Single Interdigital Transducer Approach for Gravimetrical SAW Sensor Applications in Liquid Environments
Abstract
:1. Introduction
2. Material and Methods
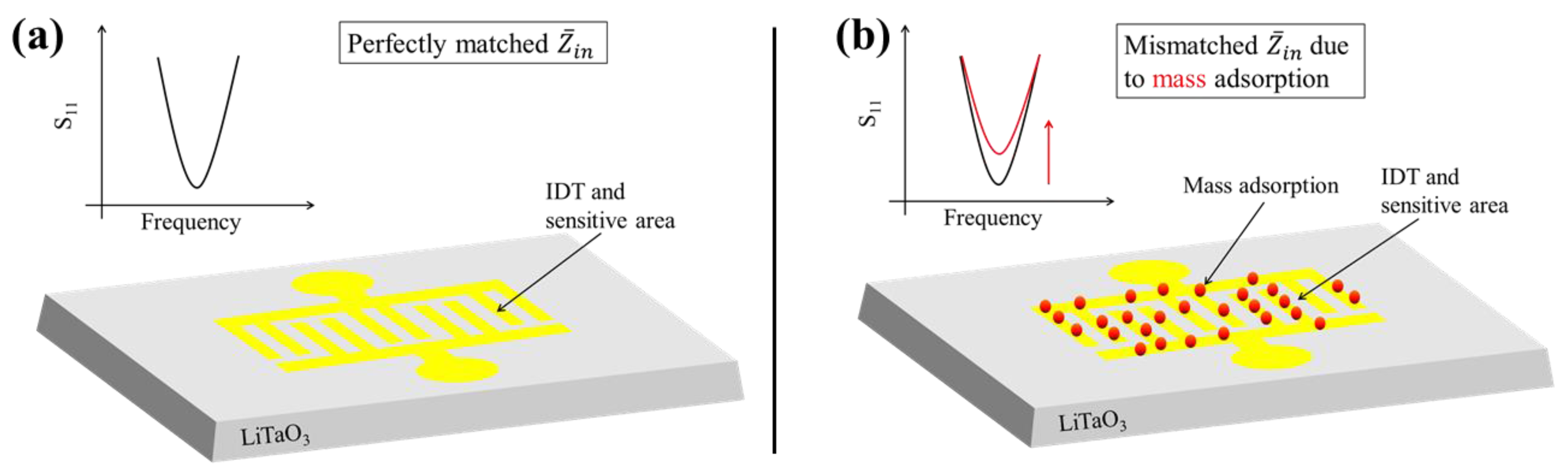
2.1. Sensor Concept
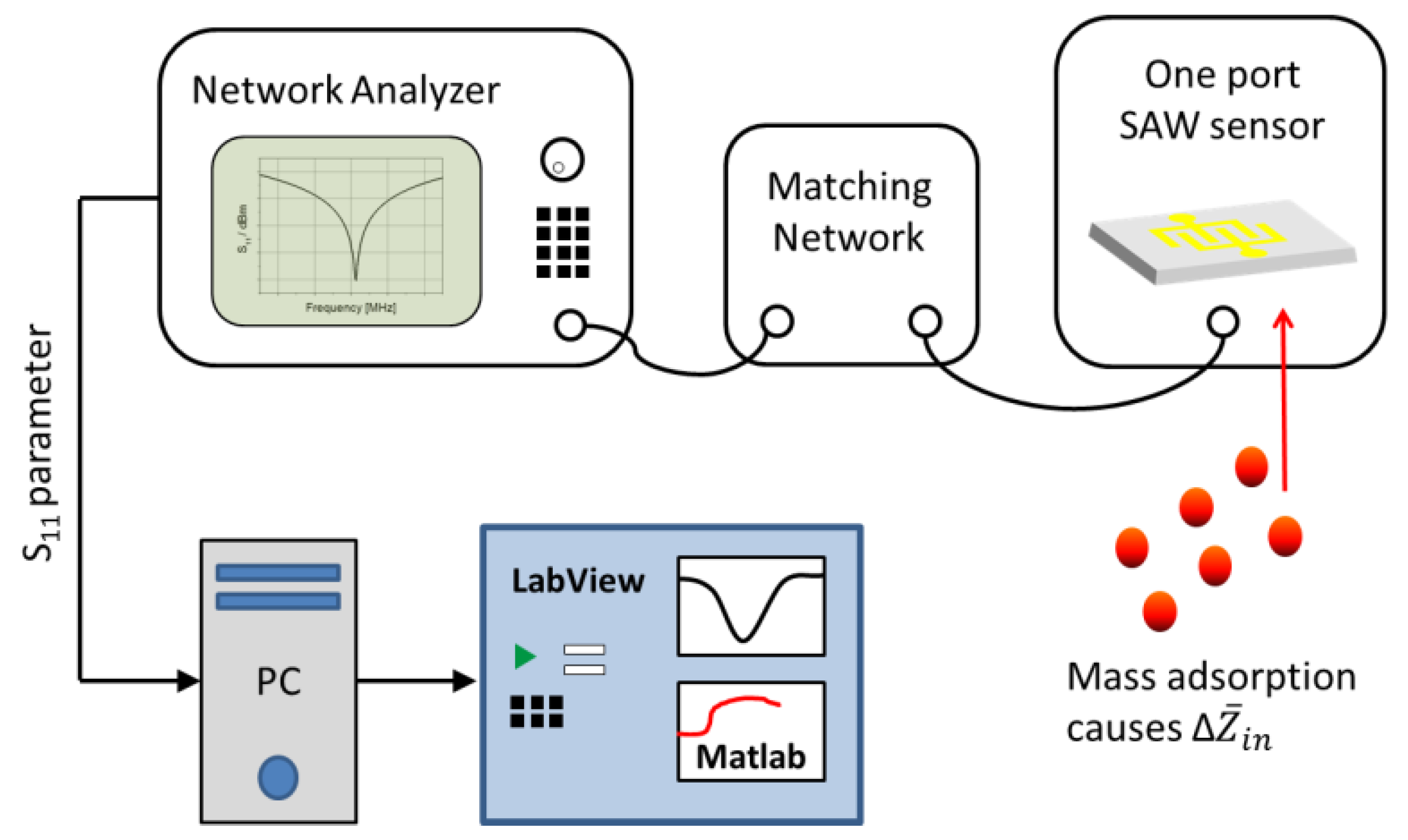
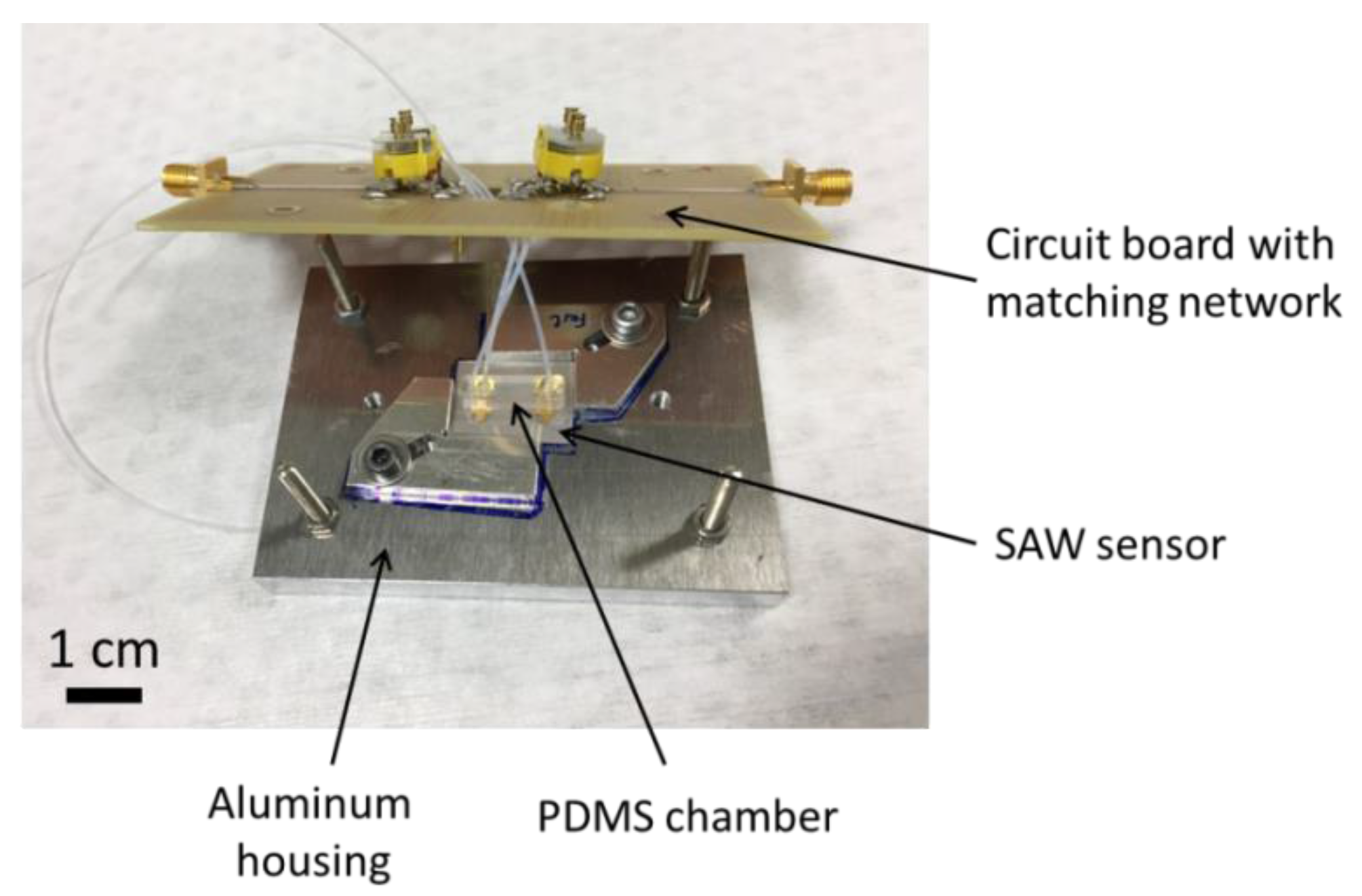
2.2. Measurement Setup
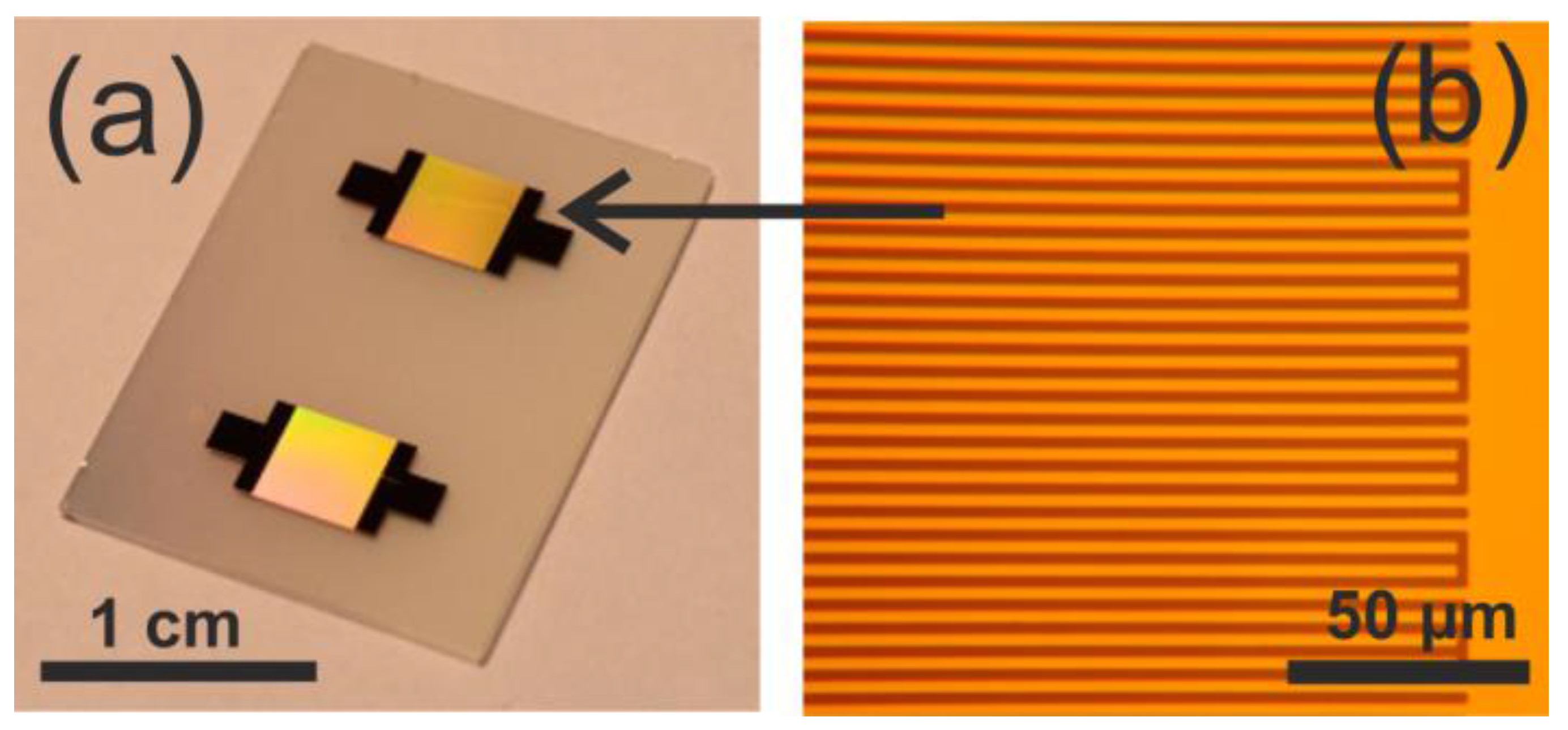
2.3. SAW Chip Fabrication and Packaging
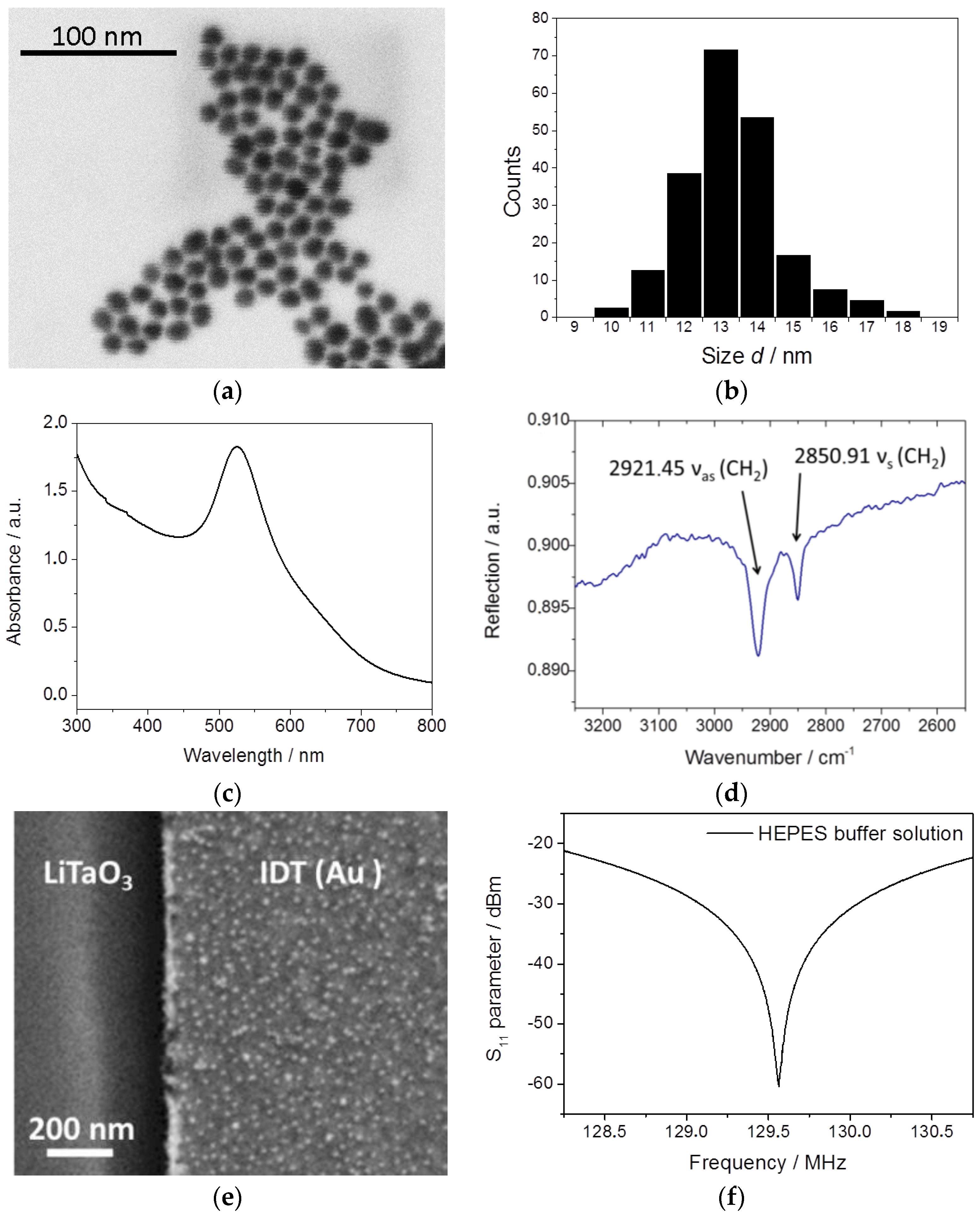
2.4. Synthesis and Characterization of 4-Mercaptophenylacetic Acid Gold Nanoparticles (MPA–AuNP)
2.4.1. Materials
2.4.2. Preparation of MPA Functionalized AuNP
2.4.3. Functionalization of the SAW Substrates with 11-Amino-1-undecanethiol (AUT)
3. Results and Discussion
3.1. Characterization of IDT Surface Functionalization and MPA–AuNP Interaction
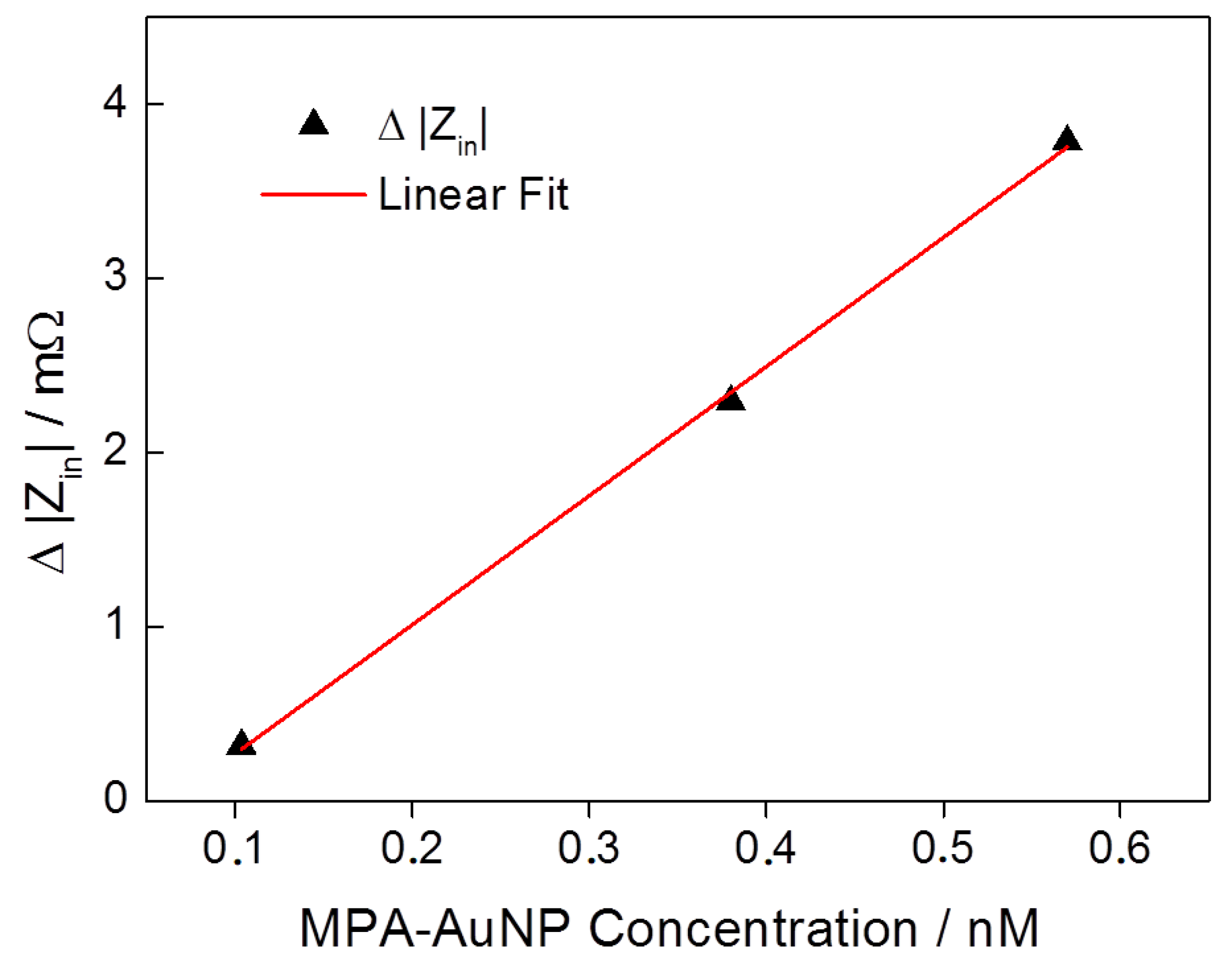
3.2. Mass Loading and Sensor Sensitivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hashimoto, K. Surface Acoustic Wave Devices in Telecommunications; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- White, R.M.; Voltmer, F.W. Direct piezoelectric coupling to surface elastic waves. Appl. Phys. Lett. 1965, 7, 314–316. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface Acoustic Wave Sensor with Pd/ZnO Bilayer Structure for Room Temperature Hydrogen Detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rodriguez, L.; Smith, M.S.; Figueroa, J.A.; Malocha, D.C.; Nam, B.H. Concrete temperature monitoring using passive wireless surface acoustic wave sensor system. Sens. Actuators A 2015, 224, 131–139. [Google Scholar] [CrossRef]
