Highly Sensitive Sensors Based on Metal-Oxide Nanocolumns for Fire Detection
Abstract
:1. Introduction
2. Experimental Procedure
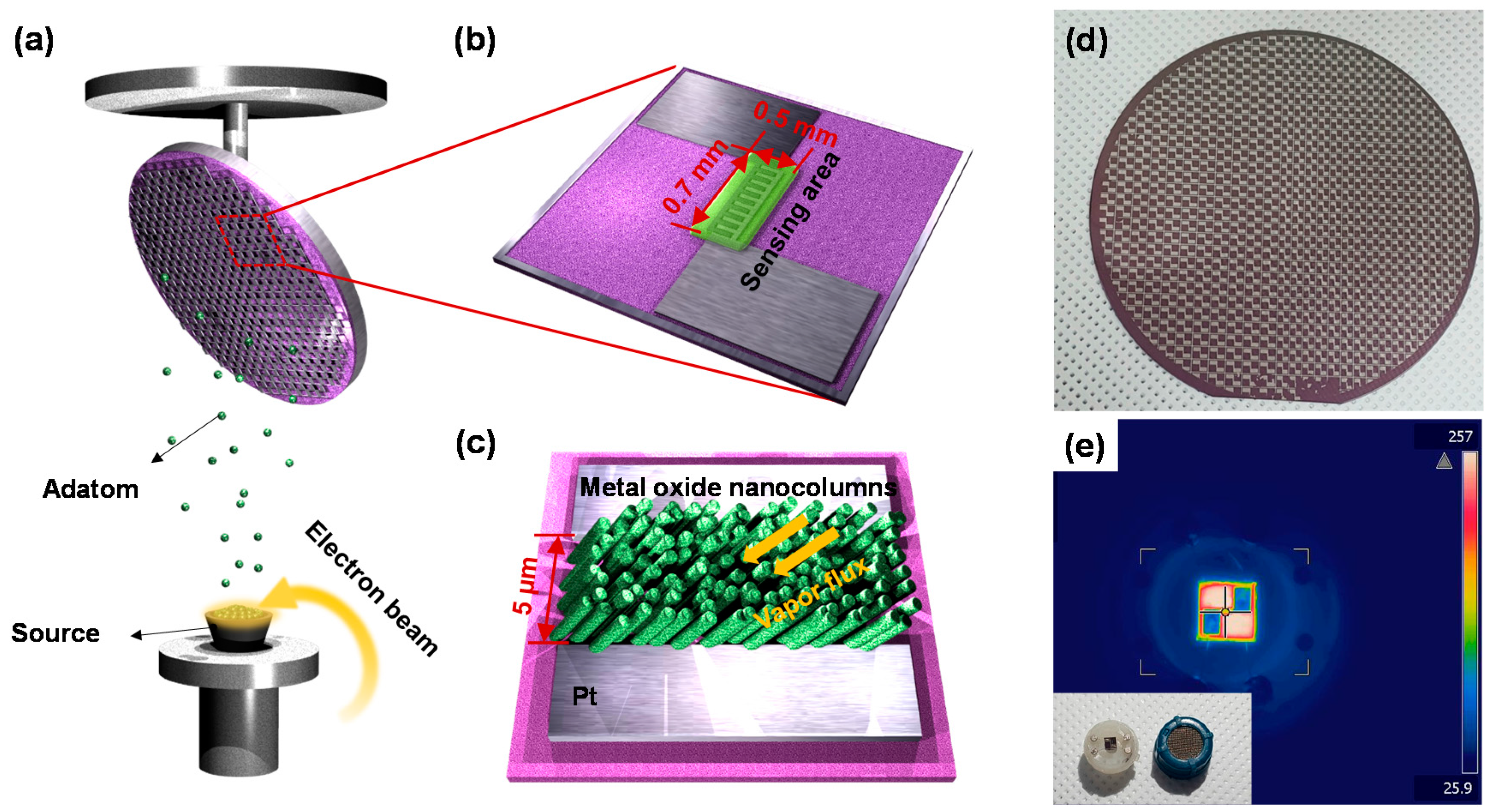
2.1. Fabrication of Sensors
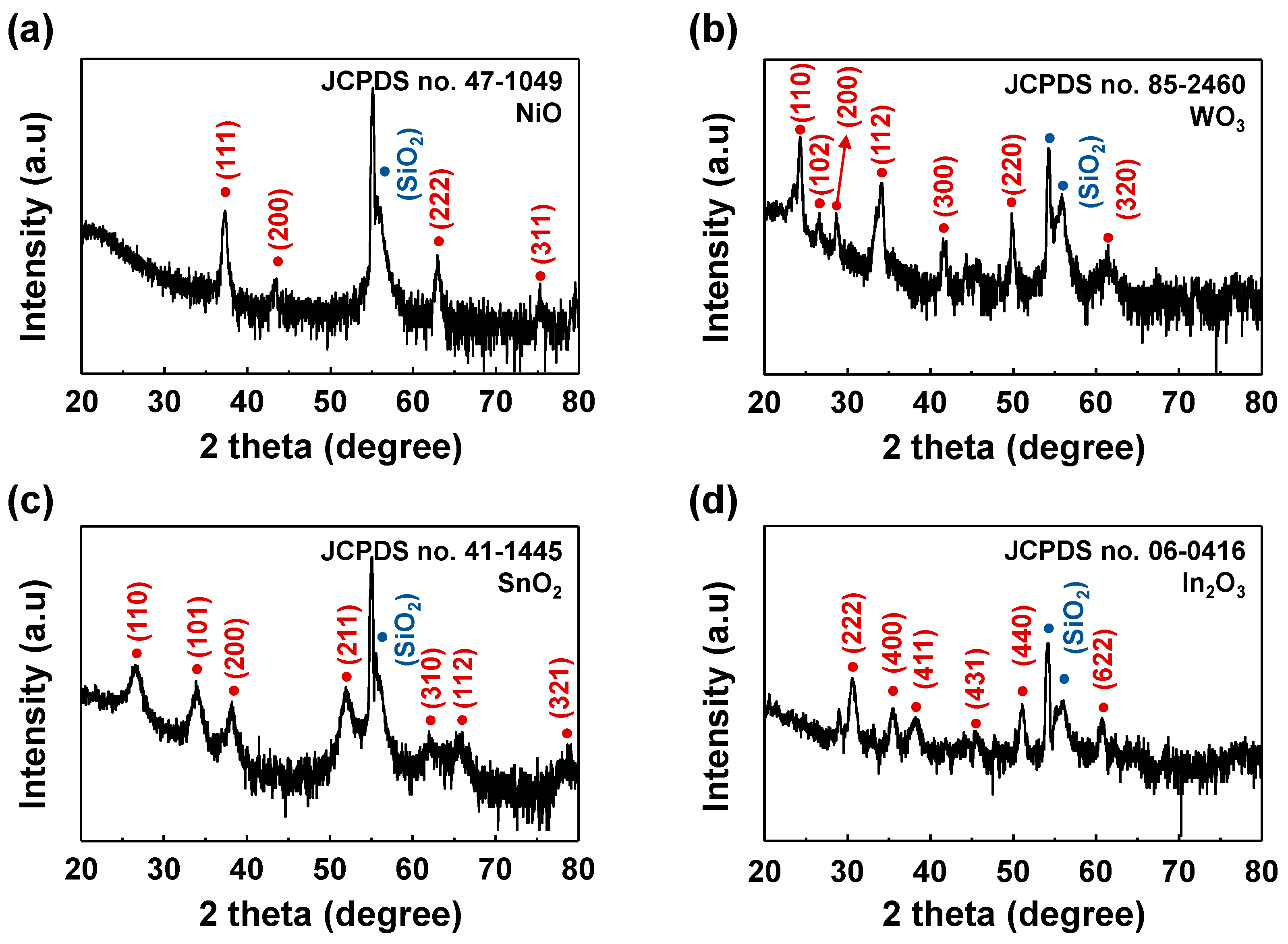
2.2. Characterization of Metal-Oxide NCs
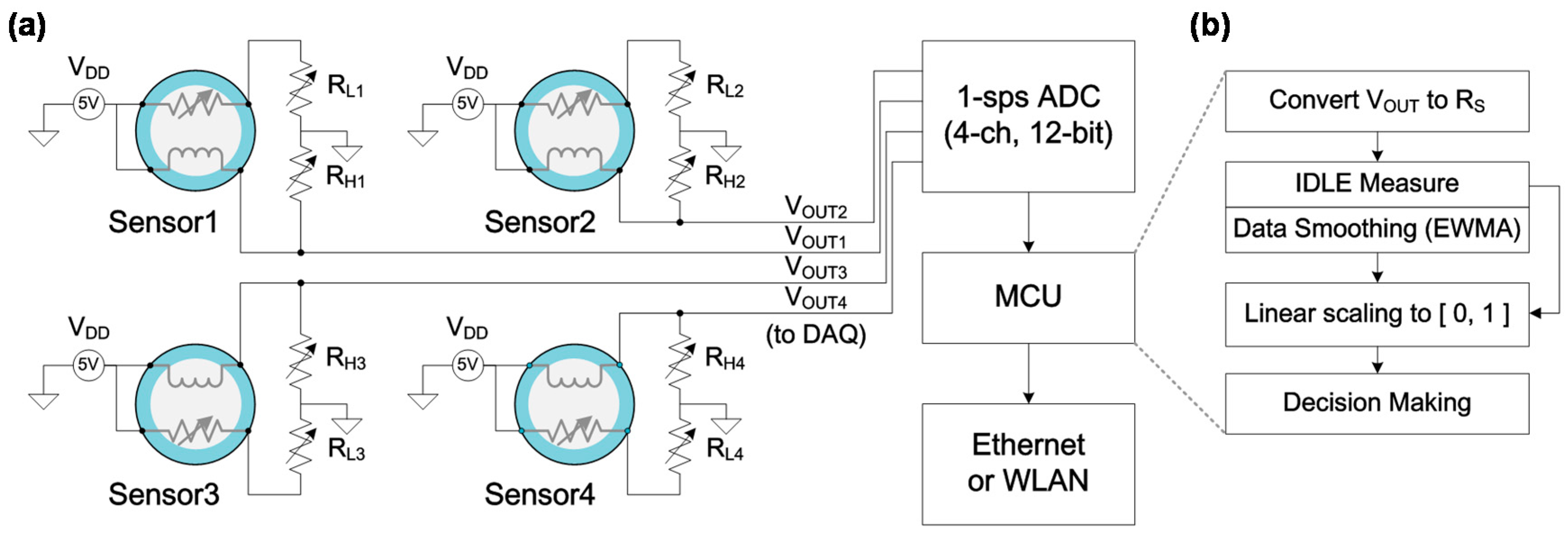
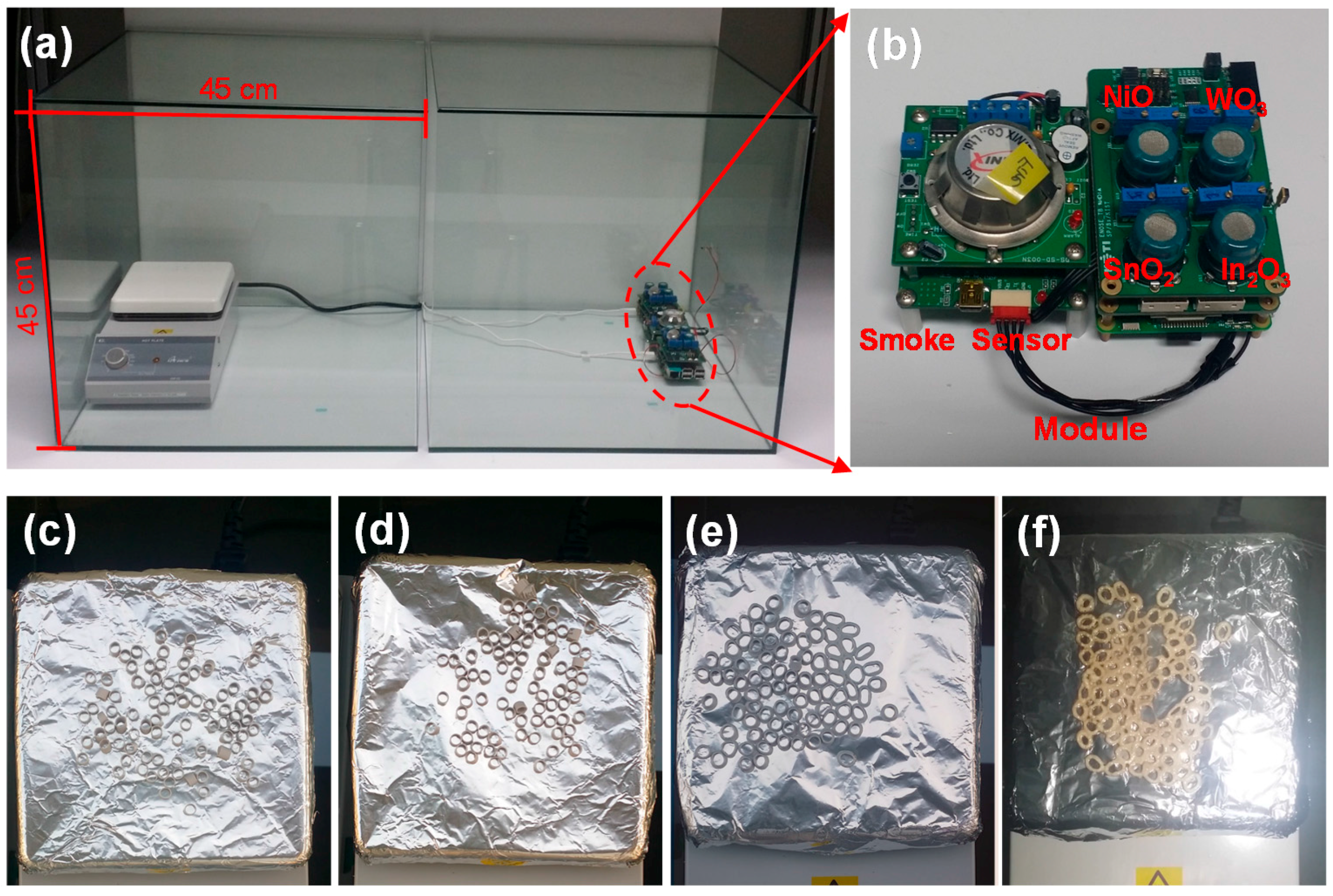
2.3. Gas-Sensing Measurement of Metal-Oxide NCs and Fire Detection Method
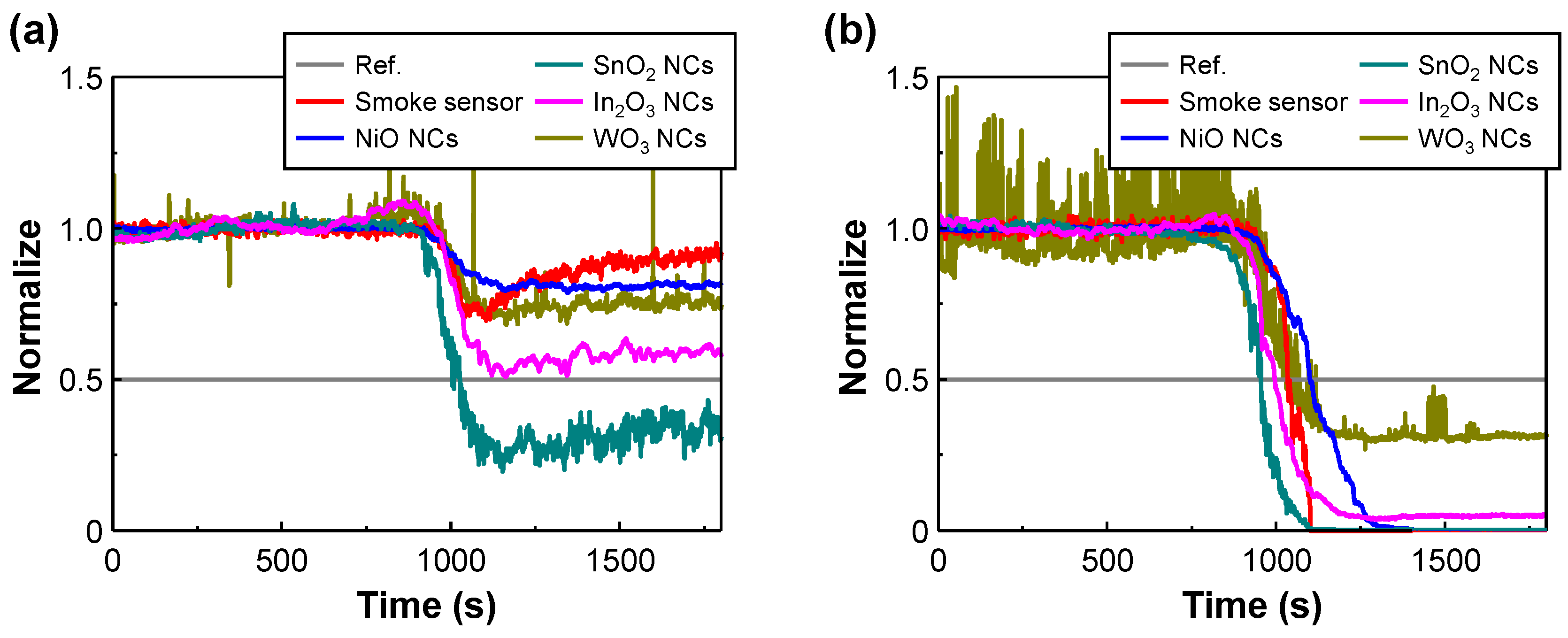
3. Results and Discussion
3.1. Gas Response of Metal-Oxide NCs as a Function of the Hot-Plate Temperature
3.2. Early Fire Detection of Metal-Oxide NC–Based Gas Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chenebert, A.; Breckon, T.P.; Gaszczak, A. A Non-temporal Texture Driven Approach to Real-time Fire Detection. In Proceedings of the IEEE International Conference on Image Processing, Brussels, Belgium, 11–14 September 2011; pp. 1781–1784.
- Chen, S.J.; Hovde, D.C.; Peterson, K.A.; Marshall, A.W. Fire detection using smoke and gas sensors. Fire Saf. J. 2007, 42, 507–515. [Google Scholar] [CrossRef]
- Warda, L.; Tenenbein, M.; Moffatt, M.E. House fire injury prevention update. Part I. A review of risk factors for fatal and non-fatal house fire injury. Injury Prev. 1999, 5, 145–150. [Google Scholar] [CrossRef]
