
| Prostate specific antigen (PSA) | Capacitive biosensor with current pulse method | MAA; EGDMA | 2.0 × 10−17 M–2.0 × 10−10 M | 16 × 10−17 M | Selectivity coefficient (k) = 2.27 for HSA, k = 2.02 for IgG | About same level during 50 injections | [58] |
| E. coli | Capacitive biosensor with current pulse method | HEMA; (2-Hydroxyethyl methacrylate), N-methacryloyl-L-histidine methyl ester (MAH), EGDMA | 1.0 × 102–1.0 × 107 CFU·mL−1 | 70 CFU·mL−1 | K = 3.14 for B. subtilis, k = 3.32 for S. aureus, k = 2.98 for S. paratyphi | About same level during 70 injections | [59] |
| Trypsin | Capacitive biosensor with current pulse method | N-isopropylacrylamide (NIPAm), N,N-methylenebisacryl, amide (MBAAm), Acrylamide, Hydroxymethylacrylamide | 1.0 × 10−13 M–1.0 × 10−7 M | 3.0 × 10−13 M | K = 733.1 for chymotrypsin (chy), k = 10.56 for BSA, k = 6.50 for lysozyme (Lyz), k = 3.46 for cytochrome c (cyt c) | The loss in performance was about 2% after 80 analyses | [60] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertürk, G.; Mattiasson, B. Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors 2017, 17, 390. https://doi.org/10.3390/s17020390
Ertürk G, Mattiasson B. Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors. 2017; 17(2):390. https://doi.org/10.3390/s17020390
Chicago/Turabian StyleErtürk, Gizem, and Bo Mattiasson. 2017. "Capacitive Biosensors and Molecularly Imprinted Electrodes" Sensors 17, no. 2: 390. https://doi.org/10.3390/s17020390