- Sayar Irani, F.; Tunaboylu, B. SAW Humidity Sensor Sensitivity Enhancement via Electrospraying of Silver Nanowires. Sensors 2016, 16, 2024. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Cassano, G. Relative humidity sensing by PVA-coated dual resonator SAW oscillator. Sens. Actuators B Chem. 2000, 68, 300–306. [Google Scholar] [CrossRef]
- Hohmann, S.; Kögel, S.; Brunner, Y.; Schmieg, B.; Ewald, C.; Kirschhöfer, F.; Brenner-Weiß, G.; Länge, K. Surface Acoustic Wave (SAW) Resonators for Monitoring Conditioning Film Formation. Sensors 2015, 15, 11873–11888. [Google Scholar] [CrossRef] [PubMed]
- Gronewold, T.M.A. Surface acoustic wave sensors in the bioanalytical field. Anal. Chim. Acta 2007, 603, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Senveli, S.U.; Tigli, O. A Novel Surface Acoustic Wave Sensor for Microparticle Sensing and Quantification. IEEE Sens. J. 2015, 15, 5748–5754. [Google Scholar] [CrossRef]
- Rapp, M.; Wessa, T.; Ache, H.J. Modification of commercially available low-loss SAW devices towards an immunosensor for in-situ. In Proceedings of the IEEE Ultrasonics Symposium, Seattle, WA, USA, 7–10 November 1995; pp. 433–436. [Google Scholar]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Shiokawa, S.; Moriizumi, T. Design of SAW Sensor in Liquid. Jpn. J. Appl. Phys. 1988, 27, 142–144. [Google Scholar] [CrossRef]