- Bowes, P.C. Casualties Attributed to Toxic Gas and Smoke at Fires a Survey of Statistics; Fire Research Station: Hertfordshire, UK, 1975. [Google Scholar]
- Irvine, D.J.; McCluskey, J.A.; Robinson, I.M. Fire hazards and some common polymers. Polym. Degrad. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Huggett, C.; Levin, B.C. Toxicity of the Pyrolysis and Combustion Products of Poly(Vinyl Chlorides): A Literature Assessment. Fire Mater. 1987, 11, 131–142. [Google Scholar] [CrossRef]
- Lestari, F.; Hayes, A.J.; Green, A.R.; Chattopadhyay, G. An Alternative Method for in Vitro Fire Smoke Toxicity Sssessment of Polymers and Composites using Human Lung Cells. Fire Mater. 2011, 35, 411–429. [Google Scholar] [CrossRef]
- Wichman, I.S. Material flammability, Combustion, Toxicity and Fire Hazard in Transportation. Prog. Energy Combust. Sci. 2003, 29, 247–299. [Google Scholar] [CrossRef]
- Ma, S.; Lu, J.; Gao, J. Study of the low temperature pyrolysis of PVC. Energy Fuels 2002, 16, 338–342. [Google Scholar] [CrossRef]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC Emission from Thermal Degradation of PVC in the Absence and Presence of Copper, Copper (II) Oxide and Copper (II) Chloride. E-J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef]