- Bender, F.; Mohler, R.E.; Ricco, A.J.; Josse, F. Identification and quantification of aqueous aromatic hydrocarbons using SH-surface acoustic wave sensors. Anal. Chem. 2014, 86, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Bisoffi, M.; Hjelle, B.; Brown, D.C.; Branch, D.W.; Edwards, T.L.; Brozik, S.M.; Bondu-Hawkins, V.S.; Larson, R.S. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens. Bioelectron. 2008, 23, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, J.; von Schickfus, M.; Hunklinger, S. A SAW immunosensor for operation in liquid using a SiO2 protective layer. Sens. Actuators B 2001, 76, 147–151. [Google Scholar] [CrossRef]
- Love, A.E.H. Some Problems of Geodynamics; Cambridge University Press: Cambridge, UK, 1911. [Google Scholar]
- Blanc, L.; Tetelin, A.; Boissiere, C.; Tortissier, G.; Dejous, C.; Rebiere, D. Love Wave Characterization of the Shear Modulus Variations of Mesoporous Sensitive Films During Vapor Sorption. IEEE Sens. J. 2012, 12, 1442–1449. [Google Scholar] [CrossRef]
- Harding, G.L.; Du, J.; Dencher, P.R.; Barnett, D.; Howe, E. Love wave acoustic immunosensor operating in liquid. Sens. Actuators A 1997, 61, 279–286. [Google Scholar] [CrossRef]
- Gizeli, E.; Bender, F.; Rasmusson, A.; Saha, K.; Josse, F.; Cernosek, R. Sensitivity of the acoustic waveguide biosensor to protein binding as a function of the waveguide. Biosens. Bioelectron. 2003, 18, 1399–1406. [Google Scholar] [CrossRef]
- Ten, S.T.; Hashim, U.; Gopinath, S.C.B.; Liu, W.W.; Foo, K.L.; Sam, S.T.; Rahman, S.F.A.; Voon, C.H.; Nordin, A.N. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sankaranarayanan, S.; Fan, C.; Su, Y.; Bhethanabotla, V.R. Achieving Lower Insertion Loss and Higher Sensitivity in a SAW Biosensor via Optimization of Waveguide. IEEE Sens. J. 2017, 17, 1608–1616. [Google Scholar] [CrossRef]
- Binhack, M.; Buff, W.; Klett, S.; Hamsch, M. A combination of SAW-resonators and conventional sensing elements for wireless passive remote sensing. In Proceedings of the IEEE Ultrasonics Symposium, San Juan, PR, USA, 22–25 October 2000; pp. 495–498. [Google Scholar]
- Yao, S.; Chen, K.; Liu, D.; Nie, L. Circuit network analysis method applied to surface acoustic wave impedance system in liquids. Anal. Chim. Acta 1994, 294, 311–318. [Google Scholar] [CrossRef]
- Chang, K.-S.; Chang, C.-K.; Chen, C.-Y. A surface acoustic wave sensor modified from a wireless transmitter for the monitoring of the growth of bacteria. Sens. Actuators B 2007, 125, 207–213. [Google Scholar] [CrossRef]
- Guliyev, E.; Klett, S. Pulling of SAW resonators for wireless sensor application. In Proceedings of the IEEE Ultrasonics Symposium, Rotterdam, The Netherlands, 18–21 September 2005; pp. 2202–2205. [Google Scholar]