- Barboni, T.; Cannac, M.; Pasqualini, V.; Simeoni, A.; Leoni, E.; Chiaramonti, N. Volatile and semi-volatile organic compounds in smoke exposure of firefighters during prescribed burning in the Mediterranean region. Int. J. Wildl. Fire 2010, 19, 606–612. [Google Scholar] [CrossRef]
- Tsyrkina, T.; Obvintseva, L.; Kuchaev, V.; Avetisov, A.; Penkin, S.; Sukhareva, I.; Dmitrieva, M. Research of the interaction mechanism between HCl and semiconductor metal oxides. Procedia Chem. 2009, 1, 184–187. [Google Scholar] [CrossRef]
- Grosshandler, W.L. A Review of Measurements and Candidate Signatures for Early Fire Detection; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995.
- Harms, M.; Goschnick, J.; Young, R.C. Early Detectiion and Distinction of Fire Gases with a Gas Sensor Microarray. In Proceedings of the 12th International Conference on Automatic Fire Detection, Gaithersburg, MD, USA, 25–28 March 2001; pp. 416–431.
- Obvintseva, L.A. Metal oxide semiconductor sensors for determination of reactive gas impurities in air. Russ. J. Gen. Chem. 2009, 78, 2545–2555. [Google Scholar] [CrossRef]