- Grate, J.W.; Klusty, M. Surface Acoustic Wave Vapor Sensors Based on Resonator Devices. Anal. Chem. 1991, 63, 1719–1727. [Google Scholar] [CrossRef]
- Reindl, L.; Ruile, W. Programmable reflectors for SAW-ID-tags. In Proceedings of the IEEE Ultrasonics Symposium, Baltimore, MD, USA, 31 October–3 November 1993; pp. 125–130. [Google Scholar]
- Gamba, P.; Goldoni, E.; Savazzi, P.; Arpesi, P.G.; Sopranzi, C.; Dufour, J.-F.; Lavagna, M. Wireless Passive Sensors for Remote Sensing of Temperature on Aerospace Platforms. IEEE Sens. J. 2014, 14, 3883–3892. [Google Scholar] [CrossRef]
- Steindl, R.; Pohl, A.; Seifert, F. Impedance loaded SAW sensors offer a wide range of measurement opportunities. IEEE Trans. Microw. Theory Tech. 1999, 47, 2625–2629. [Google Scholar] [CrossRef]
- Schimetta, G.; Dollinger, F.; Weigel, R. A wireless pressure-measurement system using a SAW hybrid sensor. IEEE Trans. Microw. Theory Tech. 2000, 48, 2730–2735. [Google Scholar] [CrossRef]
- Luo, W.; Fu, Q.; Wang, J.; Wang, Y.; Zhou, D. Theoretical Analysis of Wireless Passive Impedance-Loaded SAW Sensors. IEEE Sens. J. 2009, 9, 1778–1783. [Google Scholar] [CrossRef]
- Pohl, A. A Review of Wireless SAW Sensors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Quintero, S.; Figueiredo, S.; Takahashi, V.; Llerena, R.; Braga, A. Passive Downhole Pressure Sensor based on Surface Acoustic Wave Technology. Sensors 2017, 17, 1635. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Konstantinidis, G.; Buiculescu, V.; Dinescu, A.; Stavrinidis, A.; Stefanescu, A.; Stavrinidis, G.; Giangu, I.; Cismaru, A.; Modoveanu, A. GaN/Si based single SAW resonator temperature sensor operating in the GHz frequency range. Sens. Actuators A 2014, 209, 115–123. [Google Scholar] [CrossRef]
- Nomura, T.; Yasuda, T. Measurement of acoustic properties of liquid using liquid flow SH-SAW sensor system. Jpn. J. Appl. Phys. 1993, 32, 2372–2375. [Google Scholar] [CrossRef]
- Krischke, A. Rothammels Antennenbuch; Franckh-Kosmos Verlag: Stuttgart, Germany, 1995; pp. 118–122. [Google Scholar]
- Bristol, T.W.; Jones, W.R.; Snow, P.B.; Smith, R. Applications of double electrodes in acoustic surface wave device design. In Proceedings of the IEEE Ultrasonics Symposium, Boston, MA, USA, 4–7 October 1972; pp. 343–345. [Google Scholar]
- Turkevitch, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kaulen, C.; Babajani, N.; Bourone, S.; Homberger, M.; Karthäuser, S.; Besmehn, A.; Simon, U. Differential Adsorption of Gold Nanoparticles to Gold/Palladium and Platinum Surfaces. Langmuir 2014, 30, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Bourone, S.D.M.; Kaulen, C.; Homberger, M.; Simon, U. Directed Self-Assembly and Infrared Reflection Absorption Spectroscopy Analysis of Amphiphilic and Zwitterionic Janus Gold