- Ahmed, N.N.; Kale, G.M.; Kumar, R.V.; Fray, D.J. Development and testing of HCl gas sensor for flue gas monitoring. Solid State Ion. 1996, 86–88, 1013–1016. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Receptor Function and Response of Semiconductor Gas Sensor. J. Sens. 2009, 2009, 1–21. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U.D.O. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Kim, J.; Ju, B.K.; Yoon, S.; Jang, H.W. Highly Ordered TiO2 Nanotubes on Patterned Substrates: Synthesis-in-Place for Ultrasensitive Chemiresistors. J. Phys. Chem. C 2013, 117, 17824–17831. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodríguez, D.F. The gas sensing properties at room temperature of TiO2 nanotubes by anodization. Sens. Actuators B Chem. 2012, 171–172, 639–643. [Google Scholar] [CrossRef]
- Jin, W.; Chen, W.; Lu, Y.; Zhao, C.; Dai, Y.V. V2O5/polypyrrole Core-shell Nanotubes for Gas Sensor. J. Nanosci. Nanotechnol. 2011, 11, 10834–10838. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Shim, Y.-S.; Han, S.D.; Kim, D.H.; Kim, Y.H.; Kang, C.-Y.; Kim, J.-S.; Kim, M.; Jang, H.W. Vertically ordered SnO2 nanobamboos for substantially improved detection of volatile reducing gases. J. Mater. Chem. A 2015, 3, 17939–17945. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Yoo, H.-W.; Kim, J.Y.; Jung, W.-B.; Jin, M.L.; Kim, J.-S.; Jeon, H.-J.; Jung, H.