Nanoparticles. Langmuir 2016, 32, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Gilles, S.; Kaulen, C.; Pabst, M.; Simon, U.; Offenhäusser, A.; Mayer, D. Patterned self-assembly of gold nanoparticles on chemical templates fabricated by soft UV nanoimprint lithography. Nanotechnology 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-S.; Gwo, S. Quantitative surface acoustic wave detection based on colloidal gold nanoparticles and their bioconjugates. Anal. Chem. 2008, 80, 3318–3326. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Lazar, J.; Schnakenberg, U.; Böker, A. In situ Electrochemical Impedance Spectroscopy of Electrostatically Driven Selective Gold nanoparticle Adsorption on Block Copolymer Lamellae. ACS Appl. Mater. Interfaces 2016, 8, 27282–27290. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Jaszczolt, K.; Michna, A.; Siwek, B.; Szyk-Warszynska, L.; Zembala, M. Irreversible Adsorption of Particles on Heterogeneous Surfaces. Adv. Colloid Interface Sci. 2005, 118, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Benes, E.; Gröschl, M.; Burger, W.; Schmid, M. Sensors based on piezoelectric resonators. Sens. Actuators A 1995, 48, 1–21. [Google Scholar] [CrossRef]
- Gaso Rocha, M.I.; Jiménez, Y.; Laurent, F.A.; Arnau, A. Love Wave Biosensors: A Review; InTech: London, UK, 2013. [Google Scholar]
- Mauder, A. Comparison between delay line and two port resonator. Sens. Actuators B 1995, 26, 187–190. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Peters, O.; Schnakenberg, U. One-port portable SAW sensor system. Meas. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Peters, O.; Serve, S.; Schnakenberg, U. Portable SAW Impedance Sensor Using 1-Port Resonator Approach. In Proceedings of the Eurosensors, Paris, France, 3–6 September 2017; pp. 361–364. [Google Scholar]
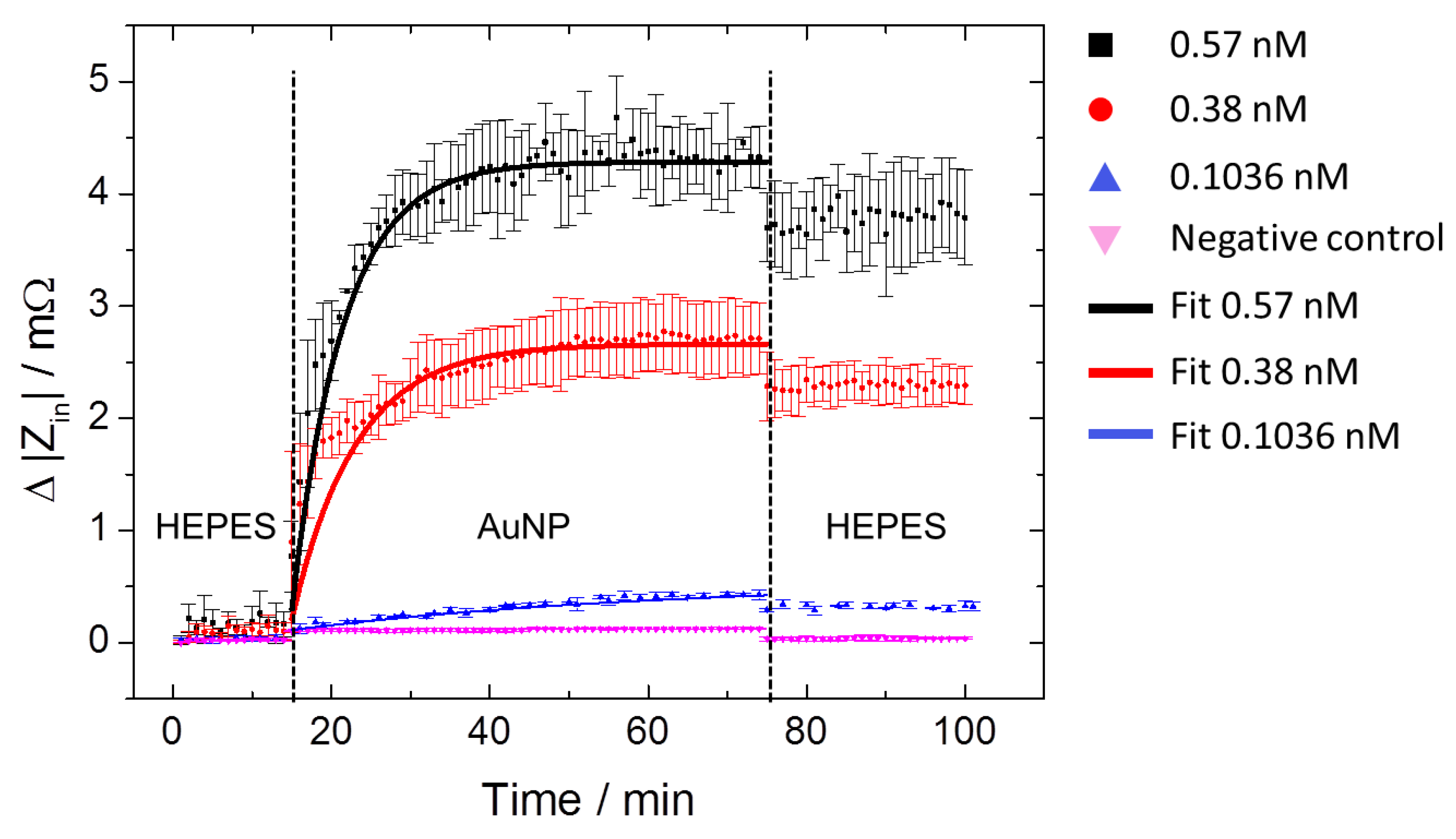
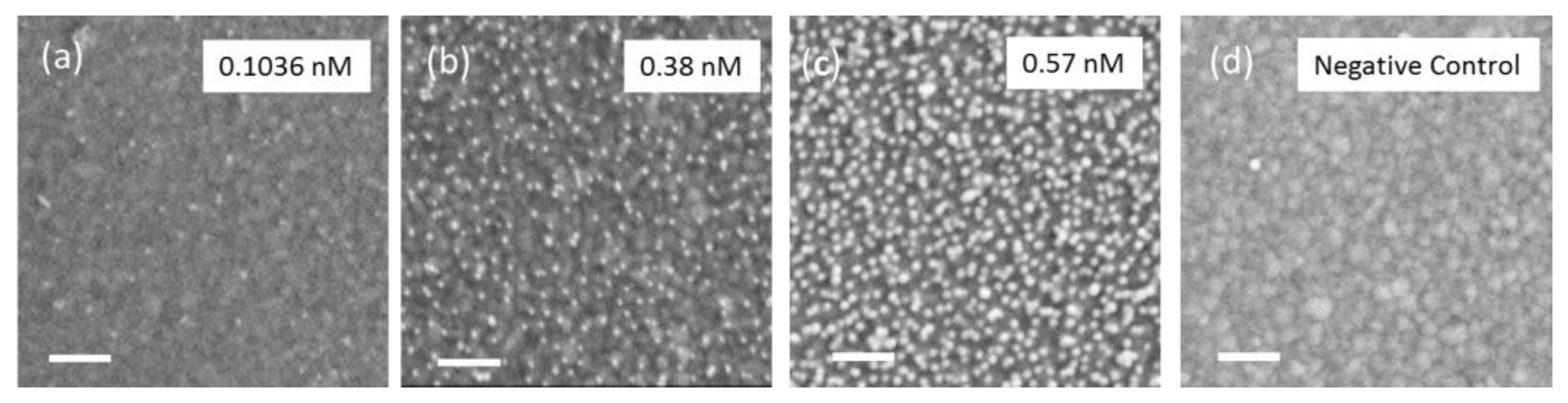
| Concentration (nM) | τ (min) | ZSat (mΩ) | AuNP Density (µm−2) |
|---|---|---|---|
| 0.1036 | 46.2 | 0.32 | 208 ± 31 |
| 0.38 | 8.01 | 2.29 | 917 ± 58 |
| 0.57 | 6.45 | 3.79 | 1333 ± 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Kaulen, C.; Simon, U.; Schnakenberg, U. Single Interdigital Transducer Approach for Gravimetrical SAW Sensor Applications in Liquid Environments. Sensors 2017, 17, 2931. https://doi.org/10.3390/s17122931
Nguyen VH, Kaulen C, Simon U, Schnakenberg U. Single Interdigital Transducer Approach for Gravimetrical SAW Sensor Applications in Liquid Environments. Sensors. 2017; 17(12):2931. https://doi.org/10.3390/s17122931
Chicago/Turabian StyleNguyen, Vu Hoa, Corinna Kaulen, Ulrich Simon, and Uwe Schnakenberg. 2017. "Single Interdigital Transducer Approach for Gravimetrical SAW Sensor Applications in Liquid Environments" Sensors 17, no. 12: 2931. https://doi.org/10.3390/s17122931