-T. High-Resolution p-Type Metal Oxide Semiconductor Nanowire Array as an Ultrasensitive Sensor for Volatile Organic Compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.J.; Longo, E.; Tuller, H.L.; Varela, J.A. The role of hierarchical morphologies in the superior gas sensing performance of CuO-based chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Moon, H.G.; Choi, Y.R.; Shim, Y.S.; Choi, K.I.; Lee, J.H.; Kim, J.S.; Yoon, S.J.; Park, H.H.; Kang, C.Y.; Jang, H.W. Extremely sensitive and selective NO probe based on villi-like WO3 nanostructures for application to exhaled breath analyzers. ACS Appl. Mater. Interfaces 2013, 5, 10591–10596. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.S.; Shin, B.; Lee, T.; Kim, J.S.; Lee, S.; Jun, S.C.; Park, H.H.; et al. Chemiresistive Electronic Nose toward Detection of Biomarkers in Exhaled Breath. ACS Appl. Mater. Interfaces 2016, 8, 20969–20976. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.G.; Shim, Y.-S.; Kim, D.H.; Jeong, H.Y.; Jeong, M.; Jung, J.Y.; Han, S.M.; Kim, J.K.; Kim, J.-S.; Park, H.-H.; et al. Self-activated ultrahigh chemosensitivity of oxide thin film nanostructures for transparent sensors. Sci. Rep. 2012, 2, 588. [Google Scholar] [CrossRef] [PubMed]
- Hawkeye, M.M.; Brett, M.J. Glancing angle deposition: Fabrication, properties, and applications of micro- and nanostructured thin films. J. Vac. Sci. Technol. A 2007, 25, 1317. [Google Scholar] [CrossRef]
- Robbie, K. Sculptured thin films and glancing angle deposition: Growth mechanics and applications. J. Vac. Sci. Technol. A Vac. Surf. Film 1997, 15, 1460. [Google Scholar] [CrossRef]
- Robbie, K.; Sit, J.C.; Brett, M.J. Advanced techniques for glancing angle deposition. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 1998, 16, 1115. [Google Scholar] [CrossRef]
- Pashami, S.; Lilienthal, A.J.; Trincavelli, M. Detecting Changes of a Distant Gas Source with an Array of MOX Gas Sensors. Sensors 2012, 12, 16404–16419. [Google Scholar] [CrossRef] [PubMed]
- Benrekia, F.; Attari, M.; Bouhedda, M. Gas Sensors Characterization and Multilayer Perceptron (MLP) Hardware Implementation for Gas Identification Using a Field Programmable Gate Array (FPGA). Sensors 2013, 13, 2967–2985. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, X.; Duan, S.; Jia, P.; Wang, L.; Peng, C.; Zhang, S. Electronic Nose Feature Extraction Methods: A Review. Sensors 2015, 15, 27804–27831. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.H. High-performance, flexible hydrogen sensors that use carbon nanotubes decorated with palladium nanoparticles. Adv. Mater. 2007, 19, 2818–2823. [Google Scholar] [CrossRef]
- Song, Y.G.; Shim, Y.; Han, S.D.; Lee, H.R. Metal Oxide Nanocolumns for Extremely Sensitive Gas Sensors. J. Sens. Sci. Technol. 2016, 25, 184–185. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Haensch, A.; Kim, I.D.; Barsan, N.; Weimar, U.; Lee, J.H. The role of NiO doping in reducing the impact of humidity on the performance of SnO2-based gas sensors: Synthesis strategies, and phenomenological and spectroscopic studies. Adv. Funct. Mater. 2011, 21, 4456–4463. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stǎnoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Shim, Y.-S.; Song, Y.G.; Han, S.D.; Lee, Y.-S.; Kang, C.-Y. Highly Sensitive Sensors Based on Metal-Oxide Nanocolumns for Fire Detection. Sensors 2017, 17, 303. https://doi.org/10.3390/s17020303
Lee K, Shim Y-S, Song YG, Han SD, Lee Y-S, Kang C-Y. Highly Sensitive Sensors Based on Metal-Oxide Nanocolumns for Fire Detection. Sensors. 2017; 17(2):303. https://doi.org/10.3390/s17020303
Chicago/Turabian StyleLee, Kwangjae, Young-Seok Shim, Young Geun Song, Soo Deok Han, Youn-Sung Lee, and Chong-Yun Kang. 2017. "Highly Sensitive Sensors Based on Metal-Oxide Nanocolumns for Fire Detection" Sensors 17, no. 2: 303. https://doi.org/10.3390/s17020